Abstract
The field of polymer therapeutics has evolved over the past decade and has resulted in the development of polymer-drug conjugates with a wide variety of architectures and chemical properties. Whereas traditional non-degradable polymeric carriers such as poly(ethylene glycol) (PEG) and N-(2-hydroxypropyl methacrylamide) (HPMA) copolymers have been translated to use in the clinic, functionalized polymer-drug conjugates are increasingly being utilized to obtain biodegradable, stimuli-sensitive, and targeted systems in an attempt to further enhance localized drug delivery and ease of elimination. In addition, the study of conjugates bearing both therapeutic and diagnostic agents has resulted in multifunctional carriers with the potential to both “see and treat” patients. In this paper, the rational design of polymer-drug conjugates will be discussed followed by a review of different classes of conjugates currently under investigation. The design and chemistry used for the synthesis of various conjugates will be presented with additional comments on their potential applications and current developmental status.
Keywords: polymer-drug conjugates, polymer therapeutics, polymeric prodrugs, nanomedicine, bioconjugation, drug delivery
INTRODUCTION
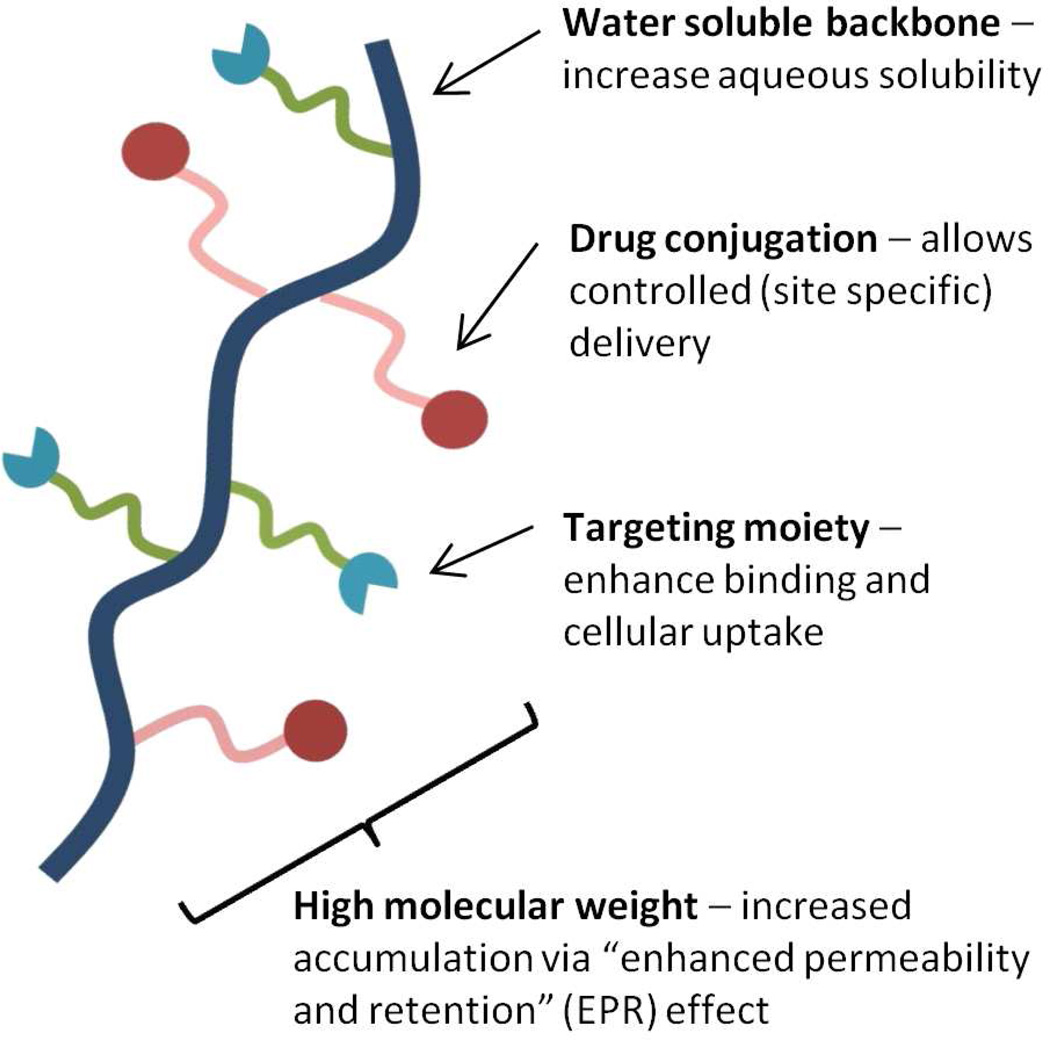
Concepts utilizing polymers in the design of therapeutic agents have been widely investigated for a number of decades. Initial work of the 1960s focused on utilizing polymers as blood plasma expanders, wound dressings, and injectable or implantable depots1, 2. In 1975, a rational model for pharmacologically active polymers was first proposed by Helmut Ringsdorf 3. His concept of covalently bound polymer-drug conjugates still forms the basis for much of the work in this area performed today. The Ringsdorf model (Figure 1) primarily consists of a biocompatible polymer backbone bound to three components: 1) a solubilizer, which serves the purpose of imparting hydrophilicity and ensuring water solubility, 2) a drug, usually bound to the polymeric backbone via a linker, and 3) a targeting moiety whose function is to provide transport to a desired physiological destination or bind to a particular biological target.
Figure 1.
Rationale for drug delivery via polymer-drug conjugates.
The Ringsdorf model is particularly attractive to drug delivery scientists, whose primary objective is the specific delivery of therapeutic agents to their intended site of action in an attempt to improve efficacy and reduce toxicity. These polymer-drug conjugates offer several significant advantages over traditional small molecule therapeutics. First, the aqueous solubility of a drug can be dramatically improved following conjugation to a water soluble polymer4, 5. This is of significant relevance as it has been estimated that 40–60% of drugs in development exhibit poor bioavailability due to low aqueous solubility6. Next, polymer-drug conjugates offer the potential for a drug to be delivered in a controlled manner, with drug release from the conjugate occurring over a defined time interval. In this way, the rate and duration of delivery can be custom designed to achieve the desired therapeutically effective concentration. Thus it is possible to avoid large fluctuations associated with periodic administration which can lead to high systemic drug concentrations resulting in undesired side effects, organ damage, or toxicity. Polymer conjugation also provides an opportunity to alter drug pharmacokinetics and biodistribution. This is particularly useful for drugs which exhibit a short blood plasma half-life due to rapid metabolism or clearance or for drugs which exhibit off target toxicities (i.e. anticancer agents). As previously mentioned, another major advantage that can be realized through drug-polymer conjugation is the inclusion of targeting moieties, which function to carry the drug to the site of pharmacological action.
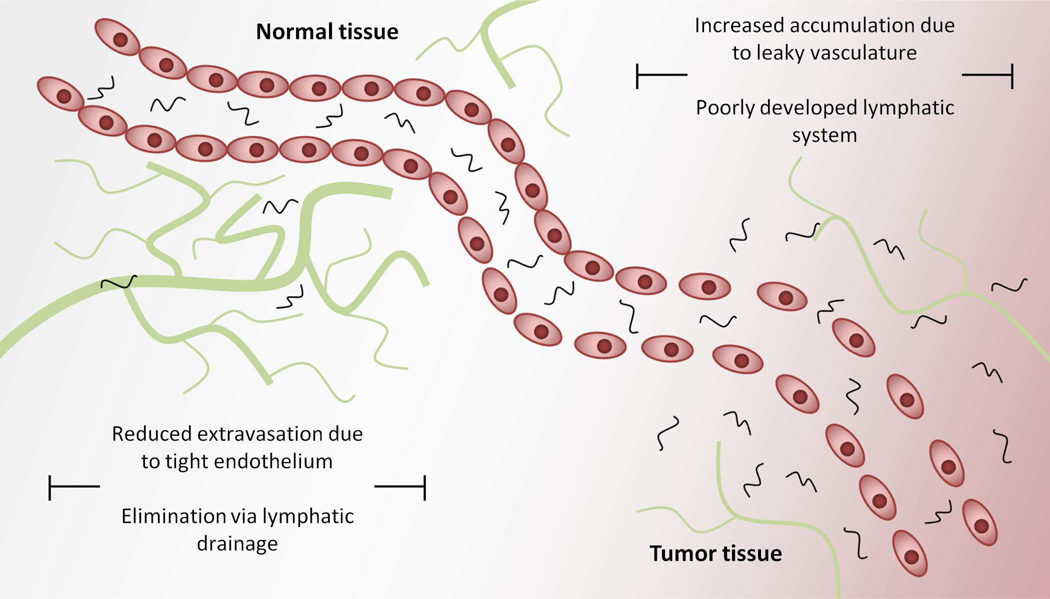
A substantial amount of effort is currently directed toward developing anticancer polymer-drug conjugates. Anticancer agents are often limited by poor water solubility and metabolic instability, and their clinical use is often limited by dose dependant toxicity. The therapeutic index of a given drug is defined as the ratio between its toxic and therapeutic dose. For the clinician, the goal is to deliver an anticancer agent at a dose high enough to achieve cytotoxicity within tumor tissues. However, the actual dose administered is very often limited by toxicity to other vital organs. Thus, any improvement in the therapeutic index for such drugs which allows the clinician the ability to deliver higher drug concentrations to tumor tissue while maintaining manageable side effects can yield benefits for cancer patients. One of the primary ways in which polymer-drug conjugates can increase the therapeutic index of anticancer agents is via the “enhanced permeability and retention (EPR) effect” first described by Matsumura and Maeda in 19867. They proposed that increased uptake of macromolecules by solid tumors can occur due to a combination of poor lymphatic drainage and increased vascular permeability present within the tumor microenvironment (Figure 2). Detailed information about the EPR effect and its implications in cancer chemotherapy has been the subject of previous reviews8–11.
Figure 2.
The enhanced permeability and retention or “EPR” effect; increased tumor accumulation of macromolecules occurs via a combination of increased extravasation and reduced lymphatic drainage in tumor tissues.
Despite the vast amount of effort directed toward the development of therapeutic polymer-drug conjugates, success in terms of translation to clinical practice has been slow due to a variety of unique challenges. Many conjugates are, for example, complex multi-component drug delivery systems that must ultimately satisfy the identity and purity regulatory requirements necessary for any new chemical entity (NCE). Validated methods for reproducible synthesis and characterization such as reversible-addition fragmentation chain-transfer (RAFT) polymerization12–14 or atom transfer radical polymerization (ATRP)15 need to be further utilized to satisfy drug product quality requirements. In addition to this, regulatory requirements require studies examining the metabolic fate of such conjugates, which can become increasingly difficult to characterize for multi-component systems as compared to traditional small molecule therapeutics.
Safety and efficacy of polymer-drug conjugates, as with other more traditional therapeutics, is obviously of the utmost concern during the drug development process. Those conjugates that have progressed to clinical trials have primarily utilized previously approved drugs16–22. Although a polymer-drug conjugate is defined as a NCE for regulatory purposes23, information pertaining to the safety of the free drug can be utilized as a guide in designing toxicity studies. However, careful consideration must be taken as polymer-drug conjugates frequently show altered biodistribution and pharmacokinetic patterns. A critical parameter directly associated with both safety and efficacy is release of the drug from the polymeric carrier. Generally, drug release from a polymeric carrier is necessary for the drug to elicit its pharmacological effect24, 25. This is advantageous as a conjugate will be mostly inactive during systemic transport. If release of the drug from the conjugate occurs prematurely during systemic transport, undesired toxicities may result, and the overall safety profile of the conjugate will be poor18, 26. Therefore, the stability of the conjugate is one critical parameter. However, upon reaching the desired target destination, release of the drug is then required to achieve efficacy. There is therefore a critical balance between conjugate stability and drug release that directly impacts safety and efficacy.
The focus of this article will be to review the rational design of polymer-drug conjugates and their biological evaluation. Polymer-drug conjugates of various chemistries and architectures will be reviewed with emphasis given on the design of each system and potential applications. Stimuli-sensitive systems, the role of targeting, and the emerging field of theranostics based on polymeric systems will also be discussed.
LINEAR POLYMERS
Many different drug conjugates have been synthesized utilizing water soluble linear polymers. While many polymeric carriers have been described such as poly(vinyl pyrrolidone) (PVP)27, 28, poly(vinyl alcohol)29, polyglutamic acid (PGA)30, and poly(malic acid)31, two of the most widely investigated chemistries are those based on poly(ethylene glycol) (PEG)21 and N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers32, which will be reviewed in detail.
Poly(ethylene glycol)
Although numerous different polymer compositions have been synthesized and studied, some of the simplest polymers, such as poly(ethylene glycol) (PEG), maintain widespread use and versatility. PEG-protein conjugates have gained particular importance due to the ability of PEG to protect against protein enzymatic degradation and reduce uptake by the reticuloendothelial system (RES)33, 34, both properties imparted via simple steric hindrance. Protein PEGylation has led to the development of numerous therapeutics, including the FDA approved products PEG-asparaginase (Oncaspar®)35, PEG-adensoine deaminase (Adagen®)36, PEG-interferon α-2a (Pegasys®)37, PEG-interferon α-2b (PEG-Intron®)38, PEG-granulocyte colony-stimulating factor (Neulasta®)39, and PEG-growth hormone receptor antagonist (Somavert®)40. Most commonly, conjugation to PEG is performed via coupling to the end chains41.
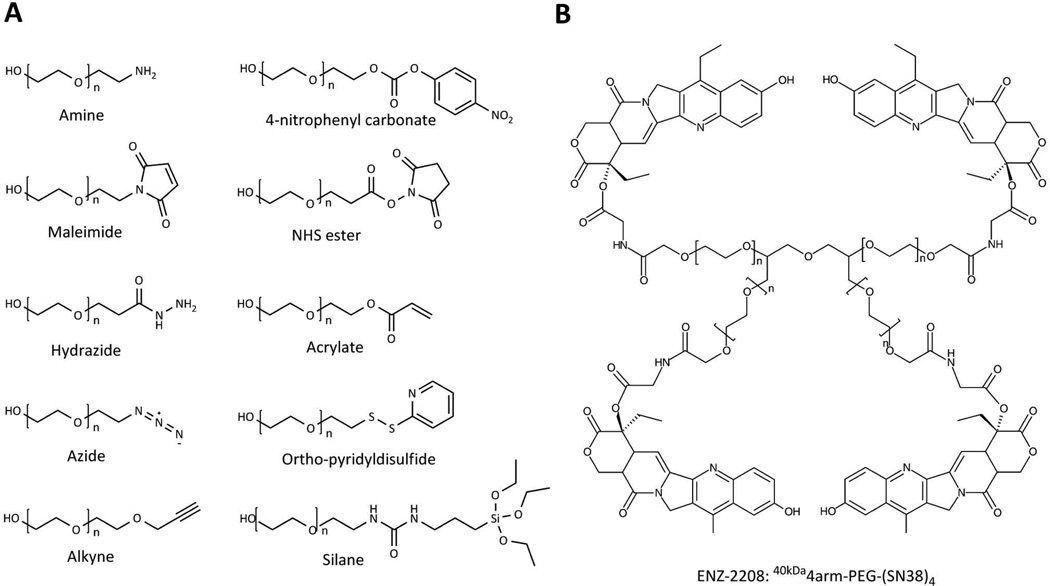
Functionalities such as N-hydroxysuccinimide (NHS) esters or aldehydes allow conjugation to the amine of lysine residues whereas maleimides react readily with the thiol of cysteine residues. Numerous functionalized PEGs are available to aid in conjugation (Figure 3A). Whereas some functionalities allow conjugation to biomolecules such as proteins and antibodies, others can be more generally applied in the synthesis of novel biomaterials. One such approach widely utilized recently involves “click” chemistry42. A click reaction is a highly specific, high yield conjugation reaction wherein mild conditions are commonly used, and by-products are easily removed. The most prevalent example is the 1,3-dipolar cycloaddition of alkynes and azides43. A number of other conjugation chemistries have also been investigated44–46.
Figure 3.
A) Examples of commercially available functionalized PEGs. B) ENZ-2208, a 40 kDa multiarm PEG conjugate currently under clinical investigation; SN-38 is bound to each PEG arm via a glycine spacer. The use of a multiarm PEG allows a degree of biodegradation and results in higher drug loading (3.7 wt% SN-38) than conjugation to linear PEGs (1.7 wt% SN38). Adapted and reprinted with permission from Ref [21].
PEG-drug conjugates have several advantages. First and foremost, the use of PEG as a biocompatible polymer has been established clinically47. Due to the widespread use of PEGs in drug conjugation, an array of functional PEGs are now commercially available44. In addition, large scale synthesis of PEGs are generally routine, and they can be readily synthesized with narrow molecular weight and molecular weight distribution specifications48. These properties make PEG-drug conjugates attractive for pharmaceutical applications.
The use of PEG in conjugation is, however, not without limitations. A fundamental disadvantage of PEG is its non-biodegradability. As previously mentioned, conjugates with a hydrodynamic radius of approximately 3.5 nm are preferred in many applications due to their ability to avoid renal filtration49. However, conjugates of this size have the potential for long term accumulation, resulting in toxic side effects. Another significant disadvantage of traditional PEG-drug conjugates is the low drug loading that is achieved due to conjugation at only the end chains of PEG50. In an effort to overcome these limitations, branched51–53 and multiarm54, 55 PEGs have been investigated which can be excreted more easily following biodegradation.
EZN-2208 is an example of a drug-multiarm PEG conjugate currently under clinical investigation21. This conjugate was synthesized by coupling a 40 kDa 4 pronged multiarm PEG with the camptothecin (CPT) derivative SN38, a potent topoisomerase II inhibitor56 with poor water solubility (Figure 3B). A glycine spacer was utilized to provide a link between the 20-hydroxyl group of SN-38 and each PEG arm. This conjugate demonstrated a drug loading of 3.7 wt% as compared to 1.7 wt% for a previously reported linear PEG-CPT conjugate. Conjugation to this multiarm PEG resulted in approximately 1000 fold increase in the aqueous solubility of SN-3857. EZN-2208 showed a longer blood circulation half-life which resulted in a 207-fold increase in tumor exposure to SN-38 as compared to camptothecin-11 (CPT-11), a small molecule prodrug of SN-38. Antitumor efficacy was also demonstrated in xenograft models of breast, colorectal, and pancreatic cancer57. EZN-2208 is currently under phase 2 clinical investigation for patients with metastatic breast cancer.
N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers
Copolymers of HPMA, first developed by Kopecek and coworkers, have also been widely investigated as hydrophilic, biocompatible, polymeric drug carriers23, 32, 58, 59. Substitution of the α-carbon and the presence of an amide linkage in the side chain ensure hydrolytic stability. In addition, HPMA was selected over other derivatives as the presence of divinyl compounds was eliminated due to the crystalline nature of the monomer, as compared to 2-hydroxyethyl methacrylate type esters. Various functionalities may be incorporated into HPMA copolymers via functionalized comonomers, allowing control over the composition of these systems. In particular, side chains which include drugs, targeting moieties, imaging agents, or reactive groups can be combined with relative synthetic ease. A major driving force behind the continued development of HPMA copolymers as drug carriers was the development of oligopeptide sequences as drug linkers60, 61. These sequences were specifically designed to ensure hydrolytic stability during systemic transport and the ability to be enzymatically cleaved by lysosomal enzymes following cellular internalization62. In developing such a system, early studies with model enzymes demonstrated that factors such as peptide sequence structure and length, drug loading, drug structure, and steric hindrance play important roles in stability and drug release kinetics63, 64. Studies evaluating release in the presence of the lysosomal enzyme cathepsin B resulted in the isolation of the tetrapeptide sequence glycylphenylalanylleucylglycine (GFLG). Numerous HPMA copolymer-drug conjugates utilizing this lysosomally cleavable linker have been reported to date65–68, including several HPMA copolymers used in clinical trials17, 69.
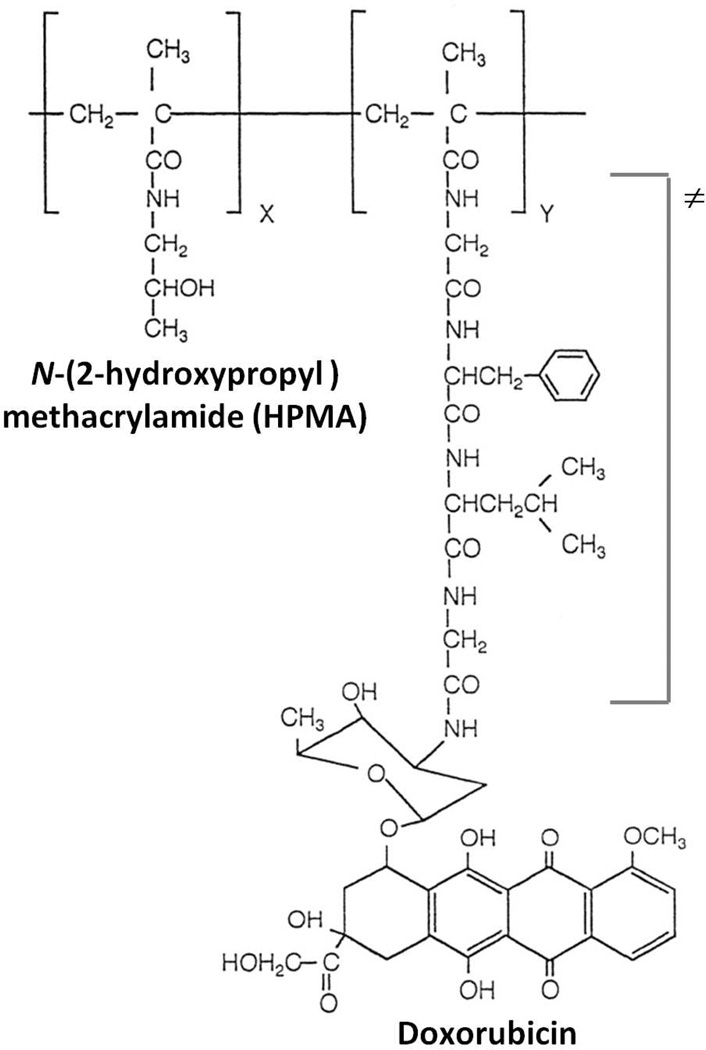
PK1 was the first clinically investigated water soluble polymer-drug conjugate for cancer therapy70. This agent consists of the anticancer anthracycline antibiotic doxorubicin attached to a HPMA copolymer backbone via the lysosomally degradable sequence GFLG (Figure 4). PK1 had a molecular weight of approximately 30 kDa and contained 8.5% doxorubicin by weight71. The stability of the GFLG linkage to doxorubicin was demonstrated following intravenous administration, with no release of free doxorubicin and biological inactivity of covalently bound doxorubicin71. PK1 in comparison with free doxorubicin also demonstrated decreased cardio- and bone marrow toxicity in animals. In addition, tumor accumulation of doxorubicin was increased 17–70 fold as compared to free doxorubicin in melanoma tumor bearing mice72. These promising results led to the further clinical development of PK117, 73. PK1 was generally well tolerated with no alopecia until doses greater than 180 mg/m2, and no anthracycline related cardiotoxicity until doses greater than 1680 mg/m2. Efficacy was marginal with 2 partial and 2 minor responses out of 36 patients observed during phase I studies, and 6 partial responses out of 56 evaluable patients during phase II studies. Building on the experience gained during the evaluation of PK1, a number of HPMA copolymer-drug conjugates have entered clinical evaluation as anticancer agents23, 32, 59.
Figure 4.
Representative structure of PK1 (FCE28068), a HPMA copolymer conjugate bearing the anticancer agent doxorubicin bound via the lysosomally degradable Gly-Phe-Leu-Gly (GFLG) linker (≠). PK1 was the first anticancer polymer-drug conjugate evaluated clinically. Adapted and reprinted with permission from Ref [203].
In the recent decade, strategies to improve on the first generation of HPMA based polymer conjugates have been investigated. For example, HPMA copolymers exhibiting pH dependant drug release have been described (see section on pH sensitive systems). The versatility of HPMA copolymer design has also allowed a wide array of conjugates containing a variety of drugs including taxanes18, 68, 74, camptothecin75, 76, platinates19, 20, dexamethasone77–79, gemcitabine80, 81, and geldanamycin66, 82, 83. Conjugates bearing a combination of drugs have also been investigated80, 84, 85. A “drug-free” strategy has recently been reported, wherein apoptosis of cancer cells is induced via crosslinking of cell surface CD20 using a coiled-coil peptide approach86. More recent attention has also focused on developing backbone degradable HPMA copolymer-drug conjugates (see section on biodegradable polymers).
DENDRIMERS
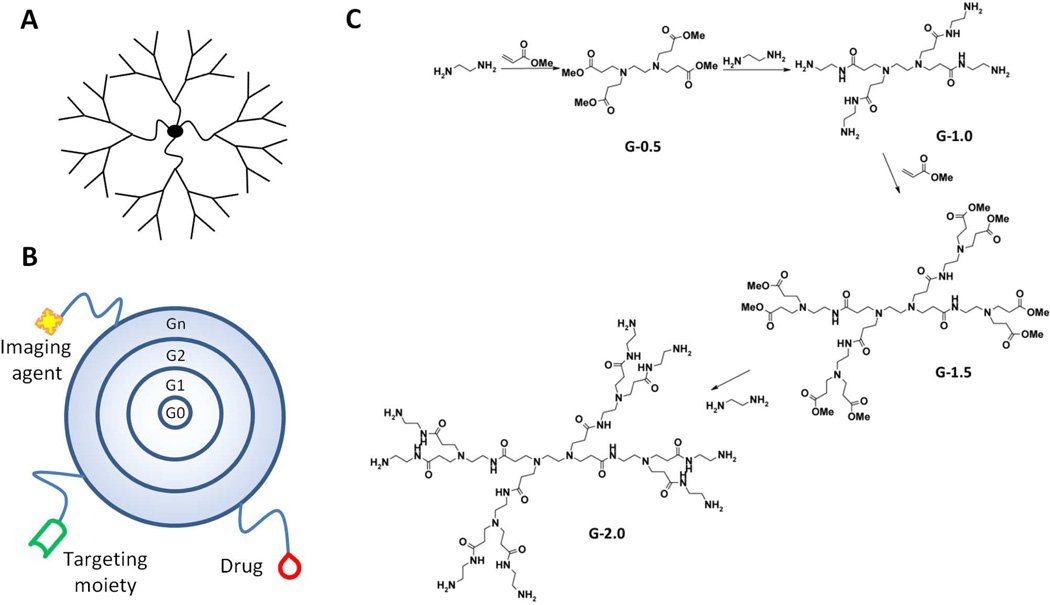
Dendrimers are branched polymeric macromolecules forming a star-like structure (Figure 5A). Such unique structures allow conjugation of drugs to the surface, thus maximizing the potential for biological interactions. A wide array of chemistries can be employed in the synthesis of dendrimers, where the core, monomer units, and surface functionality determine physiochemical characteristics. However, for use in drug delivery applications, it is necessary to maintain biocompatibility. Physiochemical properties such as solubility, surface group functionality, surface charge density, and stability must therefore be considered. Tomalia et al. first described the synthesis of poly(amido amine) (PAMAM) dendrimers in 198587, 88. Synthesis, which occurs for each “generation” in a step-wise fashion (Figure 5B), can result in dendrimers with precisely defined structures. With each synthetic step, the generation increases resulting in a linear increase in radius and an exponential increase in surface groups89. For example, PAMAM dendrimers are synthesized from an ethylenediamine core followed by subsequent half-generation addition by reaction with methyl acrylate and complete generation synthesis by reaction with ethylenediamine (Figure 5C). A major advantage of dendrimers, as compared to most linear polymers, is their synthetic precision, often yielding monodispered structures having polydispersity indices (Mw/Mn) less than 1.0590. In addition, the large number and density of functional groups at the dendrimer surface provides opportunities for conjugation of drugs91, 92, targeting moieties93, imaging agents94, etc. (Figure 5B).
Figure 5.
A) Dendrimers are hyperbranched, star-link polymers. Drugs can be either conjugated to the dendrimer surface or encapsulated within “void” spaces between adjacent branches. B) Dendrimers grow linearly in size and exponentially in surface area with each successive “generation.” They can be utilized as multifunctional nanocarriers, bearing drugs, imaging agents, and/or targeting moieties. C) Synthesis of poly(amido amine) (PAMAM) dendrimers occurs from a ethylenediamine core with alternating reactions with methyl acrylate and ethylenediamine to produce each generation.
In the field of drug delivery, much of the work with dendrimers has focused on their use in the encapsulation and formulation of drugs95. Due to their hyper branched structure, dendrimers often possess open cavities between adjacent branches, thus allowing encapsulation of drugs96. This can aid in the solubilization of poorly water soluble drugs. In addition, dendrimers formulated (physically mixed) with drugs have been investigated as both transdermal97 and oral98 delivery systems. Dendrimers with positively charged surface functionalities, such as poly(ethyleneimine) and PAMAM dendrimers, have also been investigated as gene carriers99, due to their ability to complex with negatively charged DNA.
The covalent attachment of drugs to dendrimers has been widely investigated. Numerous chemotherapeutics have been attached to the surface of dendrimers in an attempt to increase aqueous solubility and provide specific delivery to tumor tissues. For example multifunctional drug delivery platforms utilizing dendrimers conjugated with imaging agents, drugs, and targeting moieties have been investigated.93 Synthesis of such conjugates begins with an ethylenediamine (EDA) initiator core followed by repeated Michael addition of methyl acrylate and subsequent methyl ester condensation with EDA to produce increasing generation PAMAM dendrimers (Figure 5C). In theory, a resulting generation 5 (G5) dendrimer will possess 128 primary amino groups on its surface, thus providing ample opportunity for further modification. Partial acetylation of the primary amino groups has been performed to reduce the positive surface charge in an effort to improve biocompatibility. This is followed by formation of a thiourea bond with fluorescein isothiocyanate (FITC) to enable in vitro imaging. Folic acid as a targeting moiety was then conjugated to PAMAM dendrimers by reaction of the surface primary amines with an activated ester derivative of folic acid. Glycidolation was performed to transform the remaining primary amines into hydroxyl groups, followed by attachment of the chemotherapeutic methotrexate via formation of an ester bond. Characterization of such constructs is generally carried out by size exclusion chromatography coupled with multi-angle laser light scattering (SEC-MALLS), nuclear magnetic resonance (NMR), and UV spectrometry to determine the content of drug, fluorescent probes, and targeting agent100. Confocal images obtained in vitro demonstrated increased cellular uptake of the tri-functional dendrimers in folic acid receptor expressing cells as compared to untargeted controls101. Evaluation for in vivo efficacy was then performed in CB-17 SCID mice bearing human KB cell (human carcinoma over-expressing folic acid receptor) xenografts. Mice treated with the tri-functional dendrimer conjugate showed slowing of tumor growth and increased survival out to 84 days as compared to mice treated with equivalent doses of free methotrexate102. These results demonstrate that dendrimer-drug conjugates can be synthesized and utilized as multi-functional drug delivery platforms.
Dendrimers have also been increasingly investigated for their potential to facilitate drug delivery across biological membranes including skin (transdermal)97, 103, intestinal epithelia104, 105, human placenta106, and the blood-brain barrier107–109. Biodegradable dendrimers110–112, glycodendrimers113, 114, amphiphilic dendrimers115–117, and asymmetric dendrimers118 have also been investigated as potential drug carriers. The further development of dendrimers as multifunctional drug delivery systems functionalized with drugs, targeting moieties, and imaging agents have been the subject of several recent reviews97, 119, 120.
Several studies have also compared dendritic carriers to other more traditional polymeric carriers such as HPMA copolymers and PEG. For example, linear and branched HPMA copolymer-doxorubicin (DOX) conjugates were compared in terms of anticancer activity against lymphoma and colorectal carcinoma cell lines, wherein branched HPMA copolymer-DOX conjugates demonstrated a 3 to 11 fold increase in activity as compared to linear HPMA copolymer-DOX121. Another comparative study evaluated the anticancer activity of G4-paclitaxel dendrimers and PEG-paclitaxel, wherein the G4-paclitaxel demonstrated enhanced activity as compared to free paclitaxel. PEG-paclitaxel, however, showed significantly reduced activity as compared to free paclitaxel122. These results demonstrate the unique potential of dendritic polymeric architectures as drug carriers.
Despite much progress, clinical translation of dendrimer based drug delivery systems has been limited due to concerns over their biocompatibility and toxicity. Dendrimers have been shown to exhibit high affinity for metal ions, lipids, bile salts, proteins, and nucleic acids, resulting in the disrupting of biological processes and leading to toxicity123. The molecular toxicity of dendrimers depends primarily upon their surface functionalization. In particular, dendrimers with a highly positive surface charge have been shown to elicit toxicities in vitro124, 125 and in vivo126, 127. Therefore, much effort is currently focused towards the design of biocompatible dendrimers surface modification to increase biocompatibility. In addition, the difficulty and expense associated with dendrimer synthesis needs to be addressed before clinical translation can be achieved.
POLYMERIC MICELLES
Micelles are colloidal particles with a size of about 5–150 nm that consist of self assembled aggregates of amphiphilic molecules or surfactants. Amphiphiles, at low concentrations in aqueous media exist as unimers in solution. However, as their concentration is increased, thermodynamic processes drive the formation of aggregates which sequester hydrophobic regions into core like structures surrounded by a hydrophilic corona or shell. The concentration at which aggregation occurs is commonly referred to as the critical micelle concentration (CMC). Traditionally, low molecular weight surfactants (i.e. polysorbates, sodium dodecyl sulfate, etc.) with relatively high CMCs in the range of 10−3 to 10−4 M have been used extensively in pharmaceutical formulations, primarily as excipients to increase the aqueous solubility of poorly water soluble drugs128. Hydrophobic drugs are contained within and associate with the hydrophobic regions of the micelle. However, following administration, dilution of a given pharmaceutical formulation occurs rapidly, and as the micelle concentration drops below its CMC, its stability is compromised.
Early work by Kataoka 129, Kabanov 130, and coworkers described the potential use of amphiphilic polymers as drug carriers. These polymeric micelles are primarily composed of block-copolymers with hydrophilic and hydrophobic units that also self assemble into a hydrophobic core surrounded by a hydrophilic shell (Figure 6A). Each micellar unimer unit can be assembled in various fashions such as A–B diblock copolymers, A-B-A triblock copolymers, and grafted copolymers. A major advantage of polymeric micelles as compared to traditional low molecular weight surfactant derived systems is their increased stability. Polymeric micelles commonly exhibit CMCs in the 10−6 to 10−7 M range131. The ideal polymeric micelle should demonstrate high drug loading ability, controlled drug release, and suitable biological compatibility and stability. Physiochemical properties of polymeric micelles are primarily based on the characteristics and lengths of the hydrophilic and hydrophobic blocks. PEG is the most commonly employed hydrophilic polymer, due to highly hydrated nature and ability to resist uptake by the reticuloendothelial system (RES). However, a number of other hydrophilic polymer chemistries have been applied including poly(N-vinyl-2-pyrrolidone) (PVP)132, poly(vinyl alcohol) (PVA)133, and poly(ethyleneimine) (PEI)134. PEG remains the polymer of choice due to its widespread acceptance and availability. A large variety of unimers to form hydrophoblic blocks have been utilized in forming the hydrophobic core of polymeric micelles. Examples include propylene oxide, L-lysine, caprolactone, D,L-lactic acid, styrene, aspartic acid, β-benzoyl-L-aspartate, and spermine among others135. More hydrophobic unimers (i.e. styrene) form micelle cores spontaneously, while other less hydrophobic unimers (i.e. lysine) first interact via electrostatic interactions with hydrophobic drug molecules, followed by micelle formation136. CMC tends to depend more on the type and length of the hydrophobic block, with lower CMCs associated with greater hydrophobicity and increased hydrophobic block length137, 138.
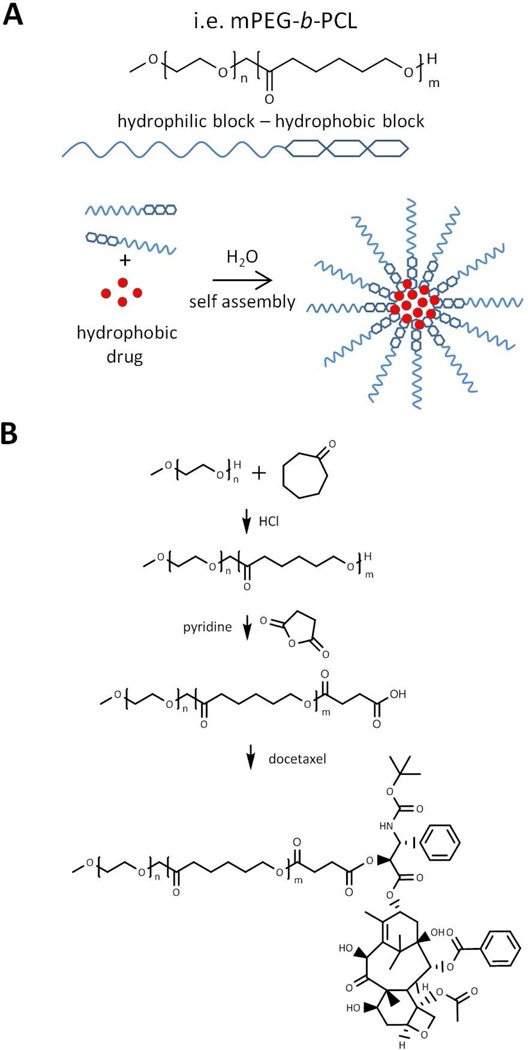
Figure 6.
A) A typical example of polymeric micelle unimer structure composed of both hydrophilic (mPEG) and hydrophobic (PCL) blocks. Hydrophobic drugs associate with hydrophobic domains of the micelle following self assembly in aqueous conditions. B) Synthetic scheme for mPEG-b-PCL-docetaxel micelle unimer. Adapted and reprinted with permission from Ref [139].
The majority of polymeric micelles investigated cannot technically be classified as polymer-drug conjugates, as no covalent bonds exist between the drug and the micellar carrier. However, a number of polymeric micelles wherein the drug is covalently bound to the hydrophobic chains in the micelle core have also been described. For example, recent work has focused on poly(ethylene glycol)-b-poly(ε-caprolactone) polymeric micelles containing chemically conjugated docetaxel (Figure 6B)139. Synthesis of PEG-b-polycaprolactone (PCL) was first achieved via cationic ring-opening polymerization. Following polymerization, the terminal hydroxyl of PCL was reacted with succinic anhydride to generate a terminal carboxylic acid, which was then subsequently coupled to 2’ hydroxyl of docetaxel, a potent anti-mitotic chemotherapy agent (Figure 6B). Conjugation performed in this scenario resulted in an increase in the drug loading of docetaxel as well as increased stability as evidenced by a reduced release rate of docetaxel from the micelle core.
Continued advances are being made in the development of polymeric micelles. Increasingly, there is a trend towards “smart” polymeric micelles in terms of their response to various biological stimuli (see section on pH-sensitive systems) and their ability to target specific tissues (see section on targeting). Another interesting application involves the use of polyion complex (PIC) micelles, wherein the micelle core is composed of a polycation block, for the delivery of negatively charged DNA or small interfering RNA (siRNA)140, 141. Polymeric micelles based on HPMA copolymers have also been described142. PolyHPMA has been used successfully in the generation of polymeric micelles either in the hydrophilic block comprising the shell143–146, or following chemical modification, as the hydrophobic core147, 148. A variety of hydrophobic drugs have also been encapsulated in these micelles; however, the majority of the data on the activity of these systems to date have been obtained in vitro142, 148, 149, and more in vivo data is needed to ascertain their potential as carriers.
An advantage of polymeric micelles as compared to other polymeric drug carriers is their relative ease of fabrication, due to their inherent self-assembly properties. This has resulted in a number of polymeric micelles currently under clinical investigation150.
BIODEGRADABLE POLYMERS
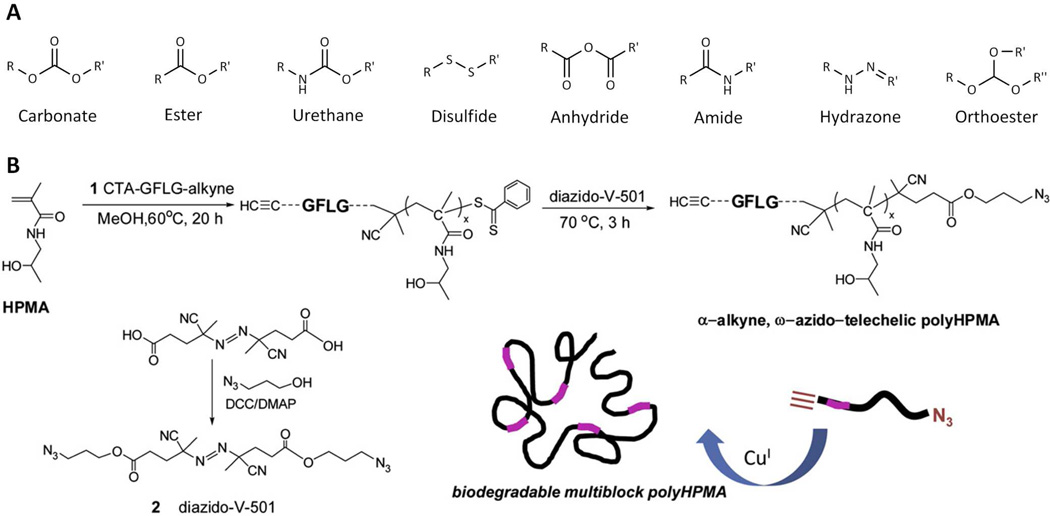
A major advantage of polymer-drug conjugates is their ability to escape filtration via the kidneys, resulting in an increased blood circulation time. For anti-cancer conjugates, an added advantage is increased tumor accumulation via the previously described EPR effect for conjugates at least greater than 3.5 nm49. However, eventual elimination from the body is also required to reduce potential long term adverse effects of these carriers. The use of biodegradable systems allows conjugates of a sufficient size to both evade renal filtration and allow subsequent degradation and elimination. Such conjugates should have degradation rates slow enough to allow adequate biodistribution, and such degradation should result in the production of non-toxic degradation products. A number of biologically degradable bonds have been described (Figure 7A). Biodegradation generally occurs via hydrolysis, enzymatic cleavage, or reductive degradation. Biodegradable polymers have been described151, 152 which include poly(α-amino acids) such as poly(L-lysine)153, poly(L-glutamic acid)154, and poly ((N-hydroxyalkyl)glutamine)155 as well as carbohydrate polymers such as dextrins156, hydroxyethylstarch (HES)157, polysialic acid158, and the polyacetal Fleximer®22.
Figure 7.
A) Examples of bonds utilized in the synthesis of biodegradable polymer-drug conjugates. Biodegradation typically occurs via hydrolysis (via reduction for disulfides). B) Overall strategy for the synthesis of multiblock polyHPMA copolymers. HPMA copolymer blocks are linked together via lysosomally degradable Gly-Phe-Leu-Gly (GFLG) linkages introduced via a combination of RAFT polymerization and click chemistry. Adapted and reprinted with permission from Ref [81].
An example of a biodegradable polymer-drug conjugate currently under phase III clinical development in the United States is OPAXIO™(formerly branded as XYOTAX), which is a conjugate of poly(L-glutamic acid) and the anticancer agent paclitaxel30. Poly(L-glutamic acid) was chosen as its breakdown product L-glutamic acid can enter normal cellular metabolism and is not cleared via the kidneys. Paclitaxel is conjugated via an ester bond to the γ-carboxylic acid side chains. In addition, because conjugation is via the 2’ hydroxyl of paclitaxel, the conjugate is unable to bind tubulin and elicit its pharmacological action, thus rendering it inactive. In one example, the poly(L-glutamic acid) conjugate had a molecular weight of 48 kDa, and contained approximately 37% by weight paclitaxel, while maintaining water solubility. During preclinical investigation, this conjugate demonstrated a higher maximum tolerated dose (MTD) and was more efficacious than paclitaxel formulated in Cremophor EL/ethanol. Clinical trials are currently underway159–163 evaluating OPAXIO™in prostate, breast, ovarian, colorectal, and lung cancers.
Strategies to produce biodegradable derivatives of more traditional polymers such as PEG and HPMA copolymers have also been investigated. As mentioned previously, biodegradable multi-arm PEGs57 (i.e. ENZ-2208, Figure 3B) containing ester bonds between PEG chains have entered clinical trials. Another strategy in which a biodegradable polymer consisting of small molecular weight PEG blocks is linked together via enzymatically cleavable oligopeptide groups, and bearing the anti-cancer agent doxorubicin, has been described164. Work by Ulbrich and coworkers has utilized a variety of approaches to generate biodegradable HPMA copolymer-drug conjugates including graft systems containing oliogopeptide sequences and/or reductive disulfide bonds165, as well as the generation of biodegradable star HPMA copolymer-drug conjugates166. In the latter, PAMAM dendrimers were modified with polyHPMA grafts via enzymatically cleavable or reducible linkers, thus enabling degradation of the high molecular weight polymer. These star polymer conjugates bearing doxorubicin exhibited prolonged blood circulation, increased tumor accumulation, and anti-tumor efficacy in lymphoma tumor bearing mice110. Other recent work on the synthesis of biodegradable multiblock poly(HPMA) conjugates generated via a combination of RAFT polymerization and click chemistry has been described81, 167. This synthesis was performed in three major steps (Figure 7B). First, RAFT polymerization of HPMA was performed using an enzyme-sensitive, GFLG containing chain transfer agent (CTA) with a terminal alkyne. Second, post-polymer modification was performed to introduce a terminal azide, resulting in an α-alkyne, ω-azido-telechelic poly(HPMA). Third, a biodegradable multiblock poly(HPMA) was synthesized by click chemistry in the presence of a copper catalyst. The resulting biodegradable multiblock poly(HPMA) exhibited a molecular weight of 291 kDa and a polydispersity index of 1.11. Following incubation with model lysosomal enzymes, poly(HPMA) segments of similar molecular weights (42 kDa) were obtained. These results demonstrate how advances in chemistry (i.e. RAFT polymerization and click chemistry) can be utilized to generate new biodegradable polymer-drug conjugates with well-defined physicochemical properties.
STIMULI SENSITIVE POLYMERS
So called “smart polymers” have been engineered to contain a vast array of properties, including the ability to respond to changes in environmental stimuli such as pH, ionic strength, temperature or externally applied heat, magnetic or electric fields, or ultrasound168. Such polymers commonly respond via conformational and/or electrostatic changes, which can be exploited to help facilitate a particular function (i.e. drug release, endosomal escape, etc.). Carriers which respond to variations in pH and temperature have found the greatest versatility in drug delivery and will be reviewed in brief below.
pH-sensitive systems
Exploiting physiological variations in pH has been widely investigated as a means to obtain site specific delivery. The pH of diseased areas such as tumors, infarcts, and sites of inflammation may drop to around 6.5, almost one full pH unit below that of normal blood (pH 7.4) due to hypoxic conditions and extensive cell death169, 170. In addition, following cellular uptake via endocytosis, the pH of late endosomes may reach values as low as 5.0, further providing a gradient over which release may be triggered171. The polymer backbone can be made pH sensitive, typically through the inclusion of acidic (i.e. carboxylic and sulfonic acids) or basic (i.e. ammonium salts) groups that undergo protonation or deprotonation in response to changes in pH. Commonly studied chemistries of this nature include poly(acrylamide) (PAAm), poly(methacrylic acid) (PMAA), poly(acrylic acid) (PAA), and poly(2-(dimethylamino)ethyl methacrylate (PDMAEMA)172. Utility is primarily found in non-conjugated systems such as micelles, liposomes, and other nanocarriers, where conformational changes disrupt the stability of the carrier resulting in drug release173.
As for polymer-drug conjugation, pH sensitivity is introduced primarily via pH sensitive chemical bonds which can result in site specific drug delivery. For example, hydrazone bonds formed via the action of hydrazine on ketones or aldehydes exhibit hydrolysis under mildly acidic conditions (pH 5–6) such as that present in lysosomes while maintaining stability at pH values found in blood (pH 7.4)174, 175. For example, pH sensitive HPMA copolymers bearing the anticancer agent doxorubicin conjugated via hydrazone bonds and cis-aconitic acid residues (aconityl conjugation) have been described176. These systems demonstrate increased drug release at pH 5 as compared to pH 7.4, suggesting potential preferential release following cellular uptake via endocytosis. In vitro assays demonstrated that cytotoxicity was related to drug release kinetics, with higher toxicity observed for faster releasing hydrazone conjugates as compared to aconityl conjugates, and these conjugates also demonstrated significant in vivo activity as determined by tumor regression in mice176.
A number of pH responsive polymeric micelles have also been described including systems in which doxorubicin was conjugated to the side chains of the micelle core-forming blocks via hydrazone bonds177. The micelles demonstrated both time and pH dependant release, with increased release under endosomal low pH conditions (5.0–5.5)177. Biodistribution studies showed minimal signs of premature drug release, and selective accumulation in tumors and the anti-tumor efficacy of these pH sensitive micelles was significantly higher than that achieved with comparable doses of free doxorubicin178.
Temperature-sensitive systems
The concept of using temperature to control drug delivery is in part due to the observation that elevated temperature can be associated with diseased tissues. In addition, the external application of hyperthermia can be utilized as a trigger to induce changes in polymer structure resulting in drug release. Water soluble temperature sensitive polymers such as those based on poly(N-isopropylacrylamide) (poly(NIPAAM)) undergo a lower critical solution temperature (LCST) phase transition, wherein polymer chains collapse and aggregate at temperatures above their LCST due to the reversible dehydration of hydrocarbon side chains179. The LCST for poly(NIPAAM) is approximately 32°C. However, the LCST of such polymers can be adjusted by changing the N-substituted carbon chain or via copolymerization180.
Over the past decade, elastin-like polypeptides (ELPs) have been investigated in drug delivery applications. ELPs are recombinant polymers produced using genetic engineering techniques181, 182, resulting in monodisperse polymers with precisely defined molecular weights and compositions183. They consist of a repeated peptide sequence based on a motif found in mammalian tropoelastin (VPGXG)n, where X is defined as any residue except proline184. ELPs exhibit an LCST above which they become insoluble. This LCST can be varied by modifications in molecular weight and composition. A number of strategies utilizing the thermo sensitive nature of ELPs have been investigated. For example, ELPs with LCST above normal body temperature but below 43°C have been utilized as anticancer drug carriers, in which systemic delivery is combined with localized hyperthermia to tumor tissue, resulting in an increased accumulation of ELP aggregates within the tumor 185. Other strategies including the use of ELP microparticles, ELP micelles, and ELP block copolymers have also been investigated186, 187.
TARGETING
A tremendous amount of effort developing therapeutic polymer-drug conjugates and other nanomedicines has focused on the inclusion of targeting moieties. In the majority of cases, physicochemical properties of polymer-drug conjugates such a size, surface charge, conformation, and biocompatibility dictate how absorption, distribution, metabolism, and excretion take place. 188. As previously described, polymer-drug conjugates with a size above renal threshold will exhibit longer blood circulation times, thereby increasing the probability for a conjugate to interact with its target. The benefits of so called “active targeting” are realized by increasing binding to and internalization into the cells of target tissues, phenomena which occur over short distances. Targeting can therefore be utilized as a way to maximize the effect of “passive targeting” mechanisms, by ensuring that physically delivered polymer-drug conjugates remain at their intended site of action.
A number of features characterize an ideal target. The target should be universally and uniquely expressed by the diseased tissue. The vast majority of targeting strategies rely on the over expression of particular cell surface markers in diseased cells as compared to normal cells. Therefore, the probability of binding and cellular uptake of a conjugate with its intended target is increased as compared to normal cells. While it is well understood that expression of a particular target is generally not entirely specific, it is nevertheless anticipated that the large relative differences in expression between diseased and normal cells can still be utilized as an effective targeting strategy. Ideally, the target should also facilitate endocytosis following binding of the conjugate, thereby allowing the agent to exert it pharmacological action within the cell.
Sugars, hormones, growth factors, antibodies, antibody fragments, peptides, or other small molecules can be utilized as targeting moieties189–192. The targeting moiety must include the necessary functionality to facilitate conjugation, and should be conjugated in a manner that will ensure its stability during systemic circulation. Different targeting moieties have distinct advantages and disadvantages. Antibodies, for example provide excellent binding affinity and target selectivity. However, their large size can drastically influence the properties of the carrier. Also, the relative cost associated with antibody production, conjugation, and concerns over their stability and immunogenicity remain important issues. The use of antibody fragments (Fab′, single-chain variable fragments (scFvs)) can partially address these concerns. These proteins retain the specificity of the original antibody, but are reduced in size, and can often be synthesized in bacterial cultures and thus reduce synthetic cost. Other targeting moieties such as peptides, sugars, and hormones can generally be readily synthesized at low cost, but they typically have reduced binding affinity and specificity as compared to antibodies and antibody fragments. These advantages and disadvantages and the choice of carrier dictate targeting moiety selection. How the binding affinity of the targeting moiety is affected following conjugation must also be evaluated.
In an attempt to find an alternative, low cost, effective targeting strategy, Torchilin and coworkers have described paclitaxel loaded polymeric micelles modified with a tumor-specific phage protein193. The amphiphilic nature of the phage fusion coat protein enabled stable incorporation into the polymeric micelles without the requirement for specific conjugation chemistry and this approach relied completely on the inherent self-assembly of the phage protein into the micelles, resulting in synthetic ease. In vitro evaluation of cellular uptake and cytotoxicity demonstrated enhanced activity as compared for free paclitaxel and non-targeted micelles193.
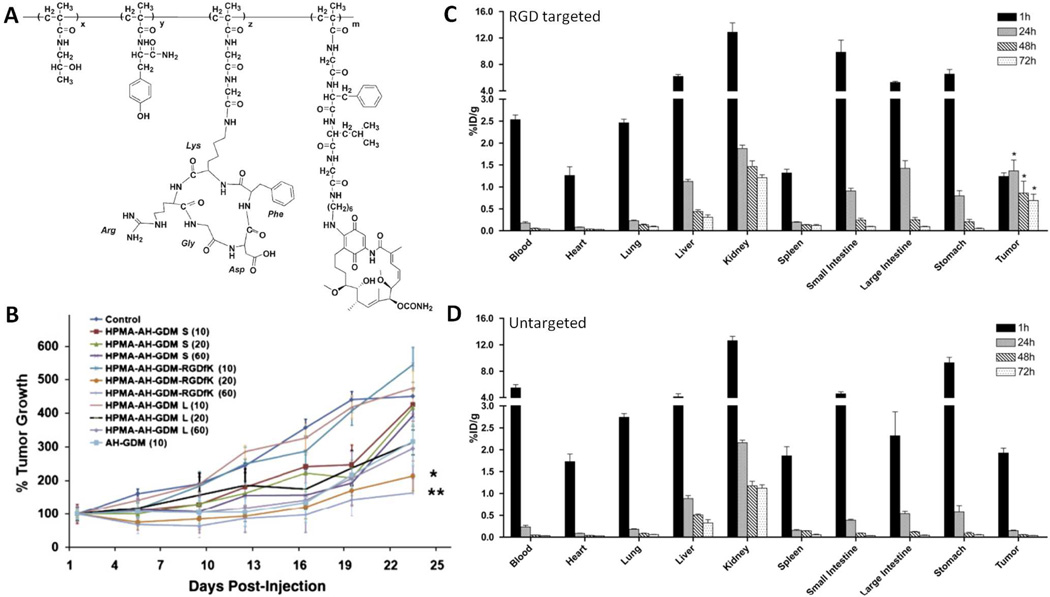
Work in our laboratory has focused on developing HPMA copolymers containing cyclic Arg-Gly-Asp (RGD) peptides that target αvβ3 integrins expressed on angiogenic tumor blood vessels and tumor cells68, 82, 194–196. Copolymers containing a derivative of the anticancer and antiangiogenic agent geldanamycin (aminohexylgeldanamycin (AHGDM)) bound to the polymer backbone via the lysosomally degradable GFLG linker were synthesized (Figure 8A) and characterized. Molecular weight was maintained below 40 kDa to allow eventual renal clearance following administration. The conjugates demonstrated the ability for drug release, binding to αvβ3 integrins, and introduced cytotoxicity in endothelial and prostate cancer cells at concentrations similar to the free drug controls. To assess the efficiency of targeting, the biodistribution of 125I-labeled copolymers was evaluated in prostate cancer bearing mice. Significantly higher localization was observed in the tumor following administration of the HPMA copolymer containing cyclic RGD peptides as compared to an untargeted control (Figure 8C, 8D). In addition, the tumor accumulation of released drug was quantified by tumor extraction followed by HPLC analysis. Significantly higher concentrations of AHGDM were observed following administration of the targeted conjugate. In vivo efficacy studies were performed in prostate cancer tumor bearing mice. Percent tumor growth as a function of time was evaluated following a single dose of HPMA copolymer-AHGDM-cyclic RGD, HPMA copolymer-AHGDM (untargeted control), AHGDM (free drug control), or saline (negative control). The study also included a large molecular weight HPMA copolymer-AHGDM conjugate so as to see how efficacy via RGD targeting would compare to conjugates relying solely on “passive” targeting via the EPR effect. Tumor growth was suppressed more for HPMA copolymers bearing cyclic RGD peptides as compared to both untargeted controls (small and large molecular weight) and free drugs (Figure 8B). The results demonstrate that an appropriately selected targeting strategy can yield increased tumor delivery resulting in the increased efficacy of cancer chemotherapy.
Figure 8.
A) HPMA copolymer conjugates bearing the antiangiogenic and anticancer agent aminohexylgeldanamycin (AH-GDM) and cyclic Arg-Gly-Asp (RGD) peptides for targeting angiogenic αvβ3 integrin expression blood vessels. B) Tumor regression as a function of time in DU145 human prostate cancer tumor bearing nu/nu mice. RGD targeted HPMA copolymers demonstrated significant efficacy as compared to untargeted HPMA copolymers and free drug controls. C) Biodistribution in DU145 bearing mice of 125I-radiolabeled HPMA copolymers bearing AH-GDM and cyclic RGD peptides. Increased accumulation was observed in tumor tissues as compared to untargeted conjugate (D). Adapted and reprinted with permission from Refs [82, 83, 195].
THERANOSTICS
In addition to their application as therapeutics agents, polymer-drug conjugates and other nanomedicines are increasingly being studied for diagnostic purposes. The combination of use of therapeutics and diagnostics has resulted in the term “theranostics” which defines delivery systems bearing both therapeutic and imaging or contrast agents197. Such systems allow for a more personalized medicine approach, wherein therapy can be directly monitored and custom tailored. Multiple benefits may be realized from these multifunctional systems. For example, biodistribution and accumulation at the target site of therapy can be monitored in a non-invasive manner. In addition, localization at the target site can be used as an accurate predictor of efficacy, thus relieving a patient from subsequent therapy that might not prove efficacious. This can be achieved by first administering a tracer version of an imaging agent labeled conjugate. Those patients who demonstrate abnormal or unfavorable biodistribution, pharmacokinetics, or localization at the target site are then disqualified from treatment with a therapeutic version of the conjugate. Imaging modalities such as optical imaging, x-ray computed tomography (CT), dynamic contrast enhanced magnetic resonance imaging (DCE-MRI), single photon emission computerized tomography (SPECT) and positron emission tomography (PET) are well established and provide the necessary tools to allow spatial visualization and quantification of delivery. For example, during the initial clinical evaluation of HPMA copolymer-doxorubicin conjugates (PK1, PK2), localization was visualized in patients following treatment with 131I radiolabeled conjugates, allowing information related to potential toxicities and tumor accumulation to be obtained early in the development process. Details regarding the use of macromolecules in theranostic applications are outside the current scope, but has been the subject of several reviews197, 198 and it is anticipated that going forward, multifunctional drug-polymer conjugates bearing imaging agents will play a role in the future of image guided drug delivery and personalized medicine.
CHALLENGES AND FUTURE OUTLOOK
All polymers exhibit some degree of heterogeneity. Traditional small molecular weight therapeutics and proteins have well defined chemical structures. In contrast, individual polymer-drug conjugate molecules vary with respect to molecular weight, drug loading, and resulting conformation. In order to satisfy strict regulatory criteria, variations in such properties must be minimized. It is therefore critical that polymeric conjugates be synthesized in a reproducible fashion. As previously discussed, modern polymerization techniques such as RAFT and ATRP provide better control over both molecular weight and molecular weight distribution. Validated methods for physiochemical characterization must also be developed to ensure reproducible product quality. As conjugates become more complex (i.e. multifunctional nanomedicines), complexities in synthesis and characterization are also increased. Those polymeric conjugates that have progressed to clinical evaluation thus far have been synthesized in a relatively simple and straightforward manner (i.e. PK1, OPAXIO). More complex conjugates currently being developed offer potential advantages such as stimuli-sensitivity, active targeting, or inclusion of imaging agents. However, increases in the complexities of synthesis and characterization need to be carefully weighed against improvements in efficacy and/or safety to ensure that an appropriate “cost to benefit” ratio is maintained. Ideally, conjugates should offer significant advantages in efficacy and safety while maintaining simple and cost effective synthesis.
The rate of drug release is directly related to the efficacy and safety of polymer-drug conjugates. As previously described, strategies for site-specific release via degradable peptide sequences or variations in pH are used frequently. However, there is considerable room for improvement with respect to linker design. The ideal linker should be stable during systemic transport, but facilitate drug release at the intended site of action. Some examples of linkers currently under investigation include a peptide based linker cleaved in the presence of tumor-associated legumain199 and peptide based coiled-coil linkers200, 201. Another interesting approach is the use of “self-immolative” linkers202 in drug conjugation, wherein changes in a “trigger” moiety rapidly induces or amplifies degradation of additional bonds. Novel approaches such as these and the design of new linker chemistries will help facilitate further development of polymer-drug conjugates.
Although many polymer-drug conjugates based on chemistries such as PEG or HPMA have progressed into clinical development, their progression towards market approval has undoubtedly been hindered by the non-biodegradable nature. Those conjugates which have been evaluated have primarily been low molecular weight conjugates, so as to allow renal elimination. However, these conjugates are therefore unable to take full advantage of potential benefits such as long blood circulation times and potential tumor accumulation via the EPR effect. In addition, persistence of the polymer backbone following treatment is a significant disadvantage, especially where drug loading is below 10%, which require high quantities of the polymeric conjugates to be administered. As previously described in detail, a number of biodegradable chemistries are currently available. Biodegradable conjugates have advantages in terms of: a) efficacy, due to the ability to fully optimize pharmacokinetics via increases in molecular weight, and b) safety, due to their biodegradation and elimination post-treatment. These advantages are significant, and it is safe to assume that going forward, those conjugates which are biodegradable will have a much higher probability of clinical success.
The field of polymer therapeutics and the rational design of polymer-drug conjugates have seen much progress over the past 2 decades, with an increasing number of candidates currently under clinical investigation. A number of different polymer architectures and chemistries are being developed and new applications are being realized. The continued development and success of this field in particular is dependent on a multidisciplinary approach, where collaboration between polymer chemists, medicinal chemists, pharmaceutical scientists, biologists, and clinicians is critical in the design, development, and clinical translation of polymer-drug conjugates. Such collaboration, however, will continue to yield success in the synthesis of novel biomaterials and further aid in the development of controlled and site specific drug delivery technologies.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grats (R01 EB007171 and R01 EB007470) and the Utah Science Technology and Research (USTAR) initiative.
REFERENCES
- 1.Shelanski HA, Shelanski MV. PVP-iodine: history, toxicity and therapeutic uses. J Int Coll Surg. 1956;25(6):727–734. [PubMed] [Google Scholar]
- 2.Ravin HA, Seligman AM, Fine J. Polyvinyl pyrrolidone as a plasma expander. New Eng J Med. 1952;247(24):921–929. doi: 10.1056/NEJM195212112472403. [DOI] [PubMed] [Google Scholar]
- 3.Ringsdorf H. Structure and properties of pharmacologically active polymers. Journal of Polymer Science: Polymer Symposia. 1975;51(1):135–153. [Google Scholar]
- 4.Van S, Das SK, Wang X, Feng Z, Jin Y, Hou Z, Chen F, Pham A, Jiang N, Howell SB, Yu L. Synthesis, characterization, and biological evaluation of poly(L-gamma-glutamyl-glutamine)-paclitaxel nanoconjugate. Int J Nanomedicine. 2010;5:825–837. doi: 10.2147/IJN.S13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Li Z, Chau Y. Synthesis, characterization, and in vivo evaluation of poly(ethylene oxide-co-glycidol)-platinate conjugate. Eur J Pharm Sci. 2010;41(3–4):464–472. doi: 10.1016/j.ejps.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Lipinski C. Poor aqueous solubility - An industry wide problem in drug discovery. Am Pharm Rev. 2002;5(3):82–85. [Google Scholar]
- 7.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 8.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15(7–8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 11.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Gregory A, Stenzel MH. The use of reversible addition fragmentation chain transfer polymerization for drug delivery systems. Expert Opin Drug Deliv. 2011;8(2):237–269. doi: 10.1517/17425247.2011.548381. [DOI] [PubMed] [Google Scholar]
- 13.Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT polymerization. Chem Rev. 2009;109(11):5402–5436. doi: 10.1021/cr9001403. [DOI] [PubMed] [Google Scholar]
- 14.York AW, Kirkland SE, McCormick CL. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: stimuli-responsive drug and gene delivery. Adv Drug Deliv Rev. 2008;60(9):1018–1036. doi: 10.1016/j.addr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Matyjaszewski K, Tsarevsky NV. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat Chem. 2009;1(4):276–288. doi: 10.1038/nchem.257. [DOI] [PubMed] [Google Scholar]
- 16.Galic VL, Herzog TJ, Wright JD, Lewin SN. Paclitaxel poliglumex for ovarian cancer. Expert Opin Investig Drugs. 2011;20(6):813–821. doi: 10.1517/13543784.2011.576666. [DOI] [PubMed] [Google Scholar]
- 17.Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schatzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtles S, Duncan R, Cassidy J. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol. 2009;34(6):1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 18.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, Rocchetti M, Rosing H, Koopman FJ, Beijnen JH. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12(4):315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, Beijnen JH, Schellens JH, Droz JP. A Phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin Cancer Res. 2004;10(10):3386–3395. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- 20.Campone M, Rademaker-Lakhai JM, Bennouna J, Howell SB, Nowotnik DP, Beijnen JH, Schellens JH. Phase I and pharmacokinetic trial of AP5346, a DACH-platinum-polymer conjugate, administered weekly for three out of every 4 weeks to advanced solid tumor patients. Cancer Chemother Pharmacol. 2007;60(4):523–533. doi: 10.1007/s00280-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 21.Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;61(13):1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Deliv Rev. 2009;61(13):1193–1202. doi: 10.1016/j.addr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Duncan R, Vicent MJ. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv Drug Deliv Rev. 2010;62(2):272–282. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Malugin A, Kopeckova P, Kopecek J. Liberation of doxorubicin from HPMA copolymer conjugate is essential for the induction of cell cycle arrest and nuclear fragmentation in ovarian carcinoma cells. J Control Release. 2007;124(1–2):6–10. doi: 10.1016/j.jconrel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson N, Ray A, Malugin A, Pike DB, Ghandehari H. HPMA copolymer-aminohexylgeldanamycin conjugates targeting cell surface expressed GRP78 in prostate cancer. Pharm Res. 2010;27(12):2683–2693. doi: 10.1007/s11095-010-0267-7. [DOI] [PubMed] [Google Scholar]
- 26.Wachters FM, Groen HJ, Maring JG, Gietema JA, Porro M, Dumez H, de Vries EG, van Oosterom AT. A phase I study with MAG-camptothecin intravenously administered weekly for 3 weeks in a 4-week cycle in adult patients with solid tumours. Br J Cancer. 2004;90(12):2261–2267. doi: 10.1038/sj.bjc.6601811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneda Y, Tsutsumi Y, Yoshioka Y, Kamada H, Yamamoto Y, Kodaira H, Tsunoda S, Okamoto T, Mukai Y, Shibata H, Nakagawa S, Mayumi T. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials. 2004;25(16):3259–3266. doi: 10.1016/j.biomaterials.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Kamada H, Tsutsumi Y, Yamamoto Y, Kihira T, Kaneda Y, Mu Y, Kodaira H, Tsunoda SI, Nakagawa S, Mayumi T. Antitumor activity of tumor necrosis factor-alpha conjugated with polyvinylpyrrolidone on solid tumors in mice. Cancer Res. 2000;60(22):6416–6420. [PubMed] [Google Scholar]
- 29.Yasukawa T, Kimura H, Tabata Y, Miyamoto H, Honda Y, Ikada Y, Ogura Y. Targeted delivery of anti-angiogenic agent TNP-470 using water-soluble polymer in the treatment of choroidal neovascularization. Invest Ophthalmol Vis Sci. 1999;40(11):2690–2696. [PubMed] [Google Scholar]
- 30.Chipman SD, Oldham FB, Pezzoni G, Singer JW. Biological and clinical characterization of paclitaxel poliglumex (PPX, CT-2103), a macromolecular polymer-drug conjugate. Int J Nanomedicine. 2006;1(4):375–383. doi: 10.2147/nano.2006.1.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljubimova JY, Fujita M, Ljubimov AV, Torchilin VP, Black KL, Holler E. Poly(malic acid) nanoconjugates containing various antibodies and oligonucleotides for multitargeting drug delivery. Nanomedicine (Lond) 2008;3(2):247–265. doi: 10.2217/17435889.3.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopecek J, Kopeckova P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev. 2010;62(2):122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasut G, Sergi M, Veronese FM. Anti-cancer PEG-enzymes: 30 years, old, but still a current approach. Adv Drug Deliv Rev. 2008;60(1):69–78. doi: 10.1016/j.addr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Veronese FM, Harris JM. Theme issue on "Peptide and Protein Pegylation". Adv Drug Deliv Rev. 2002;54(4):453–606. doi: 10.1016/s0169-409x(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 35.Graham ML. Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev. 2003;55(10):1293–1302. doi: 10.1016/s0169-409x(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 36.Levy Y, Hershfield MS, Fernandez-Mejia C, Polmar SH, Scudiery D, Berger M, Sorensen RU. Adenosine deaminase deficiency with late onset of recurrent infections: response to treatment with polyethylene glycol-modified adenosine deaminase. J Pediatr. 1988;113(2):312–317. doi: 10.1016/s0022-3476(88)80271-3. [DOI] [PubMed] [Google Scholar]
- 37.Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, Ehrlich GK, Pan W, Xu ZX, Modi MW, Farid A, Berthold W, Graves M. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001;12(2):195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- 38.Wang YS, Youngster S, Grace M, Bausch J, Bordens R, Wyss DF. Structural and biological characterization of pegylated recombinant interferon alpha-2b and its therapeutic implications. Adv Drug Deliv Rev. 2002;54(4):547–570. doi: 10.1016/s0169-409x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- 39.Roelfsema F, Biermasz NR, Pereira AM, Romijn J. Nanomedicines in the treatment of acromegaly: focus on pegvisomant. Int J Nanomedicine. 2006;1(4):385–398. doi: 10.2147/nano.2006.1.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Lee C, Sai P, Choe YH, Boro M, Pendri A, Guan S, Greenwald RB. 20-O-acylcamptothecin derivatives: evidence for lactone stabilization. J Org Chem. 2000;65(15):4601–4606. doi: 10.1021/jo000221n. [DOI] [PubMed] [Google Scholar]
- 42.Nwe K, Brechbiel MW. Growing applications of "click chemistry" for bioconjugation in contemporary biomedical research. Cancer Biother Radiopharm. 2009;24(3):289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dijk M, Rijkers DT, Liskamp RM, van Nostrum CF, Hennink WE. Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies. Bioconjug Chem. 2009;20(11):2001–2016. doi: 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]
- 44.Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem Commun (Camb) 2010;46(9):1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 45.Pasut G, Greco F, Mero A, Mendichi R, Fante C, Green RJ, Veronese FM. Polymer-drug conjugates for combination anticancer therapy: investigating the mechanism of action. J Med Chem. 2009;52(20):6499–6502. doi: 10.1021/jm900804m. [DOI] [PubMed] [Google Scholar]
- 46.Pasut G, Veronese FM. PEGylation for improving the effectiveness of therapeutic biomolecules. Drugs Today (Barc) 2009;45(9):687–695. doi: 10.1358/dot.2009.45.9.1416421. [DOI] [PubMed] [Google Scholar]
- 47.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5(1):113–128. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 48.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 49.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Rowinsky EK, Rizzo J, Ochoa L, Takimoto CH, Forouzesh B, Schwartz G, Hammond LA, Patnaik A, Kwiatek J, Goetz A, Denis L, McGuire J, Tolcher AW. A phase I and pharmacokinetic study of pegylated camptothecin as a 1-hour infusion every 3 weeks in patients with advanced solid malignancies. J Clin Oncol. 2003;21(1):148–157. doi: 10.1200/JCO.2003.03.143. [DOI] [PubMed] [Google Scholar]
- 51.Nojima Y, Suzuki Y, Yoshida K, Abe F, Shiga T, Takeuchi T, Sugiyama A, Shimizu H, Sato A. Lactoferrin conjugated with 40-kDa branched poly(ethylene glycol) has an improved circulating half-life. Pharm Res. 2009;26(9):2125–2132. doi: 10.1007/s11095-009-9925-z. [DOI] [PubMed] [Google Scholar]
- 52.Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J Am Chem Soc. 2009;131(13):4783–4787. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramon J, Saez V, Baez R, Aldana R, Hardy E. PEGylated interferon-alpha2b: a branched 40K polyethylene glycol derivative. Pharm Res. 2005;22(8):1374–1386. doi: 10.1007/s11095-005-5278-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhao H, Rubio B, Sapra P, Wu D, Reddy P, Sai P, Martinez A, Gao Y, Lozanguiez Y, Longley C, Greenberger LM, Horak ID. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19(4):849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 55.Lee JY, Bae KH, Kim JS, Nam YS, Park TG. Intracellular delivery of paclitaxel using oil-free, shell cross-linked HSA - Multi-armed PEG nanocapsules. Biomaterials. 2011;32(33):8635–8644. doi: 10.1016/j.biomaterials.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 56.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109(7):2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sapra P, Zhao H, Mehlig M, Malaby J, Kraft P, Longley C, Greenberger LM, Horak ID. Novel delivery of SN38 markedly inhibits tumor growth in xenografts. including a camptothecin-11-refractory model. Clin Cancer Res. 2008;14(6):1888–1896. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- 58.Lammers T. Improving the efficacy of combined modality anticancer therapy using HPMA copolymer-based nanomedicine formulations. Adv Drug Deliv Rev. 2010;62(2):203–230. doi: 10.1016/j.addr.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Duncan R. Development of HPMA copolymer-anticancer conjugates: clinical experience and lessons learnt. Adv Drug Deliv Rev. 2009;61(13):1131–1148. doi: 10.1016/j.addr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Subr V, Kopecek J, Pohl J, Baudys M, Kostka V. Cleavage of oligopeptide side-chains in N-2(hydroxpropyl)meth-acrylamide copolymers by mixtures of lysosomal enzymes. J Control Release. 1988;8(2):133–140. [Google Scholar]
- 61.Putnam D, Kopeček J. Biopolymers II. Vol. 122. Berlin: Springer; 1995. Polymer conjugates with anticancer activity; pp. 55–123. [Google Scholar]
- 62.Rejmanova P, Kopecek J, Duncan R, Lloyd JB. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers. Biomaterials. 1985;6(1):45–48. doi: 10.1016/0142-9612(85)90037-7. [DOI] [PubMed] [Google Scholar]
- 63.Putnam D, Kopecek J. Enantioselective release of 5-fluorouracil from N-(2-hydroxypropyl)methacrylamide-based copolymers via lysosomal enzymes. Bioconjug Chem. 1995;6(4):483–492. doi: 10.1021/bc00034a019. [DOI] [PubMed] [Google Scholar]
- 64.Kopecek J. Controlled biodegradability of polymers--a key to drug delivery systems. Biomaterials. 1984;5(1):19–25. doi: 10.1016/0142-9612(84)90062-0. [DOI] [PubMed] [Google Scholar]
- 65.Vicent MJ, Manzanaro S, de la Fuente JA, Duncan R. HPMA copolymer-1,5-diazaanthraquinone conjugates as novel anticancer therapeutics. J Drug Target. 2004;12(8):503–515. doi: 10.1080/10611860400011901. [DOI] [PubMed] [Google Scholar]
- 66.Kasuya Y, Lu ZR, Kopeckova P, Minko T, Tabibi SE, Kopecek J. Synthesis and characterization of HPMA copolymer-aminopropylgeldanamycin conjugates. J Control Release. 2001;74(1–3):203–211. doi: 10.1016/s0168-3659(01)00318-2. [DOI] [PubMed] [Google Scholar]
- 67.Hongrapipat J, Kopeckova P, Prakongpan S, Kopecek J. Enhanced antitumor activity of combinations of free and HPMA copolymer-bound drugs. Int J Pharm. 2008;351(1–2):259–270. doi: 10.1016/j.ijpharm.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ray A, Larson N, Pike DB, Gruner M, Naik S, Bauer H, Malugin A, Greish K, Ghandehari H. Comparison of active and passive targeting of docetaxel for prostate cancer therapy by HPMA copolymer-RGDfK conjugates. Mol Pharm. 2011;8(4):1090–1099. doi: 10.1021/mp100402n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Julyan PJ, Seymour LW, Ferry DR, Daryani S, Boivin CM, Doran J, David M, Anderson D, Christodoulou C, Young AM, Hesslewood S, Kerr DJ. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J Control Release. 1999;57(3):281–290. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 70.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5(1):83–94. [PubMed] [Google Scholar]
- 71.Duncan R, Seymour LW, O'Hare KB, Flanagan PA, Wedge S, Hume IC, Ulbrich K, Strohalm J, Subr V, Spreafico F, Grandi M, Ripamonti M, Farao M, Suarato A. Preclinical evaluation of polymer-bound doxorubicin. J Control Release. 1992;19(1–3):331–346. [Google Scholar]
- 72.Seymour LW, Ulbrich K, Steyger PS, Brereton M, Subr V, Strohalm J, Duncan R. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br J Cancer. 1994;70(4):636–641. doi: 10.1038/bjc.1994.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomson AH, Vasey PA, Murray LS, Cassidy J, Fraier D, Frigerio E, Twelves C. Population pharmacokinetics in phase I drug development: a phase I study of PK1 in patients with solid tumours. Br J Cancer. 1999;81(1):99–107. doi: 10.1038/sj.bjc.6690657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etrych T, Sirova M, Starovoytova L, Rihova B, Ulbrich K. HPMA copolymer conjugates of paclitaxel and docetaxel with pH-controlled drug release. Mol Pharm. 2010;7(4):1015–1026. doi: 10.1021/mp100119f. [DOI] [PubMed] [Google Scholar]
- 75.Caiolfa VR, Zamai M, Fiorino A, Frigerio E, Pellizzoni C, d'Argy R, Ghiglieri A, Castelli MG, Farao M, Pesenti E, Gigli M, Angelucci F, Suarato A. Polymer-bound camptothecin: initial biodistribution and antitumour activity studies. J Control Release. 2000;65(1–2):105–119. doi: 10.1016/s0168-3659(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 76.Gao SQ, Sun Y, Kopeckova P, Peterson CM, Kopecek J. Antitumor efficacy of colon-specific HPMA copolymer/9-aminocamptothecin conjugates in mice bearing human-colon carcinoma xenografts. Macromol Biosci. 2009;9(11):1135–1142. doi: 10.1002/mabi.200900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quan LD, Yuan F, Liu XM, Huang JG, Alnouti Y, Wang D. Pharmacokinetic and biodistribution studies of N-(2-hydroxypropyl)methacrylamide copolymer-dexamethasone conjugates in adjuvant-induced arthritis rat model. Mol Pharm. 2010;7(4):1041–1049. doi: 10.1021/mp100132h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, Wang D. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharm Res. 2008;25(12):2910–2919. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30(20):3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 81.Yang J, Luo K, Pan H, Kopeckova P, Kopecek J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. React Funct Polym. 2011;71(3):294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greish K, Ray A, Bauer H, Larson N, Malugin A, Pike D, Haider M, Ghandehari H. Anticancer and antiangiogenic activity of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer therapy. J Control Release. 2011;151(3):263–270. doi: 10.1016/j.jconrel.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borgman MP, Ray A, Kolhatkar RB, Sausville EA, Burger AM, Ghandehari H. Targetable HPMA copolymer-aminohexylgeldanamycin conjugates for prostate cancer therapy. Pharm Res. 2009;26(6):1407–1418. doi: 10.1007/s11095-009-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller K, Eldar-Boock A, Polyak D, Segal E, Benayoun L, Shaked Y, Satchi-Fainaro R. Antiangiogenic antitumor activity of HPMA copolymer-paclitaxel-alendronate conjugate on breast cancer bone metastasis mouse model. Mol Pharm. 2011;8(4):1052–1062. doi: 10.1021/mp200083n. [DOI] [PubMed] [Google Scholar]
- 85.Krakovicova H, Etrych T, Ulbrich K. HPMA-based polymer conjugates with drug combination. Eur J Pharm Sci. 2009;37(3–4):405–412. doi: 10.1016/j.ejps.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 86.Wu K, Yang J, Liu J, Kopecek J. Coiled-coil based drug-free macromolecular therapeutics: In vivo efficacy. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17(1):117–132. [Google Scholar]
- 88.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules. 1986;19(9):2466–2468. [Google Scholar]
- 89.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 90.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57(15):2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 91.Thiagarajan G, Ray A, Malugin A, Ghandehari H. PAMAM-camptothecin conjugate inhibits proliferation and induces nuclear fragmentation in colorectal carcinoma cells. Pharm Res. 2010;27(11):2307–2316. doi: 10.1007/s11095-010-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vijayalakshmi N, Ray A, Malugin A, Ghandehari H. Carboxyl-terminated PAMAM-SN38 conjugates: synthesis, characterization, and in vitro evaluation. Bioconjug Chem. 2010;21(10):1804–1810. doi: 10.1021/bc100094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Majoros IJ, Williams CR, Becker A, Baker JR., Jr Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):502–510. doi: 10.1002/wnan.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5–6):171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 95.Gajbhiye V, Palanirajan VK, Tekade RK, Jain NK. Dendrimers as therapeutic agents: a systematic review. J Pharm Pharmacol. 2009;61(8):989–1003. doi: 10.1211/jpp/61.08.0002. [DOI] [PubMed] [Google Scholar]
- 96.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257(1–2):111–124. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Y, Xu Z, Ma M, Xu T. Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci. 2008;97(1):123–143. doi: 10.1002/jps.21079. [DOI] [PubMed] [Google Scholar]
- 98.Goldberg DS, Ghandehari H, Swaan PW. Cellular entry of G3.5 poly (amido amine) dendrimers by clathrin- and dynamin-dependent endocytosis promotes tight junctional opening in intestinal epithelia. Pharm Res. 2010;27(8):1547–1557. doi: 10.1007/s11095-010-0153-3. [DOI] [PubMed] [Google Scholar]
- 99.Xu Q, Wang CH, Pack DW. Polymeric carriers for gene delivery: chitosan and poly(amidoamine) dendrimers. Curr Pharm Des. 2010;16(21):2350–2368. doi: 10.2174/138161210791920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48(19):5892–5899. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 101.Thomas TP, Majoros IJ, Kotlyar A, Kukowska-Latallo JF, Bielinska A, Myc A, Baker JR., Jr Targeting and inhibition of cell growth by an engineered dendritic nanodevice. J Med Chem. 2005;48(11):3729–3735. doi: 10.1021/jm040187v. [DOI] [PubMed] [Google Scholar]
- 102.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR., Jr Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 103.Filipowicz A, Wolowiec S. Solubility and in vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimers. Int J Pharm. 2011;408(1–2):152–156. doi: 10.1016/j.ijpharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 104.Wiwattanapatapee R, Carreno-Gomez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm Res. 2000;17(8):991–998. doi: 10.1023/a:1007587523543. [DOI] [PubMed] [Google Scholar]
- 105.Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menjoge AR, Rinderknecht AL, Navath RS, Faridnia M, Kim CJ, Romero R, Miller RK, Kannan RM. Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. J Control Release. 2011;150(3):326–338. doi: 10.1016/j.jconrel.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beg S, Samad A, Alam MI, Nazish I. Dendrimers as novel systems for delivery of neuropharmaceuticals to the brain. CNS Neurol Disord Drug Targets. 2011;10(5):576–588. doi: 10.2174/187152711796235023. [DOI] [PubMed] [Google Scholar]
- 108.He H, Li Y, Jia XR, Du J, Ying X, Lu WL, Lou JN, Wei Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Ke W, Shao K, Huang R, Han L, Liu Y, Li J, Kuang Y, Ye L, Lou J, Jiang C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials. 2009;30(36):6976–6985. doi: 10.1016/j.biomaterials.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 110.Etrych T, Kovar L, Strohalm J, Chytil P, Rihova B, Ulbrich K. Biodegradable star HPMA polymer-drug conjugates: Biodegradability, distribution and anti-tumor efficacy. J Control Release. 2011;154(3):241–248. doi: 10.1016/j.jconrel.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Cao W, Zhou J, Wang Y, Zhu L. Synthesis and in vitro cancer cell targeting of folate-functionalized biodegradable amphiphilic dendrimer-like star polymers. Biomacromolecules. 2010;11(12):3680–3687. doi: 10.1021/bm101154r. [DOI] [PubMed] [Google Scholar]
- 112.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Frechet JM. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconjug Chem. 2010;21(4):764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sebestik J, Niederhafner P, Jezek J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids. 2011;40(2):301–370. doi: 10.1007/s00726-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 114.Li Y, Cheng Y, Xu T. Design, synthesis and potent pharmaceutical applications of glycodendrimers: a mini review. Curr Drug Discov Technol. 2007;4(4):246–254. doi: 10.2174/157016307783220503. [DOI] [PubMed] [Google Scholar]
- 115.Frechet JM. Dendrimers and supramolecular chemistry. Proc Natl Acad Sci U S A. 2002;99(8):4782–4787. doi: 10.1073/pnas.082013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin Y, Ren X, Wang W, Ke L, Ning E, Du L, Bradshaw J. A 5-fluorouracil-loaded pH-responsive dendrimer nanocarrier for tumor targeting. Int J Pharm. 2011;420(2):378–384. doi: 10.1016/j.ijpharm.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 117.Raghupathi KR, Azagarsamy MA, Thayumanavan S. Guest-release control in enzyme-sensitive, amphiphilic-dendrimer-based nanoparticles through photochemical crosslinking. Chemistry. 2011;17(42):11752–11760. doi: 10.1002/chem.201101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103(45):16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medina SH, El-Sayed ME. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 2009;109(7):3141–3157. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 120.Longmire M, Choyke PL, Kobayashi H. Dendrimer-based contrast agents for molecular imaging. Curr Top Med Chem. 2008;8(14):1180–1186. doi: 10.2174/156802608785849021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jelinkova M, Strohalm J, Etrych T, Ulbrich K, Rihova B. Starlike vs. classic macromolecular prodrugs: two different antibody-targeted HPMA copolymers of doxorubicin studied in vitro and in vivo as potential anticancer drugs. Pharm Res. 2003;20(10):1558–1564. doi: 10.1023/a:1026170830782. [DOI] [PubMed] [Google Scholar]
- 122.Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, Minko T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug Chem. 2006;17(6):1464–1472. doi: 10.1021/bc060240p. [DOI] [PubMed] [Google Scholar]
- 123.Cheng Y, Zhao L, Li Y, Xu T. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 124.Goldberg DS, Vijayalakshmi N, Swaan PW, Ghandehari H. G3.5 PAMAM dendrimers enhance transepithelial transport of SN38 while minimizing gastrointestinal toxicity. J Control Release. 2011;150(3):318–325. doi: 10.1016/j.jconrel.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mukherjee SP, Lyng FM, Garcia A, Davoren M, Byrne HJ. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol Appl Pharmacol. 2010;248(3):259–268. doi: 10.1016/j.taap.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 126.Greish K, Thiagarajan G, Herd H, Price R, Bauer H, Hubbard D, Burckle A, Sadekar S, Yu T, Anwar A, Ray A, Ghandehari H. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.604442. [DOI] [PubMed] [Google Scholar]
- 127.Ziemba B, Janaszewska A, Ciepluch K, Krotewicz M, Fogel WA, Appelhans D, Voit B, Bryszewska M, Klajnert B. In vivo toxicity of poly(propyleneimine) dendrimers. J Biomed Mater Res A. 2011;99(2):261–268. doi: 10.1002/jbm.a.33196. [DOI] [PubMed] [Google Scholar]
- 128.Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 129.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjug Chem. 1992;3(4):295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 130.Kabanov AV, Vinogradov SV, Suzdaltseva YG, Alakhov V. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjug Chem. 1995;6(6):639–643. doi: 10.1021/bc00036a001. [DOI] [PubMed] [Google Scholar]
- 131.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 132.Hu Y, Jiang Z, Chen R, Wu W, Jiang X. Degradation and degradation-induced reassembly of PVP-PCL micelles. Biomacromolecules. 2010;11(2):481–488. doi: 10.1021/bm901211r. [DOI] [PubMed] [Google Scholar]
- 133.Orienti I, Zuccari G, Fini A, Rabasco AM, Carosio R, Raffaghello L, Montaldo PG. Modified doxorubicin for improved encapsulation in PVA polymeric micelles. Drug Deliv. 2005;12(1):15–20. doi: 10.1080/10717540590889574. [DOI] [PubMed] [Google Scholar]
