Abstract
Podocytes do not remain fully differentiated when cultured, and they are difficult to image in vivo, making the study of podocyte biology challenging. Zebrafish embryos are transparent and develop a single, midline, pronephric glomerulus accessible for imaging and systematic functional analysis. Here, we describe a transgenic zebrafish line that expresses green fluorescence protein (GFP) from the zebrafish podocin promoter. The line recapitulates the endogenous pronephric podocin expression pattern, showing GFP expression exclusively in podocytes starting 2 days postfertilization. Using the podocyte GFP signal as a guide for dissection, we examined the pronephric glomerulus by scanning electron microscopy; the surface ultrastructure exhibited fine, interdigitating podocyte foot processes surrounding glomerular capillaries. To determine whether the GFP signal could serve as a direct readout of developmental abnormalities or injury to the glomerulus, we knocked down the podocyte-associated protein crb2b; this led to a loss of GFP signal. Thus, podocin-GFP zebrafish provide a model for ultrastructural studies and in vivo visualization and functional analysis of glomerular podocytes. This model should also be useful for high-throughput genetic or chemical analysis of glomerular development and function.
Podocytes are specialized epithelial cells of the kidney glomerulus, which extend long branched foot processes that enclose the kidney glomerular capillary loops. The foot processes of neighboring podocytes interdigitate to form filtration slits, through which water, solutes, and proteins smaller than 60 kD pass from the blood into the primary urine, whereas larger plasma proteins are retained in the blood plasma. The podocytes, their foot processes, and the diaphragms that connect the processes and span the filtration slits (the slit diaphragms) form together the outer and size-selective layer of the glomerular filtration barrier. Proteinuria in mammals is commonly associated with disruption of the normal architecture of the podocyte foot processes and their slit diaphragms. This is observed in monogenic hereditary human nephrotic syndromes, in which podocyte-specific proteins such as nephrin and podocin are mutated,1,2 but also in more common glomerular disorders of sometimes unclear etiology and pathogenesis, such as autoimmune glomerulonephritis,3 and in toxic or inflammatory animal models of proteinuria, such as LPS-induced nephropathy.4 Podocytes are therefore recognized as critical components of the kidney filter and culprits in the development of many kidney disorders.5,6
To gain detailed insight into the functions of podocytes, cell culture systems and rodent models have been commonly applied. However, in vivo podocytes are terminally differentiated nonproliferative cells with elaborate morphologic distinctions, which are not fully recapitulated in vitro.7,8 Moreover, whereas genetically engineered mouse models have been used to demonstrate the critical importance of several podocyte proteins,9–13 a major disadvantage of mice, like almost all other mammalian systems, is that the location of the glomeruli is deep within the kidney cortex and the animals' bodies, which makes real-time studies, such as live imaging of glomeruli and podocytes, problematic. Access to Munich-Wistar rats that have glomeruli located at the renal surface circumvents some of these problems and has allowed real-time imaging of blood flow and glomerular filtration using two-photon microscopy.14 Moreover, advances in multiphoton imaging technologies now also allows imaging of glomerular function in mice, which have their glomeruli located deeper in the kidney cortex.15 Nevertheless, these types of studies remain complicated, they have limited spatial and temporal resolution, and they have low throughput.
In comparison, the small size, transparency, and external development of the zebrafish embryo offers an interesting alternative, because these embryos possess a pronephric kidney that resembles the mammalian metanephric kidney structurally and functionally.16 The pronephros is the first and simplest kidney to form during embryonic development, and unlike mammals where it constitutes a nonfunctional evolutionary residue, the pronephros is playing an important role in fish and amphibians where it constitutes the functional kidney of early larval life, regulating water homeostasis.17,18 Despite the difference in complexity between the zebrafish pronephros and the mammalian metanephric kidney, the basic glomerular structure and its cellular make-up are similar.16 Ultrafiltration in the zebrafish pronephros begins at 2 days postfertilization (dpf),19 i.e. when the embryo is fully transparent and, importantly, amenable to high throughput analyses, such as gene knockdown using morpholino antisense oligonucleotides, and drug screens using chemical compound libraries.
The molecular make-up of the podocytes and their slit diaphragms is also similar between the zebrafish pronephros and the mammalian metanephric kidney. The proteins nephrin and podocin, both key components of the slit diaphragm in mammals and implicated in hereditary nephrotic syndromes when mutated in humans, are present in the zebrafish pronephric glomerulus, and inactivation of either of them leads to renal dysfunction, with pericardial edema and early death as a result.20 Podocin is present exclusively in the glomerular podocytes1 and is therefore a specific marker for these cells.
Thus, the zebrafish pronephric glomerulus appears ideally suited for live imaging, and toward this end, transgenic fish with fluorescence reporter expression in podocytes would be helpful. A 2.5-kb DNA fragment corresponding to the 5′ end of the human podocin gene was previously shown to drive specific expression of a reporter gene specifically in podocytes in transgenic mice.21 We therefore used a similar size DNA fragment corresponding to the 5′ end of the zebrafish podocin gene to generate transgenic zebrafish expressing green fluorescence protein (GFP). The 2.5-kb fragment was cloned into a Tol2 transposon-based plasmid to generate transgenic GFP lines (Figure 1A). In total, 15 injected G0 embryos that were mosaic with regard to GFP-positive cells in the pronephric glomerulus survived into adulthood. By outcrossing, five independent founders (30%) were identified. Embryos from each of the five founders displayed identical expression patterns in which GFP was expressed specifically in the pronephric glomerulus without signs of ectopic expression (Figure 1B). The glomerular GFP signal emerged at 2 dpf and was robust thereafter. The founder with the strongest GFP expression, Tg(podocin:GFP), was used to collect homozygous fish for line maintenance and subsequent studies. To determine whether the GFP localization recapitulated the endogenous podocin expression pattern, we performed whole-mount in situ hybridization to compare the expression pattern of GFP with that of the endogenous podocin gene. These results showed a complete overlap between podocin and GFP expression (Figure 1, B and C). Consistent with the GFP expression pattern, podocin mRNA was undetectable at 1 dpf (data not shown). Thus, the 2.5-kb zebrafish podocin 5′ sequence used in this study appears to contain the transcriptional regulation elements necessary to recapitulate endogenous podocin expression in pronephric podocytes. A transgenic zebrafish expressing the fluorescence reporter mCherry from a 3.5-kb podocin promoter fragment was recently reported.22 Although that study focused mainly on the mesonephros, the expression of the 3.5- and 2.5-kb podocin promoters appears similar in the pronephros as well as in the mesonephros (reference 22 and data not shown). Our study therefore refines somewhat the region containing podocyte-specific regulatory elements. Our ongoing analyses aim at defining this region more precisely.
Figure 1.

Generation and characterization of the podocin-GFP transgenic zebrafish line showing that the GFP expression overlaps with the endogenous podocin expression. (A) The Tol2 transposon-based construct used to generate the line. A 2.5-kb zebrafish podocin 5′ sequence was cloned into the pT2KXIG plasmid. The flanking Tol2 transposon sites are indicated (red boxes). (B) GFP expression driven by the podocin promoter recapitulates endogenous podocin expression pattern. GFP is exclusively expressed in the pronephric glomerulus (arrowheads; note that fluorescence signals in yolk and eyes represent autofluorescence), illustrated in dorsal and lateral views. The location of GFP is further confirmed by in situ hybridization using GFP and podocin probes (note that the GFP in situ hybridization signal in the head represents unspecific cross-reaction). (C) Cross-sections show the presence of GFP mRNA (left panel) at the location typical for podocytes, as confirmed by the stained obtained using a podocin probe (right panel). nc, notochord.
Transmission electron microscopy (TEM) analysis has shown that, similar to podocytes of mammalian metanephric kidneys, the pronephric podocytes in zebrafish elaborate foot processes that are connected by slit diaphragms.16,20 TEM analysis of the pronephric glomerulus is relatively straightforward because it relies on sectioning of whole embryos. It is more difficult to access the three-dimensional architecture of the embryonic pronephric glomerulus by scanning electron microscopy (SEM) because this requires microdissection of a tiny transparent single glomerulus located deep inside the embryo. The brightly fluorescence glomeruli of Tg(podocin:GFP) embryos greatly facilitated glomerular dissection and cutting of the embryo to expose the glomerulus for SEM analysis in situ. The SEM image shown in Figure 2A illustrates the surface architecture of a 6-dpf pronephric zebrafish glomerulus clearly displaying interdigitating podocyte foot processes. These observations are consistent with previous data obtained by TEM.16,20 The SEM analysis thus provides further morphologic evidence for the presence of a functional filtration barrier in the embryonic zebrafish pronephric glomerulus.
Figure 2.
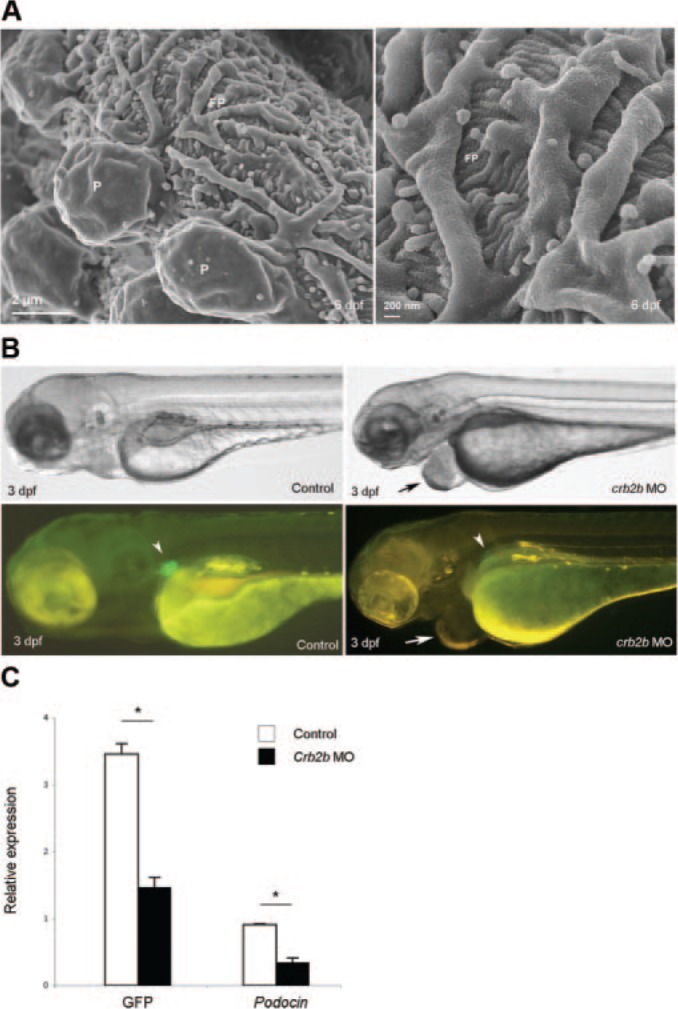
The Tg(podocin:GFP) line allows for ultrastructural studies and provides a direct readout for glomerular-specific phenotype in genetic studies. (A) Scanning electron micrograph of a 6-dpf glomerulus. Podocytes extend foot processes that interdigitate around capillary loops (left panel). The right panel shows branching foot processes at higher magnification. P, podocyte cell body; FP, primary (left panel) and interdigitating (right panel) foot processes. (B) Crb2b knockdown embryos. A crb2b morphant (right panel) shows a typical pericardial edema (arrow), coupled with loss of glomerular GFP signal (arrowhead), compared with the control (left panel). (C) Relative GFP and podocin mRNA levels in crb2b morphants (n = 5) and controls (n = 3) by determined by quantitative PCR. The error bars indicate the SD. *P < 0.01 (t test).
Pericardial edema often occurs after knockdown of glomerulus-associated genes using morpholinos.23,24 This phenotype is usually associated with loss of pronephric kidney function (osmoregulation),20,25 but it is not specific for glomerular and/or podocyte dysfunction because it generally occurs also as a result of cardiac or vascular dysfunction.26–28 Thus, the presence of pericardial edema in a genetic or chemical screen cannot be taken as evidence for kidney dysfunction without additional morphologic and/or functional analysis of the pronephros. A significant change in the GFP signal in the Tg(podocin:GFP) line could, however, be used as a direct readout of developmental aberrations or injury to the glomerulus. We tested this idea by injecting a translation-blocking morpholino against crb2b into Tg(podocin:GFP) embryos. Previous studies have demonstrated that crb2b knockdown leads to pericardial edema and pronephric cyst phenotype with compromised glomerular filtration barrier function and integrity in the early embryo.23 As shown in Figure 2B, pericardial edema in crb2b morphants correlated with loss of glomerular GFP signal in Tg(podocin:GFP) embryos. The reduced GFP signal paralleled the drop in mRNA levels for GFP and podocin (Figure 2C).
In summary, we report the generation of transgenic Tg(podocin:GFP) zebrafish lines with strong and specific GFP expression in podocytes of the pronephric glomerulus. These lines provide powerful tools for podocyte research, because they can be used for real-time imaging, FACS sorting, and molecular profiling of zebrafish pronephric podocytes, mutant and chemical screens, and for high-throughput functional analysis of genes by morpholino injections.
CONCISE METHODS
Transgenic zebrafish were generated using the Tol2 transposon system.29 A 2.5-kb DNA fragment corresponding to the zebrafish podocin promoter was amplified from AB strain genomic DNA, cloned into the pCRII-TOPO vector (Invitrogen), and confirmed by sequencing. The fragment was then transferred to the pT2KXIG vector (kindly provided by K. Kawakami, National Institute of Genetics, Shizuoka, Japan) (Figure 1A) and injected into G0 embryos, which were subsequently raised and outcrossed with TL zebrafish for the identification of germline founders. GFP expression was documented at ages 1 to 6 dpf under a stereomicroscope. For SEM, 6-dpf Tg(podocin:GFP) embryos were briefly fixed in glutaraldehyde followed by dissection under a stereomicroscope to expose the brightly fluorescence glomerulus. After further fixation, ethanol washes, infiltration with tetramethylsilane, and air-drying, specimens were mounted and analyzed in an Ultra 55 field emission SEM at 3 kV. Knockdown of Crb2b using translation-blocking morpholinos was done as recently described.23
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Dr. Arindam Majumdar for initial help with the generation of transgenic zebrafish and Susan Warner, Ulla Wargh, and Sajila Kisana at the Karolinska Institutet zebrafish core facility for maintaining fish and providing embryos. This work was supported in part by grants from the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, and the Novo Nordisk, Knut and Alice Wallenberg, IngaBritt and Arne Söderberg, and Hedlund Foundations.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Segelmark M, Hellmark T: Autoimmune kidney diseases. Autoimmun Rev 9: A366–A371, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Sun Y, He L, Takemoto M, Patrakka J, Pikkarainen T, Genové G, Norlin J, Truvé K, Tryggvason K, Betsholtz C: Glomerular transcriptome changes associated with lipopolysaccharide-induced proteinuria. Am J Nephrol 29: 558–570, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Ly J, Alexander M, Quaggin SE: A podocentric view of nephrology. Curr Opin Nephrol Hypertens 13: 299–305, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Kriz W: Progressive renal failure–inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant 11: 1738–1742, 1996 [PubMed] [Google Scholar]
- 8. Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cui S, Schwartz L, Quaggin SE: Pod1 is required in stromal cells for glomerulogenesis. Dev Dyn 226: 512–522, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM: Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 194: 13–27, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li B, Yao J, Kawamura K, Oyanagi-Tanaka Y, Hoshiyama M, Morioka T, Gejyo F, Uchiyama M, Oite T: Real-time observation of glomerular hemodynamic changes in diabetic rats: Effects of insulin and ARB. Kidney Int 66: 1939–1948, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Peti-Peterdi J, Toma I, Sipos A, Vargas SL: Multiphoton imaging of renal regulatory mechanisms. Physiology 24: 88–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drummond IA, Davidson AJ: Zebrafish kidney development. Methods Cell Biol 100: 233–260, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Howland RB: On the effect of removal of the pronephros of the amphibian embryo. Proc Natl Acad Sci U S A 2: 231–234, 1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vize PD, Seufert DW, Carroll TJ, Wallingford JB: Model systems for the study of kidney development: Use of the pronephros in the analysis of organ induction and patterning. Dev Biol 188: 189–204, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC: Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA: Organization of the pronephric filtration apparatus in zebrafish requires nephrin, podocin and the FERM domain protein Mosaic eyes. Dev Biol 285: 316–329, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F: Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol 299: F1040–F1047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A: A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol 334: 1–9, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Perner B, Englert C, Bollig F: The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309: 87–96, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Majumdar A, Drummond IA: The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol 222: 147–157, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lee HC, Tsai JN, Liao PY, Tsai WY, Lin KY, Chuang CC, Sun CK, Chang WC, Tsai HJ: Glycogen synthase kinase 3 alpha and 3 beta have distinct functions during cardiogenesis of zebrafish embryo. BMC Dev Biol 7: 93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nadauld LD, Sandoval IT, Chidester S, Yost HJ, Jones DA: Adenomatous polyposis coli control of retinoic acid biosynthesis is critical for zebrafish intestinal development and differentiation. J Biol Chem 279: 51581–51589, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Tang J, Hu G, Hanai J, Yadlapalli G, Lin Y, Zhang B, Galloway J, Bahary N, Sinha S, Thisse B, Thisse C, Jin JP, Zon LI, Sukhatme VP: A critical role for calponin 2 in vascular development. J Biol Chem 281: 6664–6672, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M: A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell 7: 133–144, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


