Abstract
An indigenous Bacillus thuringiensis strain B.t.LDC-391 producing cytocidal proteins against human colon cancer cell line, HCT-116, was subjected to phenotypic and genotypic characterization to evaluate its relatedness to B.anthracis. The morphological features of this strain were meta-analyzed with data of other parasporin and insecticidal protein producing Bacillus thuringiensis strains. The conventional biochemical analysis and antibiotic sensitivity test proved it as an ampicillin resistant which is a salient feature, absent in B.anthracis Ames. PCR analysis showed the absence of cyt and parasporin related genes in the genome of B.t.LDC-391. But the strain was positive for cap gene. The sequencing and bio-informatic analysis of cap gene and 16S rDNA of B.t.LDC-391 placed it closer to B.thuringiensis and revealed significant divergence from that of any B.anthracis strain. However our strain lacked β– hemolysis on human erythrocytes which is a common feature of B.anthracis strains and parasporin producers.
Keywords: Parasporin, B.thuringiensis, cancer cell killing, antibiotic resistance
Background
Bacillus cereus, B. thuringiensis and B. anthracis are genetically closely related members of the group 1 bacilli [1]. All three species are readily isolated from soil environments though they show different patho-physiologies in different hosts. B.thuringiensis has been widely utilized to control many agricultural and medically important insect pests whereas B. cereus and B. anthracis are considered as highly pathogenic to mammals. The presence of delta-endotoxins or crystalline proteins produced during sporulation phase is the only phenotypic criteria determining the identity of B.thuringiensis strains [2]. The genes encoding these inclusion proteins are present in the plasmid which has the ability for horizontal transfer between closely related Bacillus strains [1]. Comparative analysis of conserved genes in the core genomes and pan genomes of the related bacillus species have led to the identification of many overlapping loci in these strains [3].
Hence much debate exists in the concreteness of describing B.thuringiensis, B.cereus and B.anthracis as individual entities. However, a few sequences of specific genes such as atxA, capA capB, capC, pag, lef etc have been identified to impart high specificity to B.anthracis [4]. Cap genes are essential for synthesis of polyglutamic acid capsules which confer virulence in B.anthracis and atxA gene is a regulatory gene for anthracis toxin. Hence the presence of these genes and analysis of sequence homology could be used as a potential genetic marker in predicting the relatedness of environmental B.thuringiensis strains to that of pathogenic B.anthracis strains. The present investigation aims at identifying these gene sequences in B.t.LDC-391 and comparing its relatedness to B.anthracis.
Parasporins are a new functional category of inclusion proteins possessing cancer cell killing property, identified first in a Japanese B.thuringiensis isolate [5]. These strains are usually non haemolytic and non-motile with other phenotypic characters similar to that of insecticidal B.thuringiensis strains. B.t.LDC-391, investigated in this study is one such soil isolates from southern part of India, showing cytotoxicity against human colon cancer cell line, HCT-116 [6]. Albeit this strain possesses parasporal inclusions which is a common feature of B.thuringiensis species, the distinctness of this strain at morphological and molecular level to other related species needs to be investigated.
Methodology
Bacterial strains and culture media:
The indigenous bacterial strain B.t.LDC-391 and the reference type strain Bacillus thuringiensis israelensis, 4Q2 (Bacillus Genetic Stock center) were cultured in Nutrient broth (Hi-media, India) supplemented with 20 IU of penicillin for routine sub culturing at 37°C with 200 rpm shaking.
Morphological analysis:
Scanning Electron Microscopic analysis:
Axenic cultures of B.t.LDC-391 were obtained by streak plate method. The sporulated (72h), cells were washed thrice with 0.5M NaCl and fixed in gradients of aqueous ethanol (30%, 50%, 70%, 90%) and finally with absolute ethanol. The SEM examination was performed at the Department of Plant Sciences, Madurai Kamaraj University, and Madurai. The samples were coated with gold to a thickness of 100 Å using the Vacuum Evaporator, (Hitachi, Model HUS 5GB), and analyzed in a Scanning Electron Microscope (Hitachi, Model S-450, Japan) operated at 15 kV and photographed.
Transmission Electron Microscopy (TEM):
Sporulated cultures of B.t.LDC-391 containing intact crystals were centrifuged at 4000g for 15 min. The pellets were suspended in a buffer containing 3% glutaraldehyde, 1.5% paraformaldehyde and 0.1M PBS (pH7.2) for 3h at room temperature and centrifuged at 4000 g. The pellets were resuspended in a cocktail of 1% osmic acid and 1.5% potassium ferrocyanide for 1.5 h at 4°C. After gradient fixing, the pellets were ultra cut and serially stained with uranyl acetate and lead citrate for 5 min. The sections were examined with a transmission electron microscope (Jeol, model 1010, Japan) and photographed.
Biochemical characterization:
The biochemical tests for the bacterial characterization was performed according to the methods described in Bergey's manual of Systematic Bacteriology [7]. All the tests were done in parallel with appropriate positive and negative controls Table 1 (see supplementary material)
Characterization of salt tolerance and temperature optima:
Spizizen's media [8] containing different NaCl concentrations (0.5%-10%) were inoculated with the B.t.LDC-391 and its respective controls. Growth and optical density parameters were measured at 600 nm using spectrophotometer after 24 h. The sporulated cultures were checked for the presence of crystalline inclusions under phase contrast microscope. Sporulating cultures were monitored for their ability to grow at various temperatures ranging from 27°C - 50°C. The plates were incubated for 5 days to confirm optimal temperature and other physiological conditions. The sporulated colonies were analyzed under phase contrast microscope for the presence of spherical parasporal inclusion.
Antibiotic sensitivity test:
Different antimicrobial agents were tested by the disc-agar diffusion method in accordance with the national committee for clinical laboratory standards (NCCLS). Cultures of B.t.LDC-391 were inoculated in Mueller-Hinton Agar (Difco, BBL. USA) with discs impregnated with known concentrations of antibiotics Table 3 (see supplementary material).
PCR analysis:
Total DNA from the strain using DNA isolation kit (Chromous Biotech, India) under investigation was titrated with the below listed primers for amplification of their respective genes in Table 1 (see supplementary material). PCR was performed within a volume of 25 µl containing 500 ng of genomic DNA, 2.5 µl of 10X PCR buffer, 50 ng of forward and reverse primers of the respective gene, 10 mM of dNTP mix and 2.5U taq polymerase (Bangalore Genei, India). The samples were subjected for 30 amplification cycles at 94° C for 30s, respective annealing temperature for 40s, and 72°C for 60s in an Eppendorf Gradient Cycler. DNA from B.thuringiensis israelensis (BGSC 4Q2) was used as a control. The presence of the amplicon was observed using 1% agarose gel.
Analysis of 16S rRNA:
The 16S rRNA gene sequences for B.t.LDC-391 (EU625359.1) obtained earlier were analyzed using the BLAST program (Basic Local Alignment Search Tool) (www.ebi.ac.uk and http://www.ncbi.nlm.nih.gov). About twenty B.cereus and B.thuringiensis sequences, ten B.anthracis sequences showing maximum score in the BLAST analysis and sequence of pathogenic B.cereus strain E33L was acquired from NCBI. These sequences were aligned using ClustalW and manually corrected. Maximum likelihood (ML) analysis was performed with Molecular Evolutionary and Genome Analysis package, MEGA 5 [9, 10] using the Kimura 2-parameter model with 1000 bootstraps.
Discussion
SEM and TEM analysis:
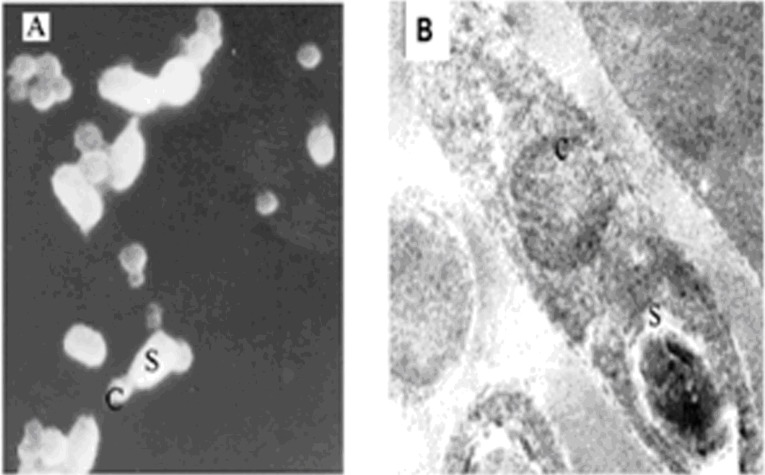
Scanning and Transmission Electron microscopic analysis of B.t.LDC-391 spore crystal complex showed spherical crystal morphology and were smaller than spores in comparison with the established parasporin producers [11]. The size of the inclusions varied from 0.5 µm to 1µm in width. There was an envelope surrounding the sporangia and they were found as detached entities from the crystalline inclusions at the fully developed stage (Figure 1A & Figure 1B).
Figure 1.
A) SEM analysis of B.t. LDC-391; B) TEM analysis of B.t. LDC-391; C-Crystalline inclusions and S-Spores
Biochemical characterization:
The biochemical and physiological characterization of B.t.LDC- 391 done in triplicates showed all the typical biochemical features exhibited by Bacillus thuringiensis strains Table 2 (see supplementary material). In sharp contrast to all the parasporin producers reported so far, B.t.LDC-391 is the only strain showing motility. Observing the motility of the strain is of special interest among group 1 Bacilli, because it is usually associated with the vegetative strains of B.thuringiensis and B.cereus but not B.anthracis [12].
Although B.t.LDC-391 was able to grow in high saline (10%) and temperature (above 35°C) conditions, the number of crystalline inclusions was reduced three to five fold when observed under phase contrast microscope. Carbon utilization potential profile of B.t.LDC-391 was similar to that of earlier observations that all parasporin producers were able to produce acid from all the monosaccharides except from arabinose [13]. B.thuringiensis strains are not capable of utilizing non-reducing sugars, lactose and galactose which is also a common feature found in B.t.LDC-391.
Antibiotic sensitivity:
B.t.LDC-391 showed resistance to ampicillin, penicillin and bacitracin but sensitive to kanamycin. Apart from the absence of δ-endotoxins, the only biochemical parameter reported to be varying from B.thuringiensis and B.anthracis is the tolerance to ampicillin as reported elsewhere [14]. The tolerance to β-lactam group of antibiotics place B.t.LDC-391 close to B.thuringiensis Table 3 (see supplementary material).
16S rDNA sequence analysis:
About 30 representative strains from B.thuringiensis, B.cereus and B.anthracis showing maximum score on the BLAST analysis of 16S rDNA sequence for B.t.LDC-391 were used for the phylogenetic tree construction.No anthracis strains were obtained within the first 50 hits. The dendrogram did not represent the strain B.t.LDC-391 in the cluster of the B.anthracis which included the pathogenic B.cereus strain E33L, with a significant bootstrap value. It is in accordance to the study reported elsewhere [15]. The strain B.t.LDC 391, was represented at a different node although it was not statistically significant (Figure 2A) implying the divergence from the pathological strains within the B.cereus group. Its exclusion from the pathogenic cluster of B.cereus group deserves special mention and confirms that the strain under investigation does not belong to the virulence causing bacillus strains.
Figure 2.
A) Phylogram analysis using using Maximum likelihood for 16s rDNA sequence of B.t.LDC-391. *B.anthricis strains. #pathogenic B.cereus strain. Bootstrap values indicated at the nodes. Scale bar indicates no.of substittions per site; B) Phylogram analysis using UPGMA for cap gene sequence of B.t.LDC-391 along with representive strains from B.cereus group. B.cereus ungrouped bacillus B.thuringiensis, B.anthracis. Bootsrap values are indicated at the nodes of the phylogram
PCR analysis:
PCR analysis of total DNA using B. thuringiensis israelensis (4Q2) as positive control confirmed that the strain B.t.LDC-391 under investigation did not possess any cyt I and cyt II related genes. B.thuringiensis israelensis (4Q2) showed the expected 480 base pairs amplicon for cyt I and a 350 base pairs amplicon for cyt II primers. Surprisingly, the strain B.t.LDC-391 was positive for the presence of cap gene, while the other primers mentioned in Table 1 (see supplementary material) did not generate amplicons, implying the absence of atxA, a regulatory gene of anthracis toxin and previously reported parasporin related genes. It is to be noted that very permissible annealing conditions were provided for the amplification of atxA, PS-1, PS-4 and PS-6 genes. Unfortunately, no reference strains are available as positive control for these reactions. Genomic analysis using cyt I, cyt II specific primers confirmed that there is no cyt related genes present in strain B.t.LDC-391. This result confirms that the protein exerting cytolytic action on cancer cells might not be due to the cyt gene. The information on the sequence of novel parasporin gene in B.t.LDC391 is yet under study.
DNA sequencing of the amplified PCR product using cap A primers revealed a high degree of sequence similarity to the gene for capsule biosynthesis protein. Cap genes are responsible for producing a protective capsule around the organism during adverse conditions, so that it can escape from host immune response [4]. The amplicon sequences obtained by the use of capA primers was submitted in GenBank and the accession number JQ319804 was obtained. The orf fragment from the amplicon revealed 98% sequence identity to the cap A genes with e-values ranging from 6e-178 to 3e-175. There was no B.anthracis hits obtained in BLAST analysis using cap sequences of B.t.LDC-391. UPGMA based analysis with 1000 boot strap using jukes cantor method was utilized to generate the phylogram. Cap sequences of representative B.anthracis strains for the tree generation were acquired from NCBI, non redundant nucleotide data base. The phylogram revealed that the cap gene sequences were placed in a different node which included only B.cereus and B.thuringiensis strains with 100% boot strap values (Figure 2B).
