Abstract
Over the course of developing a multigram scale preparation of epoxy quinol 1 via asymmetric transfer hydrogenation (ATH) using the Noyori Ru(arene)(S,S-TsDPEN) catalysts, we observed several unexpected phenomena, including (i) chemoselective alkene vs ketone reduction of an enedione, (ii) a significant arene ligand effect (p-cymene vs. mesitylene) on the reaction pathway, and (iii) solvent-based reversal of the sense of enantioinduction.
In the course of our ongoing synthesis of antascomicin B,1-3 we identified epoxy quinol 1 as an early intermediate in the synthesis (Scheme 1). Since the Noyori asymmetric transfer hydrogenation (ATH) of ketones is a widely used reaction in both industrial and academic laboratories,4,5 ATH of meso diketones 26 or 37 appeared to offer a mild, scalable method for large scale synthesis of the quinol (eq 1). Unlike aryl ketones or ynones, however, there are few examples of ATH of cyclohexenones.8,9 The ATH of ketoisophorone reported by a Hoffman-La Roche group seemed to augur well for the success of the reduction, although the reported reaction scale was only ca. 100 mg (eq 2).9
Scheme 1.

Asymmetric transfer hydrogenation substrates.
We initially sought to desymmetrize meso enedione 2 via ATH. Noyori transfer hydrogenations are typically performed using either iPrOH as both solvent and reductant or trialkylamine/formic acid as reductant with or without added solvent.4,5 To our surprise, subjection of diketone 2 to typical ATH conditions using Ru(p-cymene)(S,S-TsDPEN) as catalyst and triethylammonium formate as the reductant resulted principally in alkene rather than ketone reduction to give dihydro diketone 4,6b hydroquinone 5,10 and a small amount of recoverd starting material (Scheme 2).11 Use of 2-propanol as reductant and solvent gave similar results.
Scheme 2.

Chemoselective alkene reduction of enedione 2.
There are at least two possible mechanisms for formation of diketone 4: direct alkene reduction, or ketone reduction followed by isomerization.12 In order to distinguish the two pathways, the progress of the reduction of 2 in 2-propanol was followed directly by no-D 1H NMR analysis13 of the reaction mixture (see Supplementary data).14 Within 3 minutes of mixing the reactants, only enedione 2 and a small amount of diketone 4 were detected. After 6 hours, all of the enedione had disappeared, leading principally to diketone 4. No ketone reduction product was detectable. These results imply that either direct alkene reduction occurred, or isomerization of the keto alcohol to the diketone was very rapid. Deng et al have reported that some electron deficient enones undergo preferential alkene reduction using the Noyori Ru(arene)(TsDPEN) catalyst system, and that activated alkenes lacking ketones also undergo alkene reduction.15
The Noyori and related ATH catalysts can effect both reduction of ketones and oxidation of alcohols. In 1997 Noyori et al reported the oxidative desymmetrization of meso-diol 616 using Ru(mesitylene(S,S-TsSPEN) to give hydroxy enone 7 in 70% yield and 96% ee, although the scale of the reaction was not disclosed (Scheme 3).17 This could also lead to our desired epoxy alcohol 1, although it would require an additional undesirable reduction/oxidation sequence. While Noyori employed the Ru catalyst with the mesitylene ligand, we first examined the more widely used p-cymene variant. On a preparative scale,18,19 treatment of enediol 6 with Ru(p-cymene)(S,S-TsDPEN) in acetone at rt afforded none of the hydroxy enone 7, but rather diketones 2 and 4 in roughly equal yield, along with recovered diol (20%) and a small amount of hydroquinone 5. Use of the mesitylene catalyst did indeed provide hydroxy ketone 7, albeit in our hands in only 42% isolated yield, along with an equivalent amount of enedione 2 as well as 9% of dihydro diketone 4 and recovered diol (10%).18,19 The er of hydroxy enone 7 ranged from 87:13 to 96:4 over the course of the reactions. This is consistent with a modest intrinsic enantioselectivity in the oxidation enhanced by kinetic resolution of the minor enantiomer (vide infra).3c,17
Scheme 3.
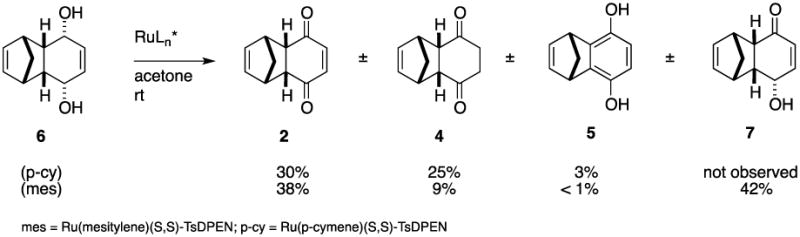
Results of attempted asymmetric oxidation of meso diol 6 using Ru(Ar)(S,S)-TsDPEN catalysts.
In order to better understand the relative rates of formation of the various products, we again followed the course of the reactions directly by no-D 1H NMR analysis using acetone as solvent (see Supplementary data).20 Using the p-cymene catalyst, within 3 minutes of mixing of the reactants, a small amount of hydroxy enone 7 was detectable, but by 60 minutes an equal amount of enedione 2 was also present. At 20 hours the reaction had proceeded to roughly 75% conversion, with enedione 2 predominating and hydroquinone 5 also present in minor amounts. Use of the mesitylene catalyst was more selective for formation of enone 7, although after 24 hours it had proceeded only to ca. 20% conversion. Interestingly, dihydro diketone 4 was not detected in either NMR experiment, although it was always present in the preparative scale reactions in significant amounts.21
In order to circumvent these problems, we examined reduction of meso epoxy diketone 3 to epoxy keto alcohol 8 using both iPrOH and NEt3/HCO2H as reductants (Scheme 4).3c,22 We found that the sense of enantioinduction reversed when changing the hydrogen source/solvent from neat iPrOH to 1:1 NEt3/HCO2H (0.2 M) in acetonitrile: 19:81 versus 82:18 8:ent-8, respectively (see Supplementary data).23 To our knowledge, a reversal in enantioselectivity as a function of solvent has not been previously noted, although solvents are known to affect the enantioselectivity of the reduction.4c
Scheme 4.

