Abstract
Whereas thousands of new neurons are generated daily during adult life, only a fraction of them survive and become part of neural circuits; the rest die, and their corpses are presumably cleared by resident phagocytes. How the dying neurons are removed and how such clearance influences neurogenesis are not well understood. Here, we identify an unexpected phagocytic role for the doublecortin (DCX)-positive neuronal progenitor cells during adult neurogenesis. Our in vivo and ex vivo studies demonstrate that DCX+ cells comprise a significant phagocytic population within the neurogenic zones. Intracellular engulfment protein ELMO1, which promotes Rac activation downstream of phagocytic receptors, was required for phagocytosis by DCX+ cells. Disruption of engulfment in vivo genetically (in Elmo1-null mice) or pharmacologically (in wild-type mice) led to reduced uptake by DCX+ cells, accumulation of apoptotic nuclei in the neurogenic niches and impaired neurogenesis. Collectively, these findings indicate a paradigm wherein DCX+ neuronal precursors also serve as phagocytes, and that their phagocytic activity critically contributes to neurogenesis in the adult brain.
The subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus represent two active neurogenic niches in the adult brain1–6. These neurogenic areas contain neural progenitor cells (NPCs) capable of generating new neurons and glial cells (astrocytes and oligodendrocytes). Thousands of new neurons are generated daily7–11, although only a fraction of the doublecortin-positive (DCX+) neuronal progenitors survive12–16. The process by which the dying DCX+ cells are removed, and how such removal impacts neurogenesis, are not known. Excess cells in many tissues die by apoptosis; the exposure of phosphatidylserine (PtdSer) on dying cells is a key ‘eat-me’ signal facilitating the recognition and phagocytic removal by resident phagocytes17–19. PtdSer on apoptotic cells can either be recognized directly (via receptors such as BAI1, TIM-4 and Stabilin-2), or indirectly through soluble bridging molecules and in turn recognized by other phagocytic receptors (such as Mer or integrin αvβ3; refs 17–19).
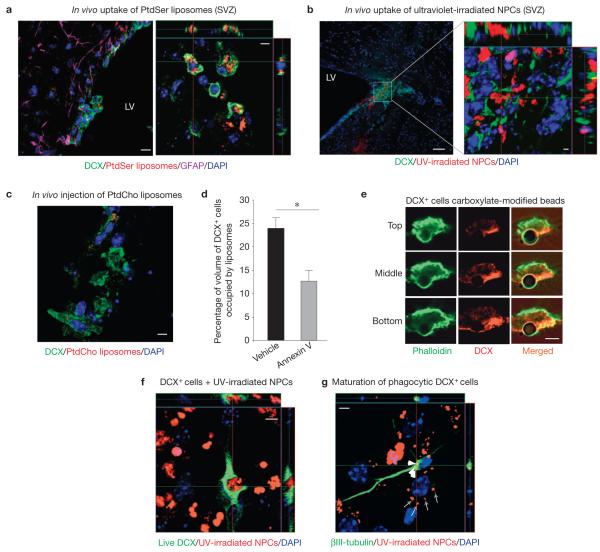
To better define the phagocytic process in the neurogenic niches, we injected fluorescently labelled PtdSer-containing liposomes (Supplementary Fig. S1a) into the SVZ of 8–12-week-old wild-type mice and examined the presence of the phagocytosed liposomes inside cells within the niche (Supplementary Fig. S1b). We initially focused on glial fibrillary acidic protein (GFAP)-expressing cells (niche astrocytes and astrocyte stem cells20–23), because glial cells in mammals24,25 and other organisms26,27 can play major phagocytic roles during development. Surprisingly, many of the ingested liposomes were found within DCX+ neuronal precursor cells in the SVZ (Fig. 1a) and the SGZ (Supplementary Fig. S1c). Among liposome-engulfing cells in the SVZ, 56±6% were DCX+ cells, whereas 44±6% were DCX− cells within the 200 μm range of the injection site. Mice injected with control phosphatidylcholine (PtdCho)-containing liposomes showed poor ingestion by DCX+ cells (Fig. 1c). Injection of ultraviolet-irradiated (apoptotic) fluorescently labelled NPCs also showed that DCX cells are phagocytic, and that they can ingest apoptotic NPCs (Fig. 1b). However, owing to relatively poor diffusion of NPCs when compared with liposomes, only 12±2% of DCX+ cells engulfed ultraviolet-irradiated progenitors within the 200 μm range of the injection site.
Figure 1.