The UPGMA and Neighbor Joining method based analysis of cap gene sequence from B.t.LDC-391 has shown that it is not grouped with any of the pathogenic or B.anthracis strains. It is also note worthy that several reports have shown that strains of B.cereus and B.thuringiensis contained similar capA sequences obtained by PCR and DNA hydridization [16, 17]. However, the functionality of the detected capA gene in B.t.LDC-391 is in question because no capsule formation was observed in both phase contrast and electron microscopic analysis. This may be perhaps due to the absence of atxA gene which regulates the capABCDE operon [18]. This gives us the notion that the cap genes may have undergone evolutionary changes resulting in divergence from B.anthracis strains.
Conclusion
In view of their natural competence, horizontal spreading of plasmids may take place and has in fact has been demonstrated for B. cereus group. The above results suggest that the strain B.t.LDC-391 might have evolved as a result of interaction with neighboring species, probably by the horizontal transfer of extraneous plasmid genes from B.cereus and B.thuringiensis as evinced by the presence of a non functional capA gene closer to B.cereus. The stand alone nature of the 16S rDNA sequence in the phylogenetic tree and the presence of the divergent cap gene along with the cancer cell killing proteins throw light into the possible permutations and combinations of nature. As hypothesized by earlier workers, what seems to be a minor problem of taxonomy may result in evolution of virulence, pathogenicity [1] and even beneficial characteristics such as novel cancer cell killing quality as in our strain B.t.LDC-391.
Supplementary material
Acknowledgments
The authors thank Department of Biotechnology (DBT), Govt. of India, New Delhi for the financial support in the form of Bioinformatics Infrastructure Facility (BTISNeT), University Grant Commission (UGC), for Major Research Grant (F.No.34- 421/2008 (SR), DST Funding vide SR/SO/HS-48/2008 and CSIR funding vide 37(1480)/11/ EMR-II.
Footnotes
Citation:Poornima et al, Bioinformation 8(10): 461-465 (2012)
References
- 1.E Helgason, et al. Appl Environ Microbiol. 2000;66:2627. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M Porcar, V Juárez-Pérez. FEMS Microbiol Rev. 2003;26:419. doi: 10.1111/j.1574-6976.2003.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 3.JE Burton, et al. J Appl Microbiol. 2006;101:754. doi: 10.1111/j.1365-2672.2006.02991.x. [DOI] [PubMed] [Google Scholar]
- 4.R Adone, et al. J Appl Microbiol. 2002;93:117. [Google Scholar]
- 5.E Mizuki, et al. J Appl Microbiol. 1999;86:477. doi: 10.1046/j.1365-2672.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- 6.Kkani Poornima, et al. J Appl Microbiol. 2010;109:348. [Google Scholar]
- 7.YC Jung, et al. J Appl Microbiol. 2007;103:65. [Google Scholar]
- 8.J Spizizen. Proc Natl Acad Sci USA. 1098;1072:1078. [Google Scholar]
- 9.M Kimura. J Mol Evol. 1980;16:111. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 10.K Tamura, et al. Mol Bio Evol. 2011;28:2731. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A Uemori, et al. Naturwissenschaften. 2007;94:34. [Google Scholar]
- 12.C Martson, et al. BMC Microbiol. 2006;6:22. doi: 10.1186/1471-2180-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M Ichikawa, et al. J Fac Agric Kyushu Univ. 2007;52:307. [Google Scholar]
- 14.Ichikawa, et al. Appl Entomol Zool. 2008;43:421. [Google Scholar]
- 15.CS Han, et al. J Bacteriol. 2006;188:3382. doi: 10.1128/JB.188.9.3382-3390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.N Sergeev, et al. J Microbiol Methods. 2006;65:488. doi: 10.1016/j.mimet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 17.D Sue, et al. J Clin Microbiol. 2006;44:3426. doi: 10.1128/JCM.00873-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JE Ibarra, et al. Appl Environ Microbiol. 2003;69:5269. doi: 10.1128/AEM.69.9.5269-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A Uemori, et al. Naturwissenschaften. 2007;94:34. [Google Scholar]
- 20.S Okumura, et al. J Agric Food Chem. 2005;53:6313. doi: 10.1021/jf0506129. [DOI] [PubMed] [Google Scholar]
- 21.Y Nagamatsu, et al. Biosci Biotechnol Biochem. 2010;74:494. doi: 10.1271/bbb.90615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.