Solvent dependent reversal in sense of absolute asymmetric induction.
The mechanism of the Noyori ATH is generally agreed to involve a concerted outer sphere transfer of hydrogen from the catalyst to a ketone that is hydrogen-bonded to the catalyst via an N-H bond (Scheme 5).24-27 Details regarding the origin of enantioselectivity are a subject of dispute, but include steric, solvent, and electrostatic effects as well as dispersion forces. In the case of aryl ketones, a favorable CH- interaction has been identified computationally as contributing to the asymmetric induction.28-30 The sense of enantioinduction relies principally on the absolute configuration of the chelating ligand,31 although Andersson reported that acetophenone and its perfluorophenyl analog gave opposite senses of asymmetric induction using the same S,S-TsDPEN ligand (95% ee S vs. 12% ee R, respectively).26b
Scheme 5.
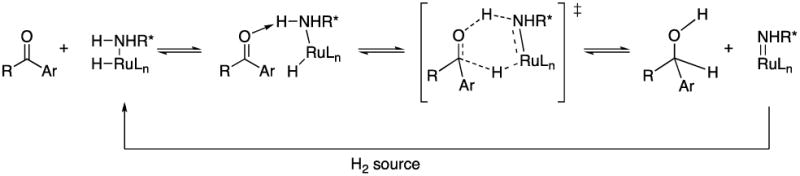
Schematic mechanism of transfer hydrogenation.
Recently, computational analysis of ATH including explicit solvent (methanol32 or water33) have shown that hydrogen bonding of the ketone oxygen to solvent lowers the transition state energies relative to the unsolvated transition states (Scheme 6).34 In the case of methanol, hydrogen bonding between solvent, ligand and ketone (transition state i), and between solvent and ketone in the case of water (transition state ii), served to lower the transition state energies.
Scheme 6.

Transfer hydrogenation transition states with explicit methanol or water molecule.
We speculate that the differing dielectric constants (iPrOH = 19.9; CH3CN = 37.5)35 and hydrogen bonding abilities of the solvents contributed to the reversal in enantioselectivity. The concentration of hydrogen bond donors also differs significantly in the two sets of conditions: neat iPrOH is 13.3 M, while the concentration of HNEt3CO2H was 0.2 M in acetonitrile. The epoxide oxygen may also serve as a hydrogen bond acceptor,36 which might further differentiate the two transition states. The latter proposal is supported by the observation that transfer hydrogenation of meso-diketone 4 reproducibly provided the known keto alcohol 937 with the same sense of asymmetric induction under both sets of conditions: 63:33 (iPrOH) and 81:19 (HCO2H/NEt3).38,39
In summary, we have identified unexpected substrate, ligand, and solvent effects in the ATH of polycyclic meso diketones and a meso diol. Given the importance of the Noyori and related ATH methods in industrial and academic synthesis, these results suggest that a careful exploration of reaction parameters may be necessary in some substrate classes to optimize chemo- and enantioselectivity.
Supplementary Material
Scheme 7.

Solvent effect on asymmetric reduction of diketone 4.
Acknowledgments
We thank NSF (CHE0911638), NIH (P30RR031154) and the Arkansas Biosciences Institute for support of this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Isolation: Fehr T, Sanglier JJ, Schuler W, Gschwