Abstract
We investigated the solution structure and dynamics of the human anti-coagulation protein Z (PZ) in the complex with protein Zdependent protease inhibitor (ZPI) to order to understand key structural changes in the presence and absence of Ca2+. Structural features of the complete complex of PZ-ZPI are poorly understood due to lack of complete atomic model of the PZ-ZPI complex. We have constructed a model of the complete PZ-ZPI complex and molecular dynamics (MD) simulation of the solvated PZ-ZPI complex with and without Ca2+ was achieved for 100ns. It is consider that the Ω-loop of GLA domains interacts with negatively charged biological membranes in the presence of Ca2+ ions. The PZ exerts its role as cofactor in a similar way. However, we used solvent-equilibrated dynamics to show structural features of the PZ-ZPI complex in the presence and the absence of Ca2+ions. We observed that the distance between the interacting sites of the ZPI with the PZ and the GLA domain decreases in the presence of Ca2+ ions. Further, we postulated that the calculated distance between the dominant plane of the Ca2+ ions and Ser196 of the pseudo-catalytic triad of the PZ is similar to the equivalent distance of FXa. This suggests that the central role of the PZ in the blood coagulation may be to align the inhibitory site of the ZPI with the active site of the FXa, which is depends on the interaction of the calcium bound GLA domain of the PZ with the active membrane.
Keywords: Protein Z, Protein Z-dependent protease inhibitor, Ca ion, Blood coagulation, Molecular Dynamics simulation
Background
Protein (PZ) is a Vitamin K-dependent protein with 360 residues and a molecular weight of 62 KD, being homolog to the coagulation factors FVII, FIX, FX, and the PC. The PZ has significant differences to other serine proteases by lack of histidine and serine residues in the catalytic triad, causing loss of proteolytic function [1, 2]. PZ serves as a cofactor, mediating inhibition of the FX by the ZPI in the presence of Ca2+ ions and phospholipids [3]. In relation to other coagulation proteins, the PZ has 4 domains, including the GLA domain (residue 1- 46), the EGF1 (47- 83), the EGF2 (85-126), and the SP domain (135- 360) with 11 disulfide bonds within and between the domains [1, 2]. The Vitamin K-Dependent (VKD) proteins contain 10-12 γ- carboxy glutamic acid (Gla) residues in the GLA domain, consisting of three turns of alpha-helix that is conserved in other VKD dependent proteins. Additional feature of PZ is the existence of 13 Gla residues in the GLA domain, which is necessary to bind to Ca2+ and membrane. A significant section in the N-terminus of the GLA domain is the ω-loop including 1- 11 residues responsible for the high-affinity binding to phospholipid membrane, and for membrane penetrating that is mediated by the so-called “keel” region of hydrophobic residues and dominant plane of Ca2+ ions [4–6]. The ω-loop contains two groups of hydrophobic and Gla residues with negative 2 charge. Previous data regarding PZ showed that binding of Ca2+ in the BHA make no structural effect, but neutralize it due to lack of Ca2+ network in this domain in opposite another VKD proteins [7]. To date, there is no data about importance and effect of Ca2+ in the complex of PZZPI and cofactor role of PZ. ZPI is a serpin with 72 KD molecular weight and 423 amino acid residues. Inhibition of FX by ZPI is PZ-dependent and increases the rate of inhibition to 1000 folds in contrast to free ZPI. It is known that the ZPI inhibit FXIa and FIXa but didn’t require PZ. Also it has been suggested that ZPI secretion, localization, clearance, even stabilization has been affected by PZ [3, 8, 9]. The ZPI inhibits factor X, while the FX inhibition takes place by anti-thrombin and the tissue factor pathway inhibitor (TFPI) [10, 11]. PZ binding to ZPI and factor X is accompanied by the SP domain and the GLA domaincalcium ions- on the same phospholipid surfaces, respectively.
The Binding of the PZ and the FX decreases the rate of the FXa inhibition by anti-thrombin and enhances the inhibition by ZPI. In the other hand, the binding of the PZ to the ZPI increases inhibition of the FX and then reduces formation of the prothrombinase complex by delay the initiation and diminish generation of thrombin. These support the anticoagulant and critical role of PZ. So this is implied that PZ accelerate inhibition FX by 3 mechanisms, 1) bind and bring ZPI to the membrane for binding to FX, 2) prevention of FX inhibition by another inhibitor like antithrombine and 3) prevention ZPI to inhibit another coagulation factor. So due to this important effect and role of PZ we prepared and investigated complete complex of PZZPI. The previous study on x-ray crystal structure of PZZPI complex is incomplete due to missing GLA domain [12, 13]. In present work we constructed full model of PZZPI complex and employed molecular dynamics (MD) simulations to investigate structural changes of GLA domain of PZ in the presence and absence of Ca2+ and relationship between GLA domain and binding site in ZPI. The results of over 100 ns of MD simulations suggest a new cofactor role for PZ. In addition homology modeling of PZ had been reported in free PZ not in the complex form. While it appears that all PZ are in bound form to ZPI in plasma [14]. Finally this study exhibit more details about structural feature and mechanisms to know more about important cofactor role of PZ.
Methodology
We add missing GLA domain of crystal structure PZZPI (3H5C.pdb with resolution 3.26Å) [12], by superimposing residues 49- 86 of full homology model PZ to same segment of crystal structure using visual molecular dynamic (VMD) (Figure 1C) [07, 15]. Then we prepared two system of complex with and without Ca2+ for MD simulation. Protein was solvated in a water box with 124, 133, 130Å dimensions. 12 Na+ and 14 Cl- ions neutralized system with Ca2+ ions but complex of PZZPI without Ca2+ neutralized by 23Na+ and 3Cl- due to lack of Ca2+ using VMD. We utilized cutoff of 12 Å for short-range non-bonded interactions. The system with Ca2+ ions contains 11 Ca2+ ions, and 57204 water molecules (TIP3W), 183453 atoms. We initially performed energy minimization for two systems (with and without Ca2+) in 10000 step in 20ps.All simulation was performed at NPT at 310k and a pressure of 1 atm and set 2fs for time step. The MD simulation was carried out for 100ns using NAMD Version 2.7 and Charmm 27 force field [16, 17].
Figure 1.
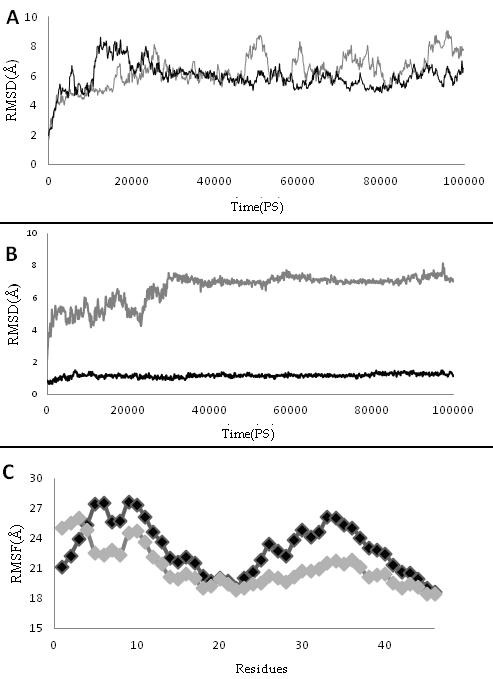
(A) the root mean square deviation (RMSDs) of the backbone atoms of protein Z and ZPI complex in the presence and absence of Ca2+ ions; (B) RMSD of GLA domain during 100 ns simulation in the presence and absence of Ca2+ ions; (C) Root mean square fluctuation (RMSF) of GLA domain in the presence and absence of Ca2+ ions. Gray curve exhibits the PZZPI without Ca2+ and Black curve is PZZPI with Ca2+ ion.