DCX-expressing neuronal progenitor cells exhibit phagocytic activity in vivo and ex vivo. (a) Wild-type mice (LV, lateral ventricle) intracranially injected with fluorescent PtdSer liposomes examined immunohistochemically in the SVZ for DCX+ and GFAP+ cells with phagocytosed liposomes. DAPI, 4,6-diamidino-2-phenylindole. Representative confocal micrographs are shown (n > 10 fields analysed per experiment; five independent experiments carried out). Scale bar, 10 μm (5 μm in inset). (b) Wild-type mice intracranially injected with fluorescently labelled ultraviolet (UV)-irradiated NPCs examined immunohistochemically in the SVZ for DCX+ cells with phagocytosed cells. A representative micrograph is shown in orthogonal projections of confocal z stacks (n > 12 fields analysed per experiment; three independent experiments carried out). Scale bar, 50 μm (2 μm in inset). (c) Wild-type mice intracranially injected with fluorescent PtdCho liposomes exhibit poor engulfment by DCX+ cells in the SVZ (n > 10 fields analysed per experiment; three independent experiments carried out). Scale bar, 5 μm. (d) Wild-type mice injected intravenously with annexin V before and immediately after intracranial injection with fluorescent PtdSer liposomes. Brain tissue examined immunohistochemically for DCX+ and GFAP+ cells containing liposomes in the SVZ. The bar graph represents a quantification of the volume of DCX+ cells containing PtdSer liposomes (mean ± s.e.m.). Student’s t -test statistical analysis was carried out for n = 4 mice in each group with at least five slices analysed for each mouse (°P < 0.05). (e) Representative confocal microscopy images (with n > 30 cells analysed), demonstrating the formation of the phagocytic cup (phalloidin staining) in DCX+ cells around the carboxylate-modified 3 μm beads (simplified apoptotic targets), are shown in top, middle and bottom planes. Scale bar, 3 μm. (f) Representative confocal microscopy images of phagocytosis by a newly differentiated neuron showing orthogonal projections of confocal z stacks (n > 20 fields analysed per experiment; seven independent experiments carried out). Scale bar, 5 μm. (g) Newly differentiated NPC cultures were fed with ultraviolet-irradiated NPCs ex vivo and washed after 6 h. Cells were analysed after a further seven days of differentiation. Representative confocal microscopy orthogonal views of maturated neurons are shown. White arrowheads point to remnants of phagocytosed cells in neurons and grey arrows those in other cells (n > 20 fields analysed per experiment; four independent experiments carried out). Scale bar, 5 μm.
It has been previously shown by several groups that injection of annexin V (a protein that binds PtdSer on apoptotic cells and inhibits engulfment) intravenously can reach the central nervous system (CNS) (refs 28,29) and other organs30 (Supplementary Fig. S2a). Intravenous injection of annexin V before intracranial injection of liposomes blocked the uptake of PtdSer liposomes by DCX+ cells in vivo (Fig. 1d). These data indicate that DCX+ cells are phagocytic cells within the neurogenic niches and that they use a PtdSer-dependent recognition for uptake.
Although microglial cells are the resident myeloid cells of the CNS with known phagocytic capacity31–33, activated microglia are hardly detectable in non-inflamed CNS, with only very few microglia phagocytosing the injected NPCs (Supplementary Fig. S2b).
To better define the phagocytic capacity of DCX+ cells, differentiated neurospheres from SVZ cells were incubated with simplified targets that mimic certain properties of apoptotic cells (negatively charged carboxylate-modified 3 μm beads, whose uptake is blocked by annexin V; refs 34,35). The DCX+ cells engulfed these targets, showing the phagocytic cup and the actin ring around the target (Fig. 1e). DCX+ neuronal precursors also efficiently engulfed apoptotic NPCs in vitro (Fig. 1f).
To determine whether early neuronal progenitors (DCX+) engulfing the dead neural precursor cells could differentiate into neurons, fluorescently labelled irradiated NPCs were added to newly differentiated dissociated neurospheres (24 h in culture) for 6 h. After washing and further 7 days, the cultures were examined for expression of a later neuronal differentiation marker ( III-tubulin). The remnants of the engulfed fluorescently labelled particles were evident in III-tubulin+ cells, indicating that DCX+ precursors that have engulfed other NPCs can differentiate into III-tubulin+ neurons (Fig. 1g). Incubation of differentiating NPC cultures with irradiated progenitors had no detectable effect on neuronal differentiation under these conditions (19±4% versus 17±4%; neuronal differentiation ±s.e.m. in control media or after treatment with irradiated progenitor cells, respectively). However, addition of a high burden of the dead progenitors resulted in accelerated death of the NPC cultures, indicating that too many dead cells create an unfavourable environment.
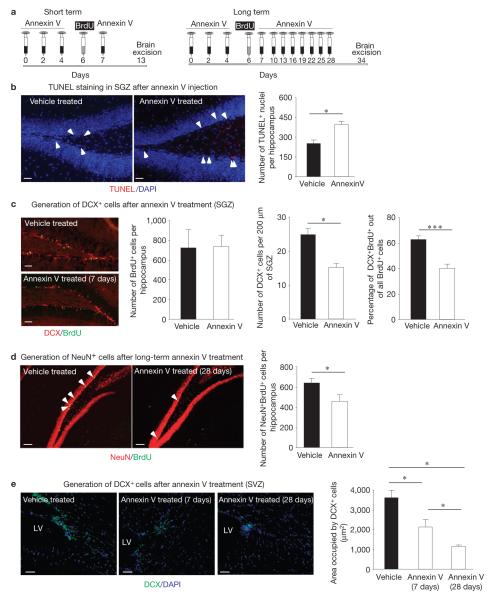
To address the physiological role for engulfment by DCX+ cells within neurogenic areas, we tested the effect of inhibiting phagocytosis on adult neurogenesis. After intravenous injection of annexin V to inhibit apoptotic cell clearance, we assessed neurogenesis (schematic representation in Fig. 2a). First, compared with the saline, annexin V treatment led to substantial accumulation of TdT-mediated dUTP nick end labelling (TUNEL)-positive nuclei in the SGZ and SVZ (Fig. 2b and Supplementary Fig. S3). Second, we observed a striking reduction in neuronal differentiation (bromodeoxyuridine (BrdU)+DCX+ cells) and survival (BrdU+NeuN+ cells) in the SGZ (Fig. 2c,d) and in neuronal differentiation (DCX+ cells) in the SVZ (Fig. 2e). Importantly, the overall number of proliferating cells (BrdU+) in the SGZ did not change on annexin V treatment. This indicates that, whereas the numbers of neuronal progenitors (DCX+ cells) are reduced, there might be an increase in the numbers of non-differentiated NPCs. These data imply that death and clearance of neurons in the neurogenic niches is an ongoing process, and that interference with phagocytic clearance significantly impacts neurogenesis.
Figure 2.
Inhibition of phagocytosis in the neurogenic niche in vivo impairs adult neurogenesis. (a) Schematic representation of short-term (7 days) and long-term (28 days) annexin V treatment to block apoptotic cell clearance, coupled with BrdU injection to monitor proliferating cells within the neurogenic zones. (b) Representative fluorescent microscopy images of accumulated apoptotic nuclei through TUNEL staining (indicated by white arrowheads) in the SGZ after short-term annexin V treatment. The bar graph represents a quantification of apoptotic (TUNEL-positive) nuclei (mean ± s.e.m.). Student’s t -test statistical analysis was carried out for n = 4 mice in each group, with at least five slices analysed for each mouse (* P < 0.05). Scale bars, 50 μm. (c) Representative fluorescent microscopy images of the SGZ from control and short-term annexin V-treated mice examined for DCX expression and BrdU incorporation. The bar graphs (means ± s.e.m.) represent quantifications of BrdU+ cells (per hippocampus), DCX+ cells (per 200 μm of subgranular layer) and the fraction of BrdU+ that were also DCX+ (indicative of neuronal differentiation; per hippocampus). Student’s t -test statistical analysis was carried out for n = 4 mice in each group with at least ten slices analysed for each mouse (* P < 0.05; *** P < 0.001). Scale bars, 50 μm. (d) Representative fluorescent microscopy images of control and long-term annexin V-treated brain slices examined for BrdU and NeuN immunoreactivity in the SGZ (BrdU+NeuN+ cells are indicated by white arrowheads). The bar graph represents quantification of BrdU+NeuN+ cells per hippocampus of vehicle- and annexin V-treated mice (mean ± s.e.m.). Student’s t -test statistical analysis was carried out for n = 7 mice in each group with at least ten slices analysed for each mouse (* P < 0.05). Scale bars, 50 μm. (e) Representative confocal microscopy images of the SVZ immunolabelled for DCX from control, short-term and long-term annexin V-treated mice (LV, lateral ventricle). The bar graph represents quantification of the areas occupied by DCX+ cells per field (mean ± s.e.m.). Student’s t -test statistical analysis was carried out between the groups for n = 5 mice in each group with at least ten slices analysed for each mouse (* P < 0.05). Scale bars, 50 μm.
We next addressed the molecular mechanism(s) contributing to phagocytosis by DCX+ cells. ELMO1 is a cytoplasmic evolutionarily conserved protein important for the clearance of dying cells35. ELMO1 binds to the cytoplasmic tail of the membrane receptor brain angiogenesis inhibitor 1 (Bai1) and activates the small GTPase Rac1, and thereby promotes cytoskeletal rearrangements to engulf apoptotic cells34. Loss of ELMO1, or mutations in ELMO1, can severely impair engulfment both in vitro35 and in vivo35,36.
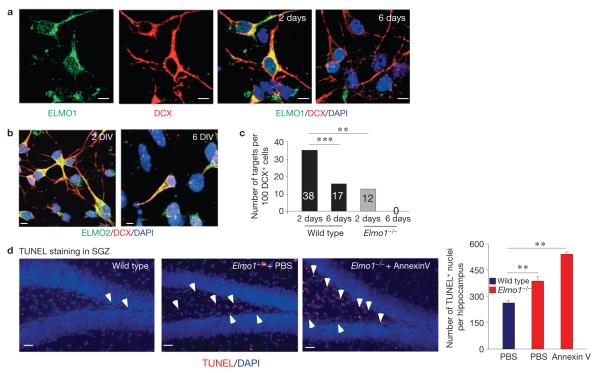
To address a possible role for ELMO1 in phagocytosis by DCX+ neuronal progenitor cells, we first examined ELMO1 expression in DCX+ cells. Primary hippocampal neurons were cultured for 2 or 6 days in vitro and examined for ELMO1 expression. Whereas high levels of ELMO1 were detected in neurons after 2 days, ELMO1 levels dropped significantly after 6 days in culture (Fig. 3a). In contrast, the level of ELMO2 was not altered under these conditions (Fig. 3b). When the DCX+ cells were fed with apoptotic targets, the phagocytic capacity of DCX+ cells after 2 days in culture was significantly higher than after 6 days, correlating with ELMO1 expression (Fig. 3c). Recently, ELMO1 null mice have been described36. DCX+ cells from mice lacking ELMO1 showed a significant reduction in phagocytic activity (2 day culture), compared with wild-type DCX+ cells. After 6 days in culture, we could not detect any apoptotic targets inside Elmo1−/− DCX+ cells (Fig. 3c). This indicated that ELMO1 is a key intracellular molecule regulating phagocytosis by DCX+ cells. Notably, the differentiated GFAP+ astrocytes from Elmo1−/− mice did not show a defect in phagocytosis when compared with astrocytes from wild-type mice (Supplementary Fig. S4).
Figure 3.
ELMO1-dependent phagocytosis of DCX+ cells in vitro. (a) Representative images of primary hippocampal neuronal cultures grown for 2 or 6 days in vitro immunolabelled for DCX and ELMO1 (for n > 30 fields). Scale bars, 5 μm. (b) Primary hippocampal neuronal cultures grown for 2 or 6 days in vitro (DIV) were immunolabelled for DCX and ELMO2. Representative confocal microscopy images (for n > 30 fields) are shown. The expression level of ELMO2 was not altered under these conditions in the DCX+ cells after 2 or 6 days in culture. Scale bars, 5 μm. (c) Cultures of neurons from a were fed with targets (carboxylate-modified beads) and assessed for engulfment. The bars represent quantifications of phagocytosed targets by primary DCX+ cells from wild-type and Elmo1−/− mice after 2 or 6 days in culture. A one-way analysis of variance with a Newman–Keuls multiple comparison test was carried out for n = 100 DCX+ cells per group (*** P < 0.01; *** P < 0.001). (d) Accumulation of apoptotic nuclei in PBS- or long-term annexin V-treated Elmo1−/− mice, compared with wild-type littermates, was examined by TUNEL immunolabelling (indicated by white arrowheads) of the SGZ. Representative images from wild-type and Elmo1−/− mice are shown. The bar graph represents a quantification of TUNEL-positive nuclei (mean ± s.e.m.). Student’s t -test was carried out for n 5 mice per group, with at least ten slices analysed for each mouse (** = P < 0.01). Scale bars, 50 μm.
When the SGZ and SVZ within the brains of Elmo1−/− mice were examined, there was a significant accumulation of TUNEL-positive nuclei (Fig. 3d and Supplementary Fig. S5a). This indicated an important role for ELMO1 in clearing apoptotic cells in the neurogenic niches. When the Elmo1−/− mice were treated long term with annexin V, there was a further increase in the accumulation of apoptotic debris (Fig. 3d), indicating that ELMO1-independent mechanisms for phagocytic clearance probably also exist.
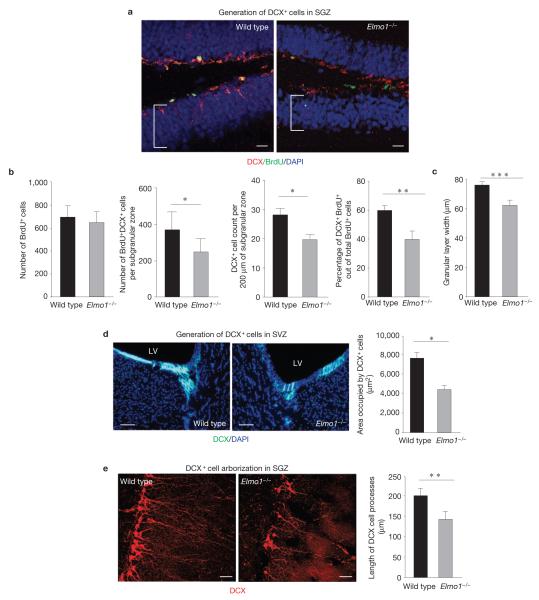
We next examined adult neurogenesis in the Elmo1−/− mice. Wild-type and Elmo1−/− mice were injected with BrdU and their brains were examined for BrdU-containing DCX+ cells after 7 days. Neuronal differentiation was substantially impaired in Elmo1−/− mice, compared with their wild-type counterparts (Fig. 4a), without affecting the total number of proliferating BrdU+ cells. Elmo1−/− mice exhibited a significant reduction in the numbers of BrdU+DCX+ cells per hippocampus, reduced neuronal differentiation (BrdU+DCX+ of total BrdU+) and lower counts of total DCX+ cells per dentate gyrus (Fig. 4b).
Figure 4.
Effect of ELMO1-dependent phagocytosis of DCX+ cells on neurogenesis in vivo. (a) Representative images of the SGZ from wild-type and Elmo1−/− mice examined for DCX and BrdU immunoreactivity 7 days after BrdU injection. The brackets indicate the width of the granular layer. Scale bars, 20 μm. (b) The bar graphs (means ± s.e.m.) represent the quantification of total numbers of BrdU+ cells (proliferating cells), total numbers of all BrdU+DCX+ cells (neuronal progenitors), total numbers of DCX+ cells per 200 μm of the SGZ (neuronal progenitors, alternative quantification) and percentages of DCX+BrdU+ out of all BrdU+ cells (neuronal differentiation of proliferating cells) per hippocampus in wild-type and Elmo1−/− mice. Student’s t -test statistical analysis was carried out for n = 6 mice in each group with at least ten slices analysed for each mouse (* P < 0.05; *** P < 0.01). (c) Mean (±s.e.m.) width of a granular cell layer. Student’s t -test was carried out for n = 6 mice per group with at least ten slices analysed for each mouse (*** P < 0.001). (d) Representative images of the SVZ immunolabelled for DCX from wild-type and Elmo1−/− mice (LV, lateral ventricle). The bar graph represents quantification of the areas occupied by DCX+ cells per field (mean ± s.e.m.). Student’s t -test statistical analysis was carried out between the groups for n = 6–8 mice in each group with at least ten slices analysed for each mouse (* P < 0.05). Representative experiment out of three independently carried out (n > 10 fields analysed in each experiment). Scale bars, 50 μm. (e) High magnification of the SGZ immunolabelled for DCX+ cells in wild-type and Elmo1−/− mice. The bar graph represents a quantification of dendritic arborization of DCX+ cells in wild-type and Elmo1−/− mice (mean s.e.m.). Student’s t -test statistical analysis was carried out for at least n± 100 DCX+ cells in each group with at least n = 5 mice per group analysed (** P < 0.01). Scale bars, 40 μm.
Newly formed neurons from the SGZ migrate and integrate into the granular layer of the dentate gyrus and maintain its size in the adult animal. One of the indications for impaired adult neurogenesis is reduced width of the granular layer. Elmo1−/− mice exhibited a significantly thinner granular layer, indicative of impaired adult neurogenesis (Fig. 4c). As we did not observe a difference in the granular layer width and the numbers of DCX+ cells in neonatal Elmo1−/− versus wild-type mice, it seems that the reduced width of the granular layer was probably due to long-lasting impaired adult neurogenesis and not a developmental defect (Supplementary Fig. S5b). As glia can play a major role in debris clearance during development25, either DCX+ cells do not have a direct role in phagocytic clearance in the developing brain or less of a role when compared with glia, or perhaps clearance in the developing brain is ELMO1 independent.
Reduced DCX+ cell numbers were also observed in the Elmo1−/− SVZ (Fig. 4d). Interestingly, the structure of DCX+ cells in the SGZ revealed fewer DCX+ cells in the Elmo1−/− hippocampus, with shorter processes and disorganized cell positioning in the SGZ (Fig. 4e). Long-term neurogenesis was also impaired without ELMO1, as determined by BrdU/NeuN labelling 28 days after BrdU injection, indicating an overall reduction in neuronal survival/maturation (Supplementary Fig. S5c).
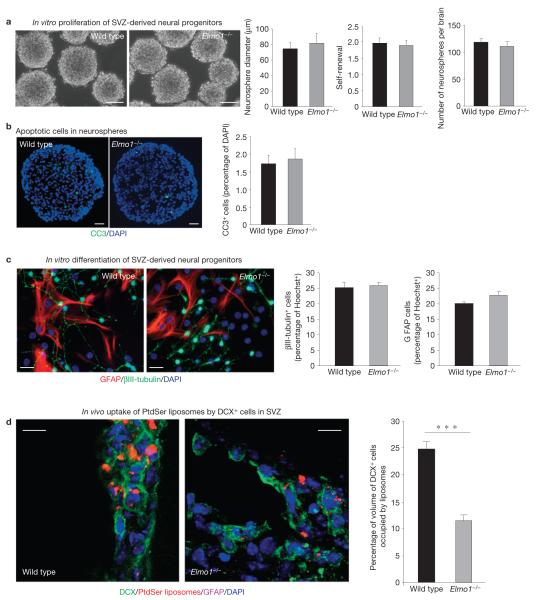
To address whether the effect of ELMO1 was cell autonomous, we established neurosphere cultures with NPCs from the SVZ of adult wild-type and Elmo1−/− mice. There was no detectable difference in the general appearance of the neurospheres (Fig. 5a), or the number of neurospheres from the brains. The comparable sizes of the neurospheres (measured by the diameters of the neurospheres), the amplification and the self-renewal capacity within the neurospheres (indicative of ‘stemness’) indicated normal proliferative capacity of the progenitor constituents in the absence of ELMO1 (Fig. 5a). Neurospheres obtained from wild-type or Elmo1−/− mice did not basally show substantial apoptosis (by cleaved caspase 3 staining of cryosectioned neurospheres; Fig. 5b), perhaps owing to the nutrient-rich in vitro conditions. Importantly, there also was no difference in neuronal or astroglial differentiation in vitro between wild-type and Elmo1−/−-derived NPCs (Fig. 5c). These data indicate that the impaired neuronal differentiation in Elmo1−/− mice in vivo probably resulted from extrinsic factors (such as impaired clearance of dying neurons) rather than intrinsic factors.
Figure 5.
ELMO1 deficiency impairs phagocytic activity of DCX+ cells in vivo. (a) Representative images of neurospheres from wild-type and Elmo1−/− mice. The bar graphs (means ± s.e.m.) show the diameter of neurospheres (indicates the ability of cells to grow and divide), the neurosphere self-renewal capacity (number of neurospheres divided by number of plated cells×100) of the first-generation (P1) neurospheres and the initial number of neurospheres obtained from the adult brains of wild-type and Elmo1−/− mice (n = 12 mice in each group, with hundreds of neurospheres analysed). Scale bars, 50 μm. (b) Neurospheres established from NPCs obtained from Elmo1−/− or wild-type mice were cryosectioned and examined for apoptotic (cleaved caspase 3 (CC3)-positive) cells in vitro. Representative images (from n > 5 neurospheres) are shown. The bar graph indicates cleaved caspase 3-positive cells (percentage of all DAPI-positive cells) in the neurosphere (mean ± s.e.m.). Multiple fields for n = 3 neurospheres from each group were analysed. Scale bars, 20 μm. (c) Neurospheres established from NPCs obtained from Elmo1−/− or wild-type mice were examined for differentiation in vitro. Representative images of differentiated NPCs after 10 days in culture, immunolabelled for III-tubulin (neurons) and GFAP (astrocytes). The bar graphs (means ± s.e.m.) indicate neuronal differentiation (percentage of III-tubulin cells) and astrocyte differentiation (percentage of GFAP cells) from wild-type and Elmo1−/− neurospheres (n = 10 fields analysed in each experiment). Scale bars, 20 μm. (d) Wild-type and Elmo1−/− mice were injected with fluorescently labelled PtdSer liposomes and examined immunohistochemically for DCX+ and GFAP+ cells containing phagocytosed liposomes in the SVZ. Representative confocal micrographs are shown. The bar graph indicates a quantification of the volume of DCX cells with PtdSer liposomes in wild-type and Elmo1−/− mice (mean±s.e.m.). Student’s t -test statistical analysis was carried out for n = 4 mice in each group with at least five slices analysed for each mouse (*** P < 0.001). Scale bars, 10 μm.
We then injected fluorescent PtdSer-containing liposomes into Elmo1−/− mice and examined the phagocytic ability of DCX+ cells in vivo. When compared with wild-type littermates, the Elmo1−/− mice showed significantly fewer PtdSer liposomes phagocytosed by DCX+ cells (>50% reduction). As the Elmo1−/− mice have fewer DCX+ cells, we also compared the fluorescence of PtdSer within the normalized volumes of individual DCX+ cells. Again, there was a substantial reduction in the phagocytosis mediated by DCX+ cells in vivo (Fig. 5d; 24.5±2% in wild-type mice, compared with 12±1.5% in Elmo1−/− littermates; P < 0.001). These data support the notion that the defective phagocytic clearance and accumulation of apoptotic nuclei are the probable underlying cause for the impaired adult neurogenesis in Elmo1−/− mice.
Taken together, the data presented in this report provide several unexpected insights into the fundamentally important process of adult neurogenesis. First, these studies reveal DCX+ cells as a critical population of phagocytes within the neurogenic niches of the adult brain. Second, these data identify the engulfment protein ELMO1 as an important molecule influencing adult neurogenesis through regulation of DCX+ cell phagocytosis. Third, when phagocytic clearance was interfered with, either pharmacologically (via annexin V injection) or genetically (in the ELMO1 null mice), the relevance of phagocytic clearance of dying neurons for continued adult neurogenesis was revealed. Collectively, these findings indicate a paradigm wherein DCX+ neuronal precursors are also phagocytes, and that such phagocytosis regulates neurogenesis in the adult brain. It is unclear whether all DCX+ cells can engulf their dying brethren. Nevertheless, those DCX+ cells that do engulf dying progenitors could differentiate and develop into neurons in vitro. Current technical challenges limit us to track the fate of phagocytic versus non-phagocytic DCX+ cells in vivo. It is possible that phagocytosis of neighbouring dying progenitors could be advantageous for the phagocytic cell as an energy source. However, precisely how the phagocytosis is advantageous for neuronal differentiation, maturation and survival in vivo remains to be defined.
DCX+ neuronal progenitor cells constitute an essential group of cells that differentiate into mature neurons during neurogenesis. Microglia have been recently shown to have close contact with dying NPCs in the SGZ and by inference were thought to mediate phagocytosis37. When we attempted to deplete ELMO1 in microglial cells (LysM-Cre mice crossed with Elmo1flox/flox mice), phagocytic clearance seemed normal without affecting adult neurogenesis (Supplementary Fig. S6). However, a caveat was that using the LysM-Cre we could only achieve partial deletion efficiency for the Elmo1 loci within the microglia. Interestingly, ELMO1 deficiency does not seem to affect the phagocytic capacity of myeloid cells36. Thus, whereas these data do not rule out a role for microglial cells in phagocytic clearance during adult neurogenesis, the impaired neurogenesis seen in Elmo1−/− mice is probably primarily due to the defective engulfment by DCX+ neuronal progenitor cells. Intriguingly, the survival of newborn neurons is improved under conditions that promote brain activity, such as hippocampus-dependent cognitive tasks, or enriched environment7,38,39. Identifying phagocytosis as an unexpected further property of neuronal cells may have a significant impact on further understanding adult neurogenesis in physiological and pathological conditions.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology
METHODS
Animals
Elmo1−/− mice and their littermates were bred in our facilities and C57Bl/6J mice (used for Annexin V injection studies) were purchased from the Jackson laboratories. Animals were housed in identical light- and temperature-controlled rooms and age-matched mice at ages 8–12 weeks were used throughout the study (except for Supplementary Fig. S2a, where 5-week-old mice were used, and this is indicated in the figure legend). All experiments were conducted under University of Virginia Animal Care and Use Committee-approved protocols. Male mice were used in the experiments in this manuscript.
Neurosphere culture
Neurospheres were generated from the SVZ of young adult mice and cultured under clonal conditions, according to previously published procedures40. Briefly, for each culture, the entire SVZ region was dissected from sagittally cut brains and incubated in DMEM containing 20 U ml−1 papain (Worthington), 1 mM N-acetyl-l-cysteine (NAC; Sigma) and 2 U ml−1 RQ1 DNase (Promega) for 1 h at 37 °C. Dissociated cells were plated in 24-well plates at a density of 2.6 × 103 cells cm−2 (5 × 103 cells in 0.5 ml per well) in high-glucose DMEM containing N2 (Invitrogen), B-27 (StemCell Technologies), 1 mM NAC, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 ng ml−1 epidermal growth factor and 20 ng ml−1 basic fibroblast growth factor (both murine growth factors were from PeproTech). The total number of neurospheres at passage 0 (P0) was determined at 7 DIV by counting all neurospheres generated from each mouse brain. In subsequent passages, the total number of neurospheres was determined by counting all neurospheres generated in a 24-well dish seeded at 5 × 103 cells per well and extrapolating for the total number of dissociated cells. Self-renewal was determined as the number of secondary neurospheres/number of seeded cells × 100 (that is, how many early precursors capable of generating a ‘daughter’ neurosphere were contained in each ‘mother’ neurosphere). Amplification was calculated as the number of viable cells from dissociated neurospheres/number of seeded cells. The number of viable cells was determined in samples of SVZ- or neurosphere-dissociated cells by trypan blue exclusion. Neurosphere diameter (>40 μm) was measured in phase-contrast pictures of individual neurospheres using QCapture Pro 6.0 software (QImaging). For the cell differentiation study, neurospheres were dissociated in papain or accutase (Invitrogen) solution. Dissociated single cells were seeded on coverslips coated with poly-l-lysine. Cells were kept in differentiation medium for either 7 or 14 days, with half of the medium changed every other day.