- 134.Mishra D, Kang HC, Bae YH. Reconstitutable charged polymeric (PLGA)(2)-b-PEI micelles for gene therapeutics delivery. Biomaterials. 2011;32(15):3845–3854. doi: 10.1016/j.biomaterials.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234(2):123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chandran T, Katragadda U, Teng Q, Tan C. Design and evaluation of micellar nanocarriers for 17-allyamino-17-demethoxygeldanamycin (17-AAG) Int J Pharm. 2010;392(1–2):170–177. doi: 10.1016/j.ijpharm.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 137.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 138.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2–3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 139.Mikhail AS, Allen C. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles containing chemically conjugated and physically entrapped docetaxel: synthesis, characterization, and the influence of the drug on micelle morphology. Biomacromolecules. 2010;11(5):1273–1280. doi: 10.1021/bm100073s. [DOI] [PubMed] [Google Scholar]
- 140.Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54(2):203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 141.Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(L-amino acid) micelles for drug delivery. Adv Drug Deliv Rev. 2002;54(2):169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 142.Talelli M, Rijcken CJ, van Nostrum CF, Storm G, Hennink WE. Micelles based on HPMA copolymers. Adv Drug Deliv Rev. 2010;62(2):231–239. doi: 10.1016/j.addr.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 143.Chytil P, Etrych T, Konak C, Sirova M, Mrkvan T, Boucek J, Rihova B, Ulbrich K. New HPMA copolymer-based drug carriers with covalently bound hydrophobic substituents for solid tumour targeting. J Control Release. 2008;127(2):121–130. doi: 10.1016/j.jconrel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 144.Jia Z, Wong L, Davis TP, Bulmus V. One-pot conversion of RAFT-generated multifunctional block copolymers of HPMA to doxorubicin conjugated acid- and reductant-sensitive crosslinked micelles. Biomacromolecules. 2008;9(11):3106–3113. doi: 10.1021/bm800657e. [DOI] [PubMed] [Google Scholar]
- 145.Chandran SS, Nan A, Rosen DM, Ghandehari H, Denmeade SR. A prostate-specific antigen activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol Cancer Ther. 2007;6(11):2928–2937. doi: 10.1158/1535-7163.MCT-07-0392. [DOI] [PubMed] [Google Scholar]
- 146.Lele BS, Leroux JC. Synthesis and micellar characterization of novel amphiphilic A–B–A triblock copolymers of N-(2-hydroxypropyl)methacrylamide or N-vinyl-2-pyrrolidone with poly(ε-caprolactone) Macromolecules. 2002;35(17):6714–6723. [Google Scholar]
- 147.Neradovic D, van Steenbergen MJ, Vansteelant L, Meijer YJ, van Nostrum CF, Hennink WE. Degradation mechanism and kinetics of thermosensitive polyacrylamides containing lactic acid side chains. Macromolecules. 2003;36(20):7491–7498. [Google Scholar]
- 148.Soga O, van Nostrum CF, Fens M, Rijcken CJ, Schiffelers RM, Storm G, Hennink WE. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J Control Release. 2005;103(2):341–353. doi: 10.1016/j.jconrel.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 149.Krimmer SG, Pan H, Liu J, Yang J, Kopecek J. Synthesis and characterization of poly(epsilon-caprolactone)-block-poly[N-(2-hydroxypropyl)methacrylamide] micelles for drug delivery. Macromol Biosci. 2011;11(8):1041–1051. doi: 10.1002/mabi.201100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun H, Meng F, Dias AA, Hendriks M, Feijen J, Zhong Z. alpha-Amino acid containing degradable polymers as functional biomaterials: rational design, synthetic pathway, and biomedical applications. Biomacromolecules. 2011;12(6):1937–1955. doi: 10.1021/bm200043u. [DOI] [PubMed] [Google Scholar]
- 152.De Souza R, Zahedi P, Allen CJ, Piquette-Miller M. Polymeric drug delivery systems for localized cancer chemotherapy. Drug Deliv. 2010;17(6):365–375. doi: 10.3109/10717541003762854. [DOI] [PubMed] [Google Scholar]
- 153.Couffin-Hoarau AC, Aubertin AM, Boustta M, Schmidt S, Fehrentz JA, Martinez J, Vert M. Peptide-poly(L-lysine citramide) conjugates and their in vitro anti-HIV behavior. Biomacromolecules. 2009;10(4):865–876. doi: 10.1021/bm801376v. [DOI] [PubMed] [Google Scholar]
- 154.Yang D, Van S, Liu J, Wang J, Jiang X, Wang Y, Yu L. Physicochemical properties and biocompatibility of a polymer-paclitaxel conjugate for cancer treatment. Int J Nanomedicine. 2011;6:2557–2566. doi: 10.2147/IJN.S25044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Metselaar JM, Bruin P, de Boer LW, de Vringer T, Snel C, Oussoren C, Wauben MH, Crommelin DJ, Storm G, Hennink WE. A novel family of L-amino acid-based biodegradable polymer-lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjug Chem. 2003;14(6):1156–1164. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 156.Baldwin AD, Kiick KL. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94(1):128–140. doi: 10.1002/bip.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kemp JD, Cardillo T, Stewart BC, Kehrberg E, Weiner G, Hedlund B, Naumann PW. Inhibition of lymphoma growth in vivo by combined treatment with hydroxyethyl starch deferoxamine conjugate and IgG monoclonal antibodies against the transferrin receptor. Cancer Res. 1995;55(17):3817–3824. [PubMed] [Google Scholar]
- 158.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99(6):2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beer TM, Ryan C, Alumkal J, Ryan CW, Sun J, Eilers KM. A phase II study of paclitaxel poliglumex in combination with transdermal estradiol for the treatment of metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Anticancer Drugs. 2010;21(4):433–438. doi: 10.1097/CAD.0b013e3283355211. [DOI] [PubMed] [Google Scholar]
- 160.Mita M, Mita A, Sarantopoulos J, Takimoto CH, Rowinsky EK, Romero O, Angiuli P, Allievi C, Eisenfeld A, Verschraegen CF. Phase I study of paclitaxel poliglumex administered weekly for patients with advanced solid malignancies. Cancer Chemother Pharmacol. 2009;64(2):287–295. doi: 10.1007/s00280-008-0869-5. [DOI] [PubMed] [Google Scholar]
- 161.Sabbatini P, Sill MW, O'Malley D, Adler L, Secord AA. A phase II trial of paclitaxel poliglumex in recurrent or persistent ovarian or primary peritoneal cancer (EOC): a Gynecologic Oncology Group Study. Gynecol Oncol. 2008;111(3):455–460. doi: 10.1016/j.ygyno.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 162.O'Brien ME, Socinski MA, Popovich AY, Bondarenko IN, Tomova A, Bilynsky BT, Hotko YS, Ganul VL, Kostinsky IY, Eisenfeld AJ, Sandalic L, Oldham FB, Bandstra B, Sandler AB, Singer JW. Randomized phase III trial comparing single-agent paclitaxel Poliglumex (CT-2103, PPX) with single-agent gemcitabine or vinorelbine for the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(7):728–734. doi: 10.1097/JTO.0b013e31817c6b68. [DOI] [PubMed] [Google Scholar]
- 163.Man M, Rugo H. Paclitaxel poliglumex. Cell Therapeutics/Chugai Pharmaceutical. IDrugs. 2005;8(9):739–754. [PubMed] [Google Scholar]
- 164.Pechar M, Ulbrich K, Subr V, Seymour LW, Schacht EH. Poly(ethylene glycol) multiblock copolymer as a carrier of anti-cancer drug doxorubicin. Bioconjug Chem. 2000;11(2):131–139. doi: 10.1021/bc990092l. [DOI] [PubMed] [Google Scholar]
- 165.Etrych T, Chytil P, Mrkvan T, Sirova M, Rihova B, Ulbrich K. Conjugates of doxorubicin with graft HPMA copolymers for passive tumor targeting. J Control Release. 2008;132(3):184–192. doi: 10.1016/j.jconrel.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 166.Etrych T, Strohalm J, Chytil P, Cernoch P, Starovoytova L, Pechar M, Ulbrich K. Biodegradable star HPMA polymer conjugates of doxorubicin for passive tumor targeting. Eur J Pharm Sci. 2011;42(5):527–539. doi: 10.1016/j.ejps.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 167.Luo K, Yang J, Kopeckova P, Kopecek J. Biodegradable multiblock poly[N-(2-hydroxypropyl)methacrylamide] via reversible addition-fragmentation chain transfer polymerization and click chemistry. Macromolecules. 2011;44(8):2481–2488. doi: 10.1021/ma102574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71(3):431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 170.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 171.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56(6):1194–1198. [PubMed] [Google Scholar]
- 172.Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4(2) doi: 10.1088/1748-6041/4/2/022001. 022001. [DOI] [PubMed] [Google Scholar]
- 173.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 174.Howard MD, Ponta A, Eckman A, Jay M, Bae Y. Polymer micelles with hydrazone-ester dual linkers for tunable release of dexamethasone. Pharm Res. 2011;28(10):2435–2446. doi: 10.1007/s11095-011-0470-1. [DOI] [PubMed] [Google Scholar]
- 175.Zhou L, Cheng R, Tao H, Ma S, Guo W, Meng F, Liu H, Liu Z, Zhong Z. Endosomal pH-activatable poly(ethylene oxide)-graft-doxorubicin prodrugs: synthesis, drug release, and biodistribution in tumor-bearing mice. Biomacromolecules. 2011;12(5):1460–1467. doi: 10.1021/bm101340u. [DOI] [PubMed] [Google Scholar]
- 176.Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. HPMA copolymers with pH-controlled release of doxorubicin: in vitro cytotoxicity and in vivo antitumor activity. J Control Release. 2003;87(1–3):33–47. doi: 10.1016/s0168-3659(02)00348-6. [DOI] [PubMed] [Google Scholar]
- 177.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42(38):4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 178.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem. 2005;16(1):122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 179.Chilkoti A, Dreher MR, Meyer DE, Raucher D. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 2002;54(5):613–630. doi: 10.1016/s0169-409x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 180.Yin X, Hoffman AS, Stayton PS. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;7(5):1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 181.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17(11):1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 182.Trabbic-Carlson K, Liu L, Kim B, Chilkoti A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004;13(12):3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McDaniel JR, Callahan DJ, Chilkoti A. Drug delivery to solid tumors by elastin-like polypeptides. Adv Drug Deliv Rev. 2010;62(15):1456–1467. doi: 10.1016/j.addr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tatham AS, Shewry PR. Elastomeric proteins: biological roles, structures and mechanisms. Trends Biochem Sci. 2000;25(11):567–571. doi: 10.1016/s0968-0004(00)01670-4. [DOI] [PubMed] [Google Scholar]
- 185.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67(9):4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 186.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130(2):687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mackay JA, Chilkoti A. Temperature sensitive peptides: engineering hyperthermia-directed therapeutics. Int J Hyperthermia. 2008;24(6):483–495. doi: 10.1080/02656730802149570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yu B, Tai HC, Xue W, Lee LJ, Lee RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol. 2010;27(7):286–298. doi: 10.3109/09687688.2010.521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 191.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;(197):3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 192.Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–172. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- 193.Wang T, Petrenko VA, Torchilin VP. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: enhanced binding to target cancer cells and increased cytotoxicity. Mol Pharm. 2010;7(4):1007–1014. doi: 10.1021/mp1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR. Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J Control Release. 2005;102(1):191–201. doi: 10.1016/j.jconrel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 195.Borgman MP, Aras O, Geyser-Stoops S, Sausville EA, Ghandehari H. Biodistribution of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer drug delivery. Mol Pharm. 2009;6(6):1836–1847. doi: 10.1021/mp900134c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pike DB, Ghandehari H. HPMA copolymer-cyclic RGD conjugates for tumor targeting. Adv Drug Deliv Rev. 2010;62(2):167–183. doi: 10.1016/j.addr.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 197.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huis In 't Veld R, Storm G, Hennink WE, Kiessling F, Lammers T. Macromolecular nanotheranostics for multimodal anticancer therapy. Nanoscale. 2011;3(10):4022–4034. doi: 10.1039/c1nr10733j. [DOI] [PubMed] [Google Scholar]
- 199.Stern L, Perry R, Ofek P, Many A, Shabat D, Satchi-Fainaro R. A novel antitumor prodrug platform designed to be cleaved by the endoprotease legumain. Bioconjug Chem. 2009;20(3):500–510. doi: 10.1021/bc800448u. [DOI] [PubMed] [Google Scholar]
- 200.Deacon SP, Apostolovic B, Carbajo RJ, Schott AK, Beck K, Vicent MJ, Pineda-Lucena A, Klok HA, Duncan R. Polymer coiled-coil conjugates: potential for development as a new class of therapeutic "molecular switch". Biomacromolecules. 2011;12(1):19–27. doi: 10.1021/bm100843e. [DOI] [PubMed] [Google Scholar]
- 201.Apostolovic B, Deacon SP, Duncan R, Klok HA. Hybrid polymer therapeutics incorporating bioresponsive, coiled coil peptide linkers. Biomacromolecules. 2010;11(5):1187–1195. doi: 10.1021/bm901313c. [DOI] [PubMed] [Google Scholar]
- 202.Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite DW. Self-immolative linkers in polymeric delivery systems. Polym Chem. 2011;2(4):773–790. [Google Scholar]
- 203.Duncan R, Coatsworth JK, Burtles S. Preclinical toxicology of a novel polymeric antitumour agent: HPMA copolymer-doxorubicin (PK1) Hum Exp Toxicol. 1998;17(2):93–104. doi: 10.1177/096032719801700204. [DOI] [PubMed] [Google Scholar]