Abstract
N-acetylcysteine (NAC) is a thiol donor with antioxidant properties that has potential use as an ergogenic aid. However, NAC is associated with adverse reactions that limit its use in humans.
Purpose
The authors evaluated NAC efficacy as a thiol donor before handgrip exercise, measuring changes in serum cysteine and glutathione status and recording adverse reactions in adult subjects across a range of doses.
Methods
Healthy individuals ingested NAC capsules (9 ± 2 or 18 ± 4 mg/kg) or solution (0, 35, 70, or 140 mg/kg). Venous blood samples were collected and subjects answered a questionnaire about adverse reactions.
Results
Low doses of NAC (capsules) did not affect plasma cysteine or glutathione or cause adverse reactions. Adverse reactions to NAC solution were predominantly mild and gastrointestinal (GI). Intensity of GI reactions to 140 mg/kg NAC was significantly higher than placebo (in a.u., 0.67 ± 0.16 vs. 0.07 ± 0.04; p < .05). Plasma cysteine concentration increased with NAC dose from 9.3 ± 0.7 μM (placebo) to 65.3 ± 6.7 μM (140 mg/kg); however, there was no difference (p > .05) in plasma cysteine for 70 mg/kg vs. 140 mg/kg. Similar increases were observed for the ratio of cysteine to total cysteine, which was directly related to handgrip exercise performance. Plasma glutathione was elevated and oxidized glutathione diminished (p < .05) with NAC 140 mg/kg vs. placebo.
Conclusion
NAC effects on plasma thiols are maximized by oral administration of 70 mg/kg, a dose that does not cause significant adverse reactions.
Keywords: glutathione, cysteine, fatigue, side effect
In the field of sport nutrition, there is growing interest in the capacity of N-acetylcysteine (NAC) to delay muscle fatigue. NAC is a thiol donor with nonspecific antioxidant properties that delays skeletal-muscle fatigue in healthy subjects (Kelly, Wicker, Barstow, & Harms, 2009; Matuszczak et al., 2005; McKenna et al., 2006; Medved et al., 2004; Reid, Stokic, Koch, Khawli, & Leis, 1994; Travaline et al., 1997) and patients with chronic obstructive pulmonary disease (Koechlin et al., 2004). These performance-enhancing effects of NAC appear to be mediated by increases in circulating and myocyte levels of cysteine and glutathione (Ferreira, Gilliam, & Reid, 2009; Ferreira & Reid, 2008; McKenna et al., 2006; Medved et al., 2004).
Use of NAC as a countermeasure for fatigue can be limited by adverse reactions associated with oral or intravenous administration (Zyoud, Awang, Syed Sulaiman, Sweileh, & Al-Jabi, 2010). NAC has been used in the clinical setting to treat acetaminophen overdose (Sandilands & Bateman, 2009) and contrast-induced nephropathy (Briguori et al., 2007) and as a mucolytic agent (Nash, Stephenson, Ratjen, & Tullis, 2009). Various doses of NAC are used in the clinical setting (Kelly, 1998): low doses (5–25 mg/kg) for mucolytic effects (Nash et al., 2009) and to prevent contrast-induced nephropathy (Sar et al., 2010; Thiele et al., 2010) and high doses (140–150 mg/kg) to minimize hepatotoxicity after acetaminophen overdose (Heard, 2008). Adverse reactions to NAC usually occur within the first hour of exposure and seem to be dose dependent (Zyoud et al., 2010). These reactions are mainly gastrointestinal (GI), such as stomach or intestinal gas, nausea, vomiting, and diarrhea (Flanagan & Meredith, 1991; Mant, Tempowski, Volans, & Talbot, 1984; Sandilands & Bateman, 2009; Zyoud et al., 2010). Other, less frequent reactions include sleepiness; metallic taste; light-headedness; redness of eye, face, or hand; cough; welts; and wheezing (Matuszczak et al., 2005; Reid et al., 1994). Adverse reactions to NAC can interfere with physical activity (Reid et al., 1994) and may be exacerbated by exercise.
NAC is marketed over the counter with the claim that it raises glutathione levels without adverse reactions when taken at doses of <1,500 mg/day. Published reports span a 15-fold range in doses used to test NAC effects on performance: from ~9 mg/kg in patients with chronic obstructive pulmonary disease (Koechlin et al., 2004) to 150 mg/kg in healthy subjects (Reid et al., 1994). However, NAC dose dependence has not been evaluated in the context of either glutathione regulation or adverse reactions during exercise.
Based on this information, we tested the hypothesis that effects of NAC on plasma cysteine and glutathione levels and adverse reactions in the context of fatiguing exercise are dose dependent. We sought to identify doses of NAC that increase plasma cysteine and glutathione in the absence of adverse reactions. According to the published range of NAC doses, we adopted a comprehensive approach and investigated doses ranging from approximately 9 to 140 mg/kg. We hypothesized that all doses of NAC would increase plasma glutathione, and adverse reactions would be most prevalent at high doses. The current study is a critical step in the development of future large-scale trials testing NAC as an adjunct therapy for muscle fatigue in rehabilitation medicine and sports sciences.
Methods
Subjects
We screened 30 individuals for inclusion in the study. Seventeen healthy subjects participated (age 30 ± 2 years, body weight 86 ± 5 kg). The study followed a double-blinded, placebo-controlled, crossover design. Subjects received NAC in capsules (Arm 1) or solution (Arm 2). Ten individuals completed each arm of the study, with 3 subjects involved in both arms. The inclusion criteria were age 21–55 years, absence of acute illness (e.g., common cold) and chronic diseases, and systolic/diastolic blood pressure ≤130/90 mm Hg. We excluded individuals taking any medication other than aspirin on a regular basis and those who could not follow our exercise protocol. Volunteers read and signed a consent form agreeing to participate in the study. All experiments and procedures were approved by the institutional review board of the University of Kentucky and performed at the institution’s General Clinical Research Center.
Arm 1
For each trial, the subjects received two capsules containing either 0 mg (placebo), 300 mg, or 600 mg of NAC yielding total doses of 0, ~9, or ~18 mg/kg per trial. The subjects ingested one capsule while in the hospital between 11 a.m. and 2 p.m. and another capsule on the same day between 9 and 11 p.m. We contacted them by phone in the evening of the trial to remind them to take the capsule and to keep track of the time of capsule intake. The subjects returned to the hospital the following morning (8–9 a.m.) for the experiments. Trials were separated by 1 week. On the day of the experiment blood was drawn from the antecubital vein and each subject performed a bout of fatiguing handgrip exercise. Five to ten minutes after finishing the exercise bout subjects answered a questionnaire about adverse reactions experienced during the trial.
Arm 2
For each trial, the subjects drank a placebo (0.9% saline) or NAC solution containing doses of 35, 70, and 140 mg/kg. Because of the characteristic sulfur smell of NAC the subjects wore a nose clip during exposure to and intake of the experimental solution. Moreover, they drank the experimental solution in a room with gauze soaked in full-strength NAC solution. Experimental visits were between 8 and 11 a.m. and separated by 1–2 weeks. As an attempt to mask the salty taste of NAC, we used saline (0.9%) to adjust the drug concentration to the desired dose. The volume of all solutions matched the highest dose of NAC. A venous blood sample was drawn 60 min after the subject drank the solution. Approximately 75–90 min after ingestion of the experimental solution, and within 5–10 min of finishing a bout of fatiguing exercise (see below), subjects answered a questionnaire about adverse reactions to NAC.
Adverse Reactions
The questionnaire on adverse reactions was derived from responses reported by subjects who received NAC in previous studies (Matuszczak et al., 2005; Reid et al., 1994). Reactions on the questionnaire included upset stomach; nausea; stomach or intestinal gas; sleepiness; metallic taste; lightheadedness; redness of eye, face, or hand; cough; welts; and wheezing. Subjects were asked to grade the intensity of each reaction and sensation as none, mild, moderate, or severe. They were asked to describe and grade any reactions that were not included in the questionnaire. Although our focus was on responses immediately after ingestion of NAC, we also contacted the subjects after their visits to inquire about adverse reactions experienced during the 24–48 hr after the experiment. We also asked the subjects if they thought they had received NAC or placebo.
Blood Sampling and Analysis
Venous blood samples were collected from the antecubital vein using a 22-gauge intravenous catheter (Jelco, Medex Medical Ltd., Rossendale, UK) connected to polyethylene tubing and a syringe. Approximately 2 ml of blood per sample were drawn slowly to prevent hemolysis. Hemolyzed samples were discarded because of plasma cysteine and glutathione contamination by lysed red blood cells. The blood was placed in a microcentrifuge tube containing a preservative solution (100 mM serine-borate [pH 8.5] containing [per ml] 0.5 mg sodium heparin, 1 mg bathophenanthroline disulfonate, and 2 mg iodoacetic acid] and gently inverted to ensure adequate mixing. After centrifugation (12,000 rpm, 60 s), 200 μl of plasma were placed in a tube containing 200 μl perchloric acid solution and stored at −80 °C until later analysis. Plasma samples were analyzed for glutathione (GSH), oxidized glutathione (GSSG), cysteine (CySH), cystine (CySS), and cysteine-glutathione disulfide (CySSG) using high-performance liquid chromatography (Clinical Biomarkers Laboratory, Emory University, Atlanta, GA). For further details on processing and analysis of blood samples see Jones (2002). Total glutathione (TGSH) was calculated as GSH + 2•GSSG + CySSG. Total cysteine (TCyS) was calculated as CySH + 2•CySS + CySSG.
Handgrip Exercise
Our focus was the perception of adverse reactions in the context of fatiguing exercise. Thus, subjects performed a handgrip exercise test. This test was conducted using a custom-made handgrip dynamometer connected to a personal computer (Matuszczak et al., 2005). The exercise protocol consisted of three maximal voluntary contractions (MVC) of ~5 s duration separated by 1 min rest followed by a sequence of repetitive isometric contractions (3 s on, 3 s off). Exercise was stopped when the subject failed to reach the target force (70% MVC) in three consecutive efforts (task failure). Subjects were familiarized with the exercise protocol before entering the study. We asked all volunteers to avoid caffeinated beverages for a minimum of 12 hr before each experimental visit.
To assess handgrip performance we determined the number of efforts in which the subject reached the target force and calculated the force-time integral and maximal rate of force development (dF/dtMAX) of each effort. All efforts before task failure were added to estimate the amount of work performed during the exercise bout (total force-time integral). The decline in dF/dtMAX during the fatigue trial was analyzed by linear regression. The slope of this relationship was used as an indicator of muscle fatigue, wherein negative slopes suggest faster fatigue.
NAC Capsules and Solution
NAC capsules and solution were obtained from PhysioLogics (Northglenn, CO) and American Regent Laboratories Inc. (Shirley, NY), respectively. Investigational Drug Services of the University of Kentucky College of Medicine prepared the solutions, capsules, and randomization sequence for each arm of the study. Investigators and subjects were blinded regarding the content of the capsules or solutions being administered during data collection and analysis. We limited our study to oral administration because in the United States, the Food and Drug Administration approves the clinical use of the intravenous route only for treatment of acetaminophen overdose.
Statistics
Comparison of plasma thiol data among doses in each experimental arm was performed using one-way repeated-measures ANOVA. Post hoc analyses were conducted using Tukey’s test. Side effects were ranked from 0 (none) to 3 (severe), categorized as GI and non-GI, and compared by Page’s test with post hoc analysis based on Hollander’s multiple comparison test. The relationship between variables was determined using Pearson’s correlation and linear regression. Values of p less than .05 were considered statistically significant. All statistics were calculated using commercially available software (Prism 5.0b for Mac OS X, GraphPad Software Inc., La Jolla, CA). Data are shown as M ± SE.
Results
Adverse Reactions to NAC
After capsule intake, 3 participants reported mild adverse reactions (Table 1). One subject experienced the majority of reactions including a reaction to placebo capsules. Reactions to solution were predominantly GI and were dose dependent in frequency and intensity (Table 2, Figure 1). The intensity of adverse reactions was mostly mild (Table 2), but some individuals experienced GI reactions of moderate intensity after the highest dose of NAC. None of the subjects vomited during the 24–48 hr after NAC ingestion. One participant had diarrhea within the first hour of receiving NAC 140 mg/kg. Non-GI reactions were predominantly sleepiness, metallic taste, and light-headedness (Table 1) and were not dose dependent. Subjects who reported sleepiness as a side effect mentioned that they could not distinguish the effects of the experimental solution from the lack of their usual intake of caffeine (one cup of coffee) in the morning. All subjects reported “none” for welts and wheezing and denied that adverse reactions interfered with performance of the handgrip exercise. Adverse reactions during the 24- to 48-hr period after ingestion of NAC were generally similar to those in Table 2 but of lesser intensity than at 1 hr postingestion.
Table 1.
Individual Adverse Reactions With Oral Ingestion of N-Acetylcysteine Capsules
| Adverse reaction | Dose
|
||
|---|---|---|---|
| 0 mg/kg | 9 ± 2 mg/kg | 18 ± 4 mg/kg | |
| Gastrointestinal | |||
| upset stomach | — | d1, h1 | d1 |
| nausea | — | d1 | d1 |
| stomach or intestinal gas | d1 | d1 | d1, i1 |
| Nongastrointestinal | |||
| sleepiness | — | — | d1, i1 |
| metallic taste | — | — | i1 |
| cough | — | h1 | — |
Note. Lowercase letters (d, h, i) refer to individual subjects from Arm 1. Superscripts show ranked intensity of adverse reaction reported by each subject. Ranked intensities are (1) mild, (2) moderate, and (3) severe. Subjects who indicated “none” (0) for an adverse reaction are omitted for clarity. None of the subjects experienced light-headedness; redness of eyes, face, or hands; welts; or wheezing. Dashes indicate that none of the subjects reported the adverse reaction listed. Subjects received placebo or 600 or 1,200 mg of N-acetylcysteine capsules. Doses are shown normalized for body weight.
Table 2.
Individual Adverse Reactions With Oral Ingestion of N-Acetylcysteine Solution
| Adverse reaction | Dose
|
|||
|---|---|---|---|---|
| 0 mg/kg | 35 mg/kg | 70 mg/kg | 140 mg/kg | |
| Gastrointestinal | ||||
| upset stomach | — | H1, I1 | F1, H1, I2 | A1, C1, D2, E1, I1, J1 |
| nausea | — | — | — | C1, H1, J1 |
| stomach or intestinal gas | F1, H1 | E1, F1 | F1, I3 | A1, C2, D2, F2, I2, J1 |
| Nongastrointestinal | ||||
| sleepiness | A1, D1, H1 | D1 | B1, C1, D1 | F1, I1 |
| metallic taste | J2 | A1 | A1, D1, I1 | A1, H1, I1, J1 |
| light-headedness | H1 | — | D1 | J1 |
| redness of eye, face, or hands | F1 | — | — | — |
| cough | — | — | D1 | — |
Note. Uppercase letters (A–J) refer to individual subjects from Arm 2. Superscripts show ranked intensity of adverse reactions reported by each subject. Ranked intensities are (1) mild, (2) moderate, and (3) severe. Subjects who indicated “none” (0) for an adverse reaction are omitted for clarity. None of the subjects experienced welts or wheezing. See Table 1 for further details.