NPC differentiation
Dissociated NPCs were plated at 8 × 103 cells cm−2 on poly-l-lysine-coated coverslips and maintained in the differentiation medium (high-glucose DMEM containing N2, B-27, 1 mM NAC, 1 mM sodium pyruvate, 2 mM l-glutamine and 1% fetal bovine serum) for 7 days. Half of the medium was replaced with fresh medium every other day. For immunofluorescent staining, the following primary antibodies were used on 4% paraformaldehyde-fixed cells: goat anti-DCX (1:200, Santa Cruz) and rabbit anti-GFAP (1:500, Millipore). Fluorescently labelled cells were visualized and imaged on a confocal microscope (Zeiss LSM510; Carl Zeiss).
NPC ultraviolet-irradiation treatment and NPC injection
Dissociated NPCs were treated with ultraviolet light for 15 min. NPCs were then stained with 5(6)-TAMRA, SE (Invitrogen) and thoroughly washed with cold PBS before they were used for either incubation with cell culture or intracranial injection. For intracranial injection, 2 μl of a 5 million cells ml−1 suspension was injected into the lateral ventricles of mice using a stereotaxic injector (Stoelting).
BrdU treatment
BrdU (Sigma) was dissolved in 0.9% saline at a concentration of 5 mg ml−1. To label proliferating cells in the brain, mice received two intraperitoneal injections of BrdU (50 mg kg−1) 2 h apart. For examination of progenitor proliferation mice were killed 24 h after the second injection, whereas for examination of progenitor differentiation mice were killed 7 days after BrdU injection. For examination of neuronal survival (NeuN+BrdU+ cells in the granular layer), animals were injected with a double dose of BrdU and killed 28 days after injection. Mice were perfused with PBS for 3 min and then with 4% paraformaldehyde (PFA) in pH 7.2 PBS for 5 min. Brains were excised and postfixed in 4% PFA for 72 h at 4 °C, then in 30% sucrose for at least 2 days. Brains were frozen in 2-methylbutane Chromasolv with dry ice, and stored at 80 °C before coronal cryostat sections (40 μm thick) were obtained. Six sequential–slices were placed into each well of a 24-well plate containing 0.02% sodium azide in PBS and stored at 4 °C. To quantify proliferating progenitor cells, every sixth section (40 μm thick) of the brain containing hippocampus or lateral ventricles was selected and immunolabelled with BrdU (rat anti-BrdU, 1:300; Abcam), DCX (goat anti-DCX, 1:200; Santa Cruz) or NeuN (1:300; Millipore) antibodies. Total numbers of positive cells in all slices per animal were multiplied by six to estimate the number of cells per hippocampus or lateral ventricles.
Immunofluorescence
For BrdU labelling on coronal free-floating sections, the sections were permeabilized with 0.5% Triton X-100 in PBS for 20 min, and then treated with 0.3% H2O2 to block endogenous peroxidases, denatured in 2 N HCl for 15 min, and rinsed twice in 0.1 M (pH 8.5) borate buffer. Sections were placed in 10% normal chicken serum in PBS for 1 h, then incubated with primary antibody rat anti-BrdU (1:300; Abcam) overnight at 4 °C. For DCX, GFAP and NeuN labelling, brain slices were permeabilized with 0.5% Triton X-100 in PBS for 1 h. Following blocking, the slices were incubated with goat anti-DCX (1:300; Santa Cruz), rabbit anti-GFAP (1:800, Millipore) or mouse anti-NeuN (1:300; Millipore) antibodies, and fluorescent-dye-conjugated secondary antibodies were used to label the cells. To detect ELMO1 and DCX in cultured hippocampus neurons, rabbit anti-ELMO1 (1:100, Sigma) and goat anti-DCX (1:300, Santa Cruz) were used to label cultured neurons on coverslips.
TUNEL assay
An in situ cell-death detection kit (catalogue no 11684795001; Roche) was used to detect cell apoptosis in the mouse brain, according to the manufacturer’s instructions.
Liposome preparation and injection
Liposomes were prepared by a classical Bangham technique; multilamellar vesicles were chosen for the study to maximize the fluorescent-dye load per particle without significant self-quenching. Briefly, dioleoyl phosphatidylcholine (Avanti Polar Lipids) and cholesterol (Sigma) were mixed in chloroform at 1:1 molar ratio, and DiIC18(3) dye (Invitrogen) was added; this dye has two stearyl residues attached to the fluorochrome, so it is tightly attached to liposome membrane and is not exchangeable. To the experimental sample but not to control, PtdSer (Avanti) was added at 1:10 PS:PC mass ratio. After chloroform removal by rotary evaporation and argon-gas flush, vacuum desiccation was applied to remove traces of organic solvent. After removal of the solvent had been completed, normal saline was added to the lipid film, and the sample was subjected to hydration under vortexing. Resulting liposomes were quite large: at normal gravity, sedimentation was observed during storage.