Discussion
Prominent differences between Ca2+-bound form and free form structure were established by following methods: 3.1 RMSD Root mean square deviations (RMSD) of protein Z and Zdependent protease inhibitor (PZZPI) complex in the presence and the absence of Ca2+ shows stability of two complexes. The RMSD ranges to 1 and 0.8 Å for pzzpica and pzzpi, respectively (Figure 1A). The RMSD of the GLA domain exposes in contrast to other domains of protein Z more differences in the presence of Ca2+ (Figure 1B). The Ca2+ bound GLA domain attains 0.12 Å, but it makes 0.91 Å in Ca2+ free conformation. We postulate therefore that Ca2+ affects on the decreasing square deviation (SD) of the GLA domain and increases its stability. 3.2 RMSF: Root mean square fluctuation (RMSF) plot of the GLA domain of two proteins exhibits different fluctuation curves, excluding the Disulfide Bridge containing regions 18 to 23, which causes rigidity in this region. We suggest further that the presence of Ca2+ ions increase the fluctuation curve of the PZZPI. Major fluctuation was observed for residues around the ω-loop. The bound form represents two distinct hot points at around 5, 6, 9, and 10 (Figure 1C). 3.3 Orientation of ω-loop residue we observed changes in orientation of two hydrophobic and negative charge groups of ω-loop in the presence and in the absence of Ca2+ (Figure 2B). Gla residues placed toward Ca2+ line within ω-loop and hydrophobic residues like leu5,6 placed to solvent and more exposed area but Gla residues in the absence of Ca2+ become more exposed and impair cohesion of the hydrophobic residues. Data of solvent accessible surface area demonstrated these result which Gla 7and 8 which are key residues in binding to Ca2+ have SASA in the absence more than presence of Ca2+. The opposite way, leu5 and 6 have SASA in the presence of Ca2+more than in its absence. The number of SASA differences for these residues is significant and this data is in agreement with Maria et al experimental results about factor X (Table 1) (see supplementary material) [18]. This imply that Ca2+ ions give a favorable conformation to ω-loop by making a powerful hydrophobic region anterior and trapping of GLA residues inside of ω-loop, which is necessary for membrane penetration.
Figure 2.
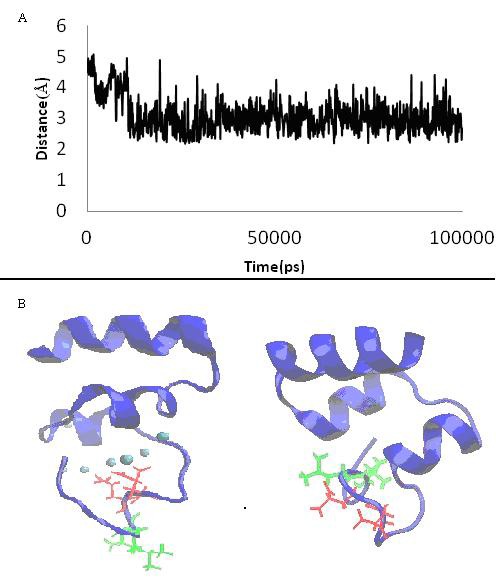
(A) Distance between ala1 (o atom) and cal364 during simulation; (B) Illustration of 3-d structure of gla domain (blue cartoon) shows orientation of gla 7, 8 (red residues) and leu 5, 6 (green residues) in the absence (right picture) and presence of Ca2+ ions (cyan sphere, left picture).
3.4 Network of Ala1 The network of Ala1 plays critical role in the conformation of interior loop of the GLA domain due to involving in different H-bond to hydrophobic and Gla residues along with Ca2+ ions [19, 20]. We observed that in the presence of Ca2+ ions Ala1 keeps binding to Gla 21, 27, an Ile22, but it losses H-bond to the Gla 27 during simulation that has effect on the conformation of the ω-loop (data has not been shown). Further, Ala1 keeps the binding to Ca2+ (Figure 2A) and participates in dominant plane in the GLA domain and binding to residue of Ile 21of first disulfide loop and Gla residues. This would underline the critical role of Ala1 in the w-loop, and explains how the Ca ions impress the network of H-bond. 3.5 Distance between center mass of GLA domain to ZPI binding residues Membrane binding of PZ causes the binding of ZPI to the membrane. The distance between the centre mass of the GLA domain and interaction region of ZPI with PZ show remarkable differences. This implies that Ca2+ may change the distance by effecting the conformation of the GLA domain (Figure 3A) [10]. The distance between the dominant planes of calcium ions in the GLA domain has been indicated in the active site of factors FX and FVII about 61 and 83Å [21– 24]. In the present study, we calculated the distance between the calcium ion plane and the Ser 196 residue in the pseudo active site of the PZ [25], which was estimated around 83Å, as it was measured for FX (Figure 3B & 3C). Apparently, the PZ situates the ZPI in a good height to bind to the FX on the surface of membrane.
Figure 3.
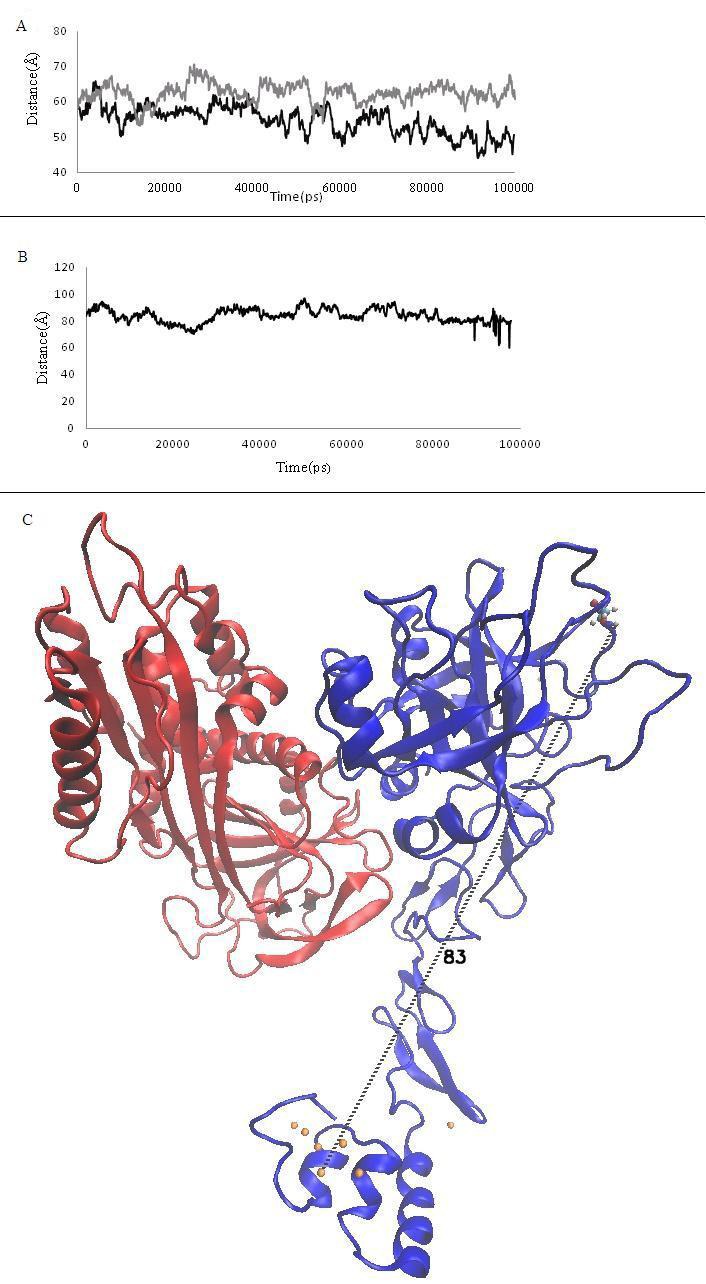
(A) Graph of Distance between center mass of Gla domain and ZPI binding residues. Gray curve is PZZPI without Ca2+ and Black curve is PZZPI with Ca2+ ion; (B) Plot of distance between Ser 196 to dominant plane of Ca2+ ions during simulation of 100ns which it has been calculated with VMD DisRg Plug-in; (C) The illustration shows distance between ser196 and center of mass of Ca2+ ions in the PZZPI complex. Protein Z and ZPI have been shown with blue and red color respectively and orange spheres show Ca2+ ions.
Conclusion
Previous studies have been investigated the role of the Ca2+ in the coagulation factors FIX, FX, protein S, and FVII [18– 19– 26– 27]. Additionally, we prepared complete PZZPI complex in the presence and in the absence of Ca2+ to investigate different structural features and elaborate the effect of Ca2+ ions on structural-functional properties of the mentioned complex. Our data revealed prominent difference between Ca2+ bound and Ca2+ free form structure. Binding of Ca2+ to the coagulation protein is a fundamental process for the membrane attaching of various blood coagulation factors. The GLA domain is a sufficient domain with Gla residues responsible for interacting to Ca2+ ions. It has been demonstrated that Ca2+ions play two distinct roles in the biology of coagulation factors. They play a critical role in folding of the GLA domain and in protein anchoring due to direct contacting to lipids [25]. Here, we constructed the full complex of protein Z and protein Zdependent protease inhibitor with Gla domain, and carried out a MD simulation for the mentioned complex in the presence and in the absence of Ca2+. Furthermore, we investigated the effect of Ca2+ions on the PZZPI complex. The presence of Ca2+ is apparently essential to the optimal orientation of PZ and ZPI to place in an appropriate height to membrane. The presented model can be utilized for simulating the entire complex at the membrane surface and to study of membrane binding and the inhibitory effect on FX.
Supplementary material
Acknowledgments
We thank Dr.zenmei and Prof.Tajkhorshid for their advices and running our systems at illinois university. We would like to give special thanks to Prof.Pedersen at university of North Carolina for his advice. Also we appreciate prof. rezaie at Saint Louis University for his comments.
Footnotes
Citation:Karimi et al, Bioinformation 8(9): 407-411 (2012)
References
- 1.H Sejima, et al. Biochem Biophys Res Commun. 1990;171:661. doi: 10.1016/0006-291x(90)91197-z. [DOI] [PubMed] [Google Scholar]
- 2.A Ichinose, et al. Biochem Biophys Res Commun. 1990;172:1139. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 3.X Han, et al. Proc Natl Acad Sci U S A. 1998;95:9250. [Google Scholar]
- 4.LA Falls, et al. J Biol Chem. 2001;276:23895. doi: 10.1074/jbc.M008332200. [DOI] [PubMed] [Google Scholar]
- 5.H Mizuno, et al. Proc Natl Acad Sci U S A. 2001;98:7230. [Google Scholar]
- 6.M Huang, et al. Nat Struct Biol. 2003;10:751. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 7.CJ Lee, et al. J Thromb Haemost. 2007;5:1558. doi: 10.1111/j.1538-7836.2007.02597.x. [DOI] [PubMed] [Google Scholar]
- 8.X Han, et al. Biochemistry. 1999;38:11073. doi: 10.1021/bi990641a. [DOI] [PubMed] [Google Scholar]
- 9.X Han, et al. Blood. 2000;96:3049. [Google Scholar]
- 10.AR Rezaie, et al. J Biol Chem. 2008;283:19922. doi: 10.1074/jbc.M802639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PK Ngai, JY Chang. Biochem J. 1991;280:805. doi: 10.1042/bj2800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.X Huang, et al. J Biol Chem. 2010;285:20399. doi: 10.1074/jbc.M110.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Z Wei, et al. Blood. 2009;114:3662. [Google Scholar]
- 14.A Tabatabai, et al. Thromb Haemost. 2001;85:655. [PubMed] [Google Scholar]
- 15.W Humphrey, et al. J Mol Graph. 1996;14:33. [Google Scholar]
- 16.JC Phillips, et al. J Comput Chem. 2005;26:1781. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AD MacKerell, et al. The Journal of Physical Chemistry B. 1998;102:3586. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 18.M Sunnerhagen, et al. Nat Struct Biol. 1995;2:504. doi: 10.1038/nsb0695-504. [DOI] [PubMed] [Google Scholar]
- 19.L Perera, et al. Biophys J. 1997;73:1847. doi: 10.1016/S0006-3495(97)78215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L Perera, et al. Biophys. 2000;79:2925. [Google Scholar]
- 21.DW Banner, et al. Nature. 1996;380:41. [Google Scholar]
- 22.D Venkateswarlu, et al. Biophys J. 2002;82:1190. doi: 10.1016/S0006-3495(02)75476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EJ Husten, et al. J Biol Chem. 1987;262:12953. [PubMed] [Google Scholar]
- 24.S Yegneswaran, et al. J Biol Chem. 1997;272:25013. doi: 10.1074/jbc.272.40.25013. [DOI] [PubMed] [Google Scholar]
- 25.YZ Ohkubo, E Tajkhorshid. Structure. 2008;16:72. [Google Scholar]
- 26.Falsafi-Zadeh, et al. Bioinformatoin. 2012;8:341. doi: 10.6026/97320630008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M Huang, et al. J Biol Chem. 2004;279:14338. doi: 10.1074/jbc.M314011200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