Figure 1.
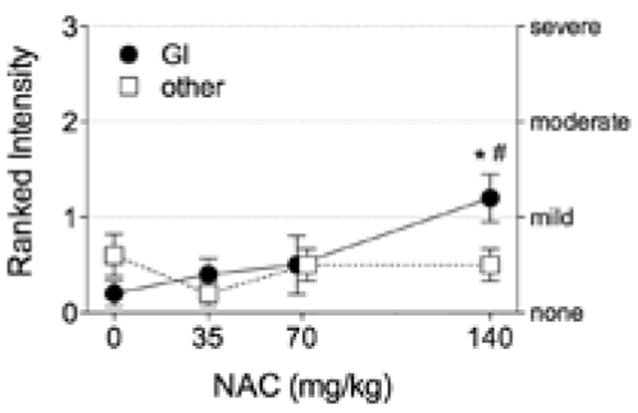
Intensity of adverse reactions to oral ingestion of N-acetylcysteine (NAC) solution. GI = gastrointestinal (upset stomach, nausea, stomach or intestinal gas); other = non-GI (sleepiness; metallic taste; light-headedness; redness of eye, face, or hand; cough; welts; and wheezing). Data are M ± SE of highest intensity of a side effect in each category. *p < .05 vs. placebo. #p < .05 vs. 35 mg/kg (Hollander post hoc test).
Plasma Redox Balance
There was no systematic effect of NAC capsules on plasma cysteine or cysteine derivatives measured 10–14 hr after intake (Table 3). GSH derivatives tended to increase with capsule dose, but these changes were not resolvable statistically (Figure 2). In contrast, 1 hr after drinking NAC solution, TCyS and CySS were increased compared with placebo (Figure 3). CySH progressively increased with NAC dose and reached a plateau at the 70 mg/kg dose. Total GSH was not affected by NAC solution, but higher doses did shift the GSH pool to a more reduced state. Plasma GSH increased after 140 mg/kg NAC; GSSG and CySSG declined after 70 or 140 mg/kg. As an index of plasma antioxidant potential, we analyzed changes in the ratio of CySH to TCyS after NAC administration. Low doses administered by capsules had no effect on CySH:TCyS (Table 3). Higher doses of NAC solution progressively increased CySH:TCyS (Figure 4, top), a response that correlated closely with the observed decline in GSSG (Figure 4, bottom).
Table 3.
Plasma Cysteine Derivatives After Ingestion of N-Acetylcysteine (NAC) Capsules
| Derivative | Dose
|
||
|---|---|---|---|
| 0 mg/kg | 9 ± 2 mg/kg | 18 ± 4 mg/kg | |
| CySH (μM) | 13.0 ± 0.99 | 11.4 ± 0.70 | 13.7 ± 1.09 |
| CySS (μM) | 71 ± 3 | 65 ± 4 | 67 ± 4 |
| TCyS (μM) | 156 ± 6 | 144 ± 8 | 151 ± 9 |
| CySH:TCyS | 0.083 ± 0.005 | 0.080 ± 0.006 | 0.091 ± 0.006 |
Note. CySH = cysteine; CySS = cystine; TCyS = total cysteine. Some samples were hemolyzed, hence, not analyzed. Data are M ± SE from N = 9 (0 mg/kg), N = 8 (NAC 9 mg/kg), and N = 8 subjects (NAC 18 mg/kg).
Figure 2.
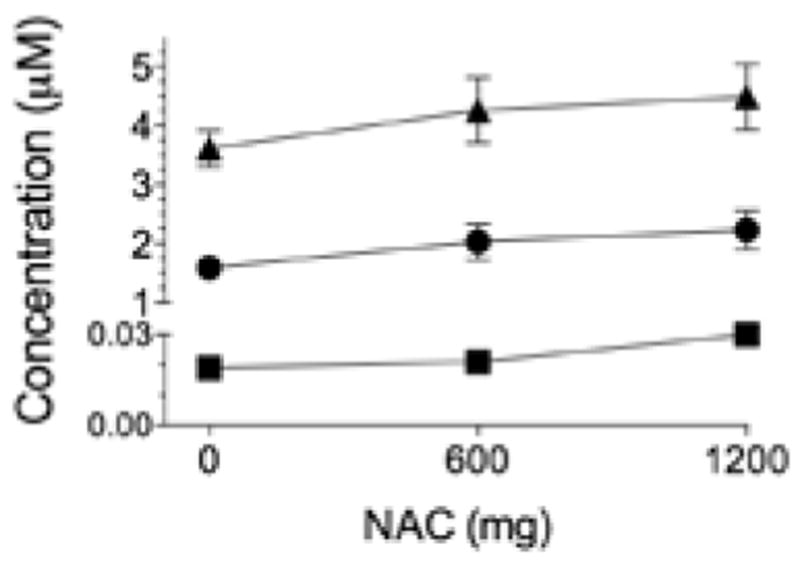
N-acetylcysteine (NAC) capsules do not alter glutathione-based thiols in plasma. Triangles = total glutathione; circles = glutathione; squares = oxidized glutathione. Placebo is represented by 0 mg. Data are M ± SE.
Figure 3.
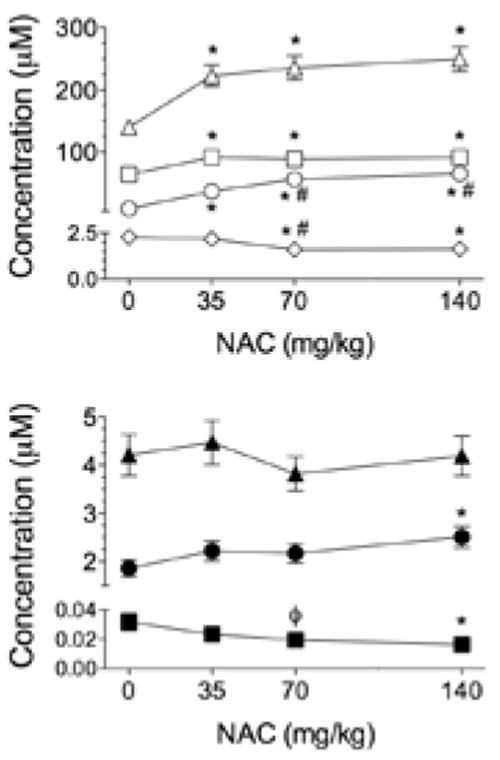
N-acetylcysteine (NAC) solution alters cysteine- and glutathione-based thiols in a dose-dependent manner. Top: triangles = total cysteine; squares = cystine; circles = cysteine; diamonds = cysteine-glutathione disulfide. Bottom: triangle = total glutathione; circles = glutathione; squares = oxidized glutathione. Placebo is represented by 0 mg/kg. Data are M ± SE. *p < .05 vs. placebo. #p < .05 vs. 35 mg/kg. ϕp < .1 vs. 0 mg/kg.
Figure 4.
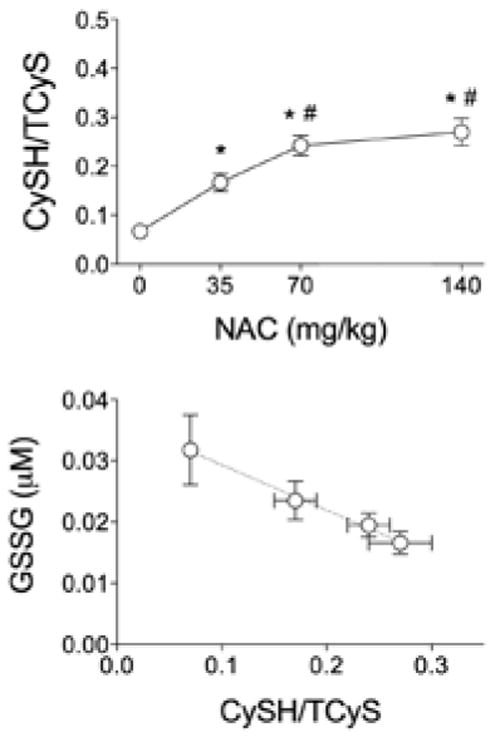
N-acetylcysteine (NAC) effects on cysteine-to-total-cysteine ratio (CySH:TCyS). Top: NAC solution increases the ratio of plasma cysteine (CySH) to total cysteine (TCyS) in a dose-dependent manner. Bottom: Negative correlation between CySH:TCyS and oxidized glutathione (GSSG). Circles represent different doses of NAC. Dotted line indicates best fit from linear regression (p < .05). Data are M ± SE. *p < .05 vs. placebo. #p < .05 vs. 35 mg/kg.
Handgrip Exercise Performance
We evaluated NAC effects in a standardized exercise environment by having subjects perform a fatiguing handgrip task (Matuszczak et al., 2005). In the placebo trial of Matuszczak et al., subjects completed an average of 48 repetitions when the target force was 50% MVC. We used a higher target force (70% MVC), and, as expected, the total number of repetitions performed by our subjects was on average lower (33 ± 4) than in the previous study from our laboratory (Matuszczak, et al., 2005).
Despite the limited sample size, plasma CySH:TCyS emerged as a novel indicator of handgrip performance. Analyses of plasma samples obtained before exercise showed strong correlations between CySH:TCyS and all three markers of handgrip fatigue (Figure 5). Even after low-dose NAC capsules, preexercise CySH:TCyS measured 10–14 hr after drug intake correlated positively with the total number of successful repetitions (p < .005; data not shown). This relationship was unique to CySH:TCyS. No other CySH or GSH derivatives were significantly related to the three fatigue indices (results not shown).
Figure 5.