In vitro and in vivo phagocytosis assay
To determine whether DCX+ cells could engulf apoptotic cells or simplified targets, NPCs were differentiated for 48 h and carboxylate-modified 3 μm beads (simplified apoptotic targets) were fed to the cells for 3 h before thoroughly washing and then fixing the cells with 4% PFA. Cells were immunolabelled with anti-DCX antibody and Alexa Fluor 488 phalloidin (1:50, Invitrogen). Beads with a clear surrounding actin ring were counted as engulfed. Alternatively, dissociated single NPCs were exposed to ultraviolet light for 10 min before their feeding to growing cultures of differentiating NPCs for 4 h, washing and fixing in 4% PFA. To assess cells’ engulfment capacity ex vivo, 1 μl of PtdSer- or PtdCho-coated liposomes (diameter 2–6 μm) were stereotaxically injected intracranially into the SVZ. Mice were killed 12 h after the injection and perfused with PBS. Brains were removed and fixed in 4% PFA. Floating sections (40 μm) were analysed for phagocytic cells as described above (every sixth slice was labelled and counted throughout the entire neurogenic niche). All of the images analysed and provided in the manuscript were taken approximately within the range of 200 μm of the injection site (liposomes were well diffused, whereas the progenitor cells did not diffuse equally well, which presumably results in the lower numbers of phagocytic DCX+ when counting progenitor-engulfing DCX+ cells as compared with liposome-engulfing DCX+ cells in vivo).
Annexin V intravenous injection
To address the role of phagocytic cell clearance in adult neurogenesis (Fig. 2), annexin V (BioVision) was intravenously injected at a dose of 5 μg per 100 μl using the scheme shown in Fig. 2a. To assess the effect of annexin V on DCX+ cell phagocytic activity in vivo, annexin V was injected three times before BrdU administration, followed by a further two injections (5 μg per 100 μl intravenously every 2 h).
Statistical tests
In this study we employed Student’s t -test for comparison between two groups and analysis of variance with the Newman-Keuls test for multiple-group comparison.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. Smith for editing the original version of the manuscript and S. Zeitlin for comments. We also thank the members of the Kipnis and Ravichandran laboratories for discussions at many stages of conducting the work and the preparation of the manuscript. This work was supported in part by an award from the National Institute of General Medical Sciences (GM55761) to K.S.R. and in part by an award from the National Institute on Aging (R01AG034113) to J.K. K.S.R. is a Bill Benter Senior Fellow of the American Asthma Foundation.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website
AUTHOR CONTRIBUTIONS Z.L. participated in the experimental design, carried out most of the experiments, analysed the data and participated in manuscript preparation; M.R.E. assisted with phagocytic assays and participated in experimental design; Y.C. assisted with immunofluorescent experiments; J.T.W. assisted with intracranial injections; A.L.K. supplied all the liposomes used in this study; K.S.R. helped with design of experiments and prepared the manuscript; J.K. designed the experiments, assisted with data analysis and prepared the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 5.Rakic P. Adult neurogenesis in mammals: an identity crisis. J. Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat. Rev. Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 7.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 8.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 9.Leuner B, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SA, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 12.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg G, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 14.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. N Y Acad. Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J. Pathol. 2011;223:177–194. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- 18.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 21.Conover JC, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 22.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 24.McFarland KN, Wilkes SR, Koss SE, Ravichandran KS, Mandell JW. Neural-specific inactivation of ShcA results in increased embryonic neural progenitor apoptosis and microencephaly. J. Neurosci. 2006;26:7885–7897. doi: 10.1523/JNEUROSCI.3524-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HH, et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 2009;12:1534–1541. doi: 10.1038/nn.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegenfuss JS, et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald JM, et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 28.D’Arceuil H, et al. 99mTc annexin V imaging of neonatal hypoxic brain injury. Stroke. 2000;31:2692–2700. doi: 10.1161/01.str.31.11.2692. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, et al. A minimally invasive, translational biomarker of ketamine-induced neuronal death in rats: microPET Imaging using 18F-annexin V. Toxicol. Sci. 2009;111:355–361. doi: 10.1093/toxsci/kfp167. [DOI] [PubMed] [Google Scholar]
- 30.Maeda Y, Shiratsuchi A, Namiki M, Nakanishi Y. Inhibition of sperm production in mice by annexin V microinjected into seminiferous tubules: possible etiology of phagocytic clearance of apoptotic spermatogenic cells and male infertility. Cell Death Differ. 2002;9:742–749. doi: 10.1038/sj.cdd.4401046. [DOI] [PubMed] [Google Scholar]
- 31.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A -stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar -amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar -amyloid through a 1 integrin-dependent mechanism. J. Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 35.Gumienny TL, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 36.Elliott MR, et al. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown J, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 39.Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr. Biol. 1998;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 40.Lu Z, Kipnis J. Thrombospondin 1—a key astrocyte-derived neurogenic factor. FASEB J. 2010;24:1925–1934. doi: 10.1096/fj.09-150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.