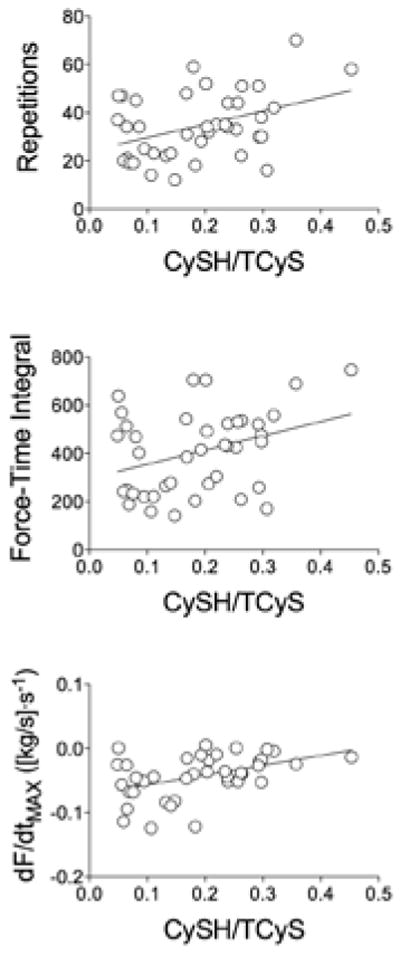
Relationship between cysteine-to-total-cysteine ratio (CySH:TCyS) and handgrip exercise performance. Top: total repetitions before task failure. Middle: total force-time integral (FTI) before task failure. Bottom: slope of decline in maximal rate of force development per repetition from onset of handgrip exercise to task failure. p < .05 for all relationships shown.
Discussion
In the current study we observed that the frequency and intensity of adverse GI reactions increased with progressively higher doses of NAC. A marked increase in adverse reactions became apparent with NAC 140 mg/kg; responses to NAC 35 mg/kg and NAC 70 mg/kg were not different from placebo. The effects of NAC on the concentration and redox state of plasma CySH and GSH variables were also dose dependent. CySH and CySH:TCyS increased for doses up to 70 mg/kg, remaining unchanged with further increase to 140 mg/kg. After intake of NAC solution, CySH:TCyS was significantly related to all three indicators of handgrip exercise performance used in our study.
Adverse Reactions to NAC
Consistent with previous studies (Pendyala & Creaven, 1995; Sandilands & Bateman, 2009), the most frequent reactions to oral NAC were GI and increased with the dose administered. Our novel observation was that subjects also reported heightened intensity of adverse reactions with increasing doses of oral NAC. The “threshold” dose beyond which there was a substantial increase in frequency and intensity of side effects was 70 mg/kg. These reactions were mainly nausea, upset stomach, and stomach or intestinal gas. Although subjects reported GI disturbances, the intensity of these reactions was mild in most cases (10 out of 15; Table 1). None of the participants in our study experienced vomiting, which is the main limitation to oral administration of NAC and can be experienced with repeated doses (Miller & Rumack, 1983). Overall, our results suggest that the oral formulation of NAC we used was well tolerated by healthy subjects.
NAC and Plasma GSH
CySH availability is the rate-limiting step for GSH synthesis in tissues (Meister & Anderson, 1983). Because NAC is a CySH donor, it has been used to promote GSH synthesis (Sen, 1997; Sen, Rankinen, Vaisanen, & Rauramaa, 1994). In our subjects, NAC tended to promote GSH reduction in plasma without affecting total GSH levels. This is generally consistent with previous observations in humans receiving oral NAC at a low dose (800 mg; Sen et al., 1994) or a single high dose (150 mg/kg; Matuszczak et al., 2005).
The stability of total GSH suggests that NAC solution may have altered GSH redox status by directly reacting with oxidized GSH derivatives. Consistent with this interpretation, the observed increase in GSH (~0.75 μM) was similar to the increase predicted from decrements in GSSG plus CySSG (0.69–0.74 μM). Our study design could not resolve the effects of low-dose NAC capsules on plasma GSH levels or redox status. Post hoc power calculations suggest that 20–25 subjects would have been required to detect significance in the trends that we observed.
NAC and Plasma CySH
NAC increases CySH levels in plasma and cells (Burgunder, Varriale, & Lauterburg, 1989; Meister & Anderson, 1983; Raftos, Whillier, Chapman, & Kuchel, 2007; Sen, 1997) by two principal mechanisms: deacetylation to CySH (Raftos et al., 2007) and release of free CySH by reacting with plasma CySS (Burgunder et al., 1989). The plasma concentrations of CySH derivatives that we measured after placebo are in close agreement with previous studies in healthy subjects (Burgunder et al., 1989; Medved et al., 2003; Mills & Lang, 1996). Administration of 600 or 1,200 mg NAC (approximately 9 or 18 mg/kg) via capsules did not alter plasma CySH derivatives measured the following day. These doses are consistent with some clinical uses (Koechlin et al., 2004) and with manufacturer suggestions for over-the-counter consumption. Under the current conditions, our data show that these doses do not augment plasma thiols in healthy young individuals.
High doses of NAC did increase CySH derivatives. All CySH derivatives increased within 1 hr of administering 35 mg/kg of NAC solution. NAC 70 mg/kg further increased CySH but not TCyS when compared with 35 mg/kg. However, there were no differences between the effects of NAC 70 mg/kg and NAC 140 mg/kg on cysteine-related thiols in the plasma. Perhaps NAC in excess of concentrations elicited by 70 mg/kg predominantly underwent formation of NAC–NAC disulfides or mixed disulfides between NAC and other plasma thiols not measured in our study. These effects of NAC would yield no change in TCyS as calculated herein because we did not measure acetylated derivatives of CySH. Overall, our study shows that the main effects of oral NAC on plasma CySH were achieved by administering a dose of 70 mg/kg.
CySH Redox State
We calculated CySH:TCyS to assess the antioxidant potential of NAC in the plasma. CySH:TCyS is conceptually similar, and correlated (data not shown), to the reduction potential calculated by the Nernst equation (Jones, 2002). We chose to focus on the CySH-to-TCyS ratio because it incorporates all CySH metabolites measured in our study into one value. In contrast, reduction potentials must be calculated separately for each CySH redox couple (CySH-CySS and CySH-CySSG). Our analysis showed an inverse relationship between GSSG and CySH:TCyS measured across the full range of clinically approved NAC doses. This supports the notion that the proportion of reduced to total CySH serves as a proxy for the antioxidant effects of NAC.
Handgrip Performance
High-dose NAC solution delays muscle fatigue in humans (Kelly et al., 2009; McKenna et al., 2006; Medved et al., 2004; Reid et al., 1994; Travaline et al., 1997), an effect that is generally attributed to support of GSH homeostasis (Ferreira & Reid, 2008). In the current study, circulating GSH derivatives showed no consistent relationship to exercise performance after NAC ingestion. In contrast, plasma CySH:TCyS was robustly linked to fatigue, correlating with each of three indices we measured. This discovery agrees with recent observations that a CySH donor can slow muscle fatigue without altering intracellular GHS content (Ferreira et al., 2009). In combination, these results suggest that CySH redox state may play an important role in the fatigue process.
Adverse effects of NAC at 70 mg/kg remain insignificant, whereas plasma levels of CySH and CySH:TCyS are maximized. These findings suggest that 70 mg/kg may be the optimal NAC dose for ergogenic testing. Emerging data support this notion. In healthy young individuals, 70 mg/kg NAC elicited no adverse reactions and increased cycling time to fatigue (Corn, 2009). This intriguing observation illustrates how our current results set the stage for formal tests of NAC in sports nutrition, exercise performance, and clinical rehabilitation.
Methodological Considerations
Because of the smell and taste of NAC and adverse reactions to it, it was difficult to ensure subject blindness during the experiments, especially when the drug was administered in solution. In all experiments with solutions the subjects wore a nose clip to prevent smell recognition, and we mixed NAC in saline to mask the taste of the drug. Despite our efforts we were not able to blind all subjects regarding the content of capsule or solution.
In Arm 1, the subjects were correct about the content of the capsules in 37% ± 10% of the visits (range 0–100%). In Arm 2, subjects were able to determine correctly whether the solution they had ingested contained NAC or placebo in 73% ± 7% of the visits (range 25–100%).
In the current study we used a custom-developed questionnaire to investigate the frequency and intensity of adverse reactions. To minimize variability and influence of study personnel on subjective responses, the same investigator gave standardized instructions about the questionnaire to all subjects. Data in Tables 1 and 2 show that, in general, study participants were consistent in the nature of reactions reported. We also obtained similar results from a secondary questionnaire using a 1–10 scale for scored intensity (data not shown). Consistent responses among subjects and between questionnaires suggest that our approach to evaluating adverse reactions to oral NAC was valid.
We did not control for dietary intake of CySH, which could influence the effects of NAC on CySH and GSH concentration. After placebo capsules or solution the levels of CySH and TCyS ranged from 6.7 to 17.2 μM and from 123 to 176 μM, respectively, which may be partially caused by differences in daily dietary intake of CySH. However, the individual responses to oral NAC were variable and independent of CySH or TCyS levels before administration of the oral solution (assessed from placebo data). Therefore, it is unlikely that end points of our study were confounded by differences in dietary intake of CySH.
Conclusions
This study shows that oral administration of NAC solution causes dose-dependent increases in the frequency and intensity of GI adverse reactions and in plasma CySH concentration. NAC did not affect total GSH concentration but promoted a decrease in oxidized GSH and increase in GSH at a dose of 140 mg/kg. Among all doses compared with placebo, 70 mg/kg was the highest tolerated without an increase in adverse reactions in the context of fatiguing exercise. At this dose there was also a plateau in the response of plasma CySH concentration and CySH-to-TCyS ratio. The latter was directly related to handgrip exercise performance, suggesting a potential role for the redox state of plasma CySH in the modulation of skeletal-muscle fatigue. Overall, our findings suggest that 70 mg/kg may be the optimal dose for future testing of NAC effects on human exercise performance.
Acknowledgments
We thank Lisa Chamblin, RN, and Dr. Stephen Sitzlar for their technical support and Dr. Richard Kryscio for performing statistical analysis of side effects. This study was funded by a National Space Biomedical Research Institute grant to M.B.R. (NASA MA00209). L.F.F. was supported by a postdoctoral fellowship from the American Heart Association (0725334B).
Footnotes
The authors declare no conflict of interest.
References
- Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, Colombo A, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): A randomized comparison of 3 preventive strategies. Circulation. 2007;115(10):1211–1217. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. European Journal of Clinical Pharmacology. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Corn SD. Master’s thesis. Kansas State University; Manhattan, KS: 2009. Effects of N-acetylcysteine on fatigue, critical power, and muscle energy stores. http://hdl.handle.net/2097/1632. [Google Scholar]
- Ferreira LF, Gilliam LA, Reid MB. L-2-oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. Journal of Applied Physiology (Bethesda, Md) 2009;107:211–216. doi: 10.1152/japplphysiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. Journal of Applied Physiology (Bethesda, Md) 2008;104(3):853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. The American Journal of Medicine. 1991;91(3C):131S–139S. doi: 10.1016/0002-9343(91)90296-a. [DOI] [PubMed] [Google Scholar]
- Heard KJ. Acetylcysteine for acetaminophen poisoning. New England Journal of Medicine. 2008;359(3):285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods in Enzymology. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Clinical applications of N-acetylcysteine. Alternative Medicine Review. 1998;3(2):114–127. [PubMed] [Google Scholar]
- Kelly MK, Wicker RJ, Barstow TJ, Harms CA. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respiratory Physiology and Neurobiology. 2009;165(1):67–72. doi: 10.1016/j.resp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? American Journal of Respiratory and Critical Care Medicine. 2004;169(9):1022–1027. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. British Medical Journal (Clinical Research Ed) 1984;289(6439):217–219. doi: 10.1136/bmj.289.6439.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle & Nerve. 2005;32(5):633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Gong X, et al. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. The Journal of Physiology. 2006;576(Pt 1):279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ. N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. Journal of Applied Physiology (Bethesda, Md) 2003;94(4):1572–1582. doi: 10.1152/japplphysiol.00884.2002. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, McKenna MJ, et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. Journal of Applied Physiology (Bethesda, Md) 2004;97(4):1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annual Review of Biochemistry. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Miller LF, Rumack BH. Clinical safety of high oral doses of acetylcysteine. Seminars in Oncology. 1983;10(1, Suppl 1):76–85. [PubMed] [Google Scholar]
- Mills BJ, Lang CA. Differential distribution of free and bound glutathione and cyst(e)ine in human blood. Biochemical Pharmacology. 1996;52(3):401–406. doi: 10.1016/0006-2952(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Nash EF, Stephenson A, Ratjen F, Tullis E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database of Systematic Reviews. 2009;1 doi: 10.1002/14651858.CD007168.pub2. CD007168. [DOI] [PubMed] [Google Scholar]
- Pendyala L, Creaven PJ. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a Phase I trial. Cancer Epidemiology, Biomarkers & Prevention. 1995;4(3):245–251. [PubMed] [Google Scholar]
- Raftos JE, Whillier S, Chapman BE, Kuchel PW. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. International Journal of Biochemistry and Cell Biology. 2007;39(9):1698–1706. doi: 10.1016/j.biocel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. The Journal of Clinical Investigation. 1994;94(6):2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clinical Toxicology. 2009;47(2):81–88. doi: 10.1080/15563650802665587. [DOI] [PubMed] [Google Scholar]
- Sar F, Saler T, Ecebay A, Saglam ZA, Ozturk S, Kazancioglu R. The efficacy of N-acetylcysteine in preventing contrast-induced nephropathy in Type 2 diabetic patients without nephropathy. Journal of Nephrology. 2010;23(4):478–482. [PubMed] [Google Scholar]
- Sen CK. Nutritional biochemistry of cellular glutathione. The Journal of Nutritional Biochemistry. 1997;8:660–672. [Google Scholar]
- Sen CK, Rankinen T, Vaisanen S, Rauramaa R. Oxidative stress after human exercise: Effect of N-acetylcysteine supplementation. Journal of Applied Physiology (Bethesda, Md) 1994;76(6):2570–2577. doi: 10.1152/jappl.1994.76.6.2570. [DOI] [PubMed] [Google Scholar]
- Thiele H, Hildebrand L, Schirdewahn C, Eitel I, Adams V, Fuernau G, Schuler G. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (prospective, single-blind, placebo-controlled, randomized Leipzig Immediate Percutaneous Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. Journal of the American College of Cardiology. 2010;55(20):2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. American Journal of Respiratory and Critical Care Medicine. 1997;156(5):1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- Zyoud SH, Awang R, Syed Sulaiman SA, Sweileh WM, Al-Jabi SW. Incidence of adverse drug reactions induced by N-acetylcysteine in patients with acetaminophen overdose. Human and Experimental Toxicology. 2010;29(3):153–160. doi: 10.1177/0960327109359642. [DOI] [PubMed] [Google Scholar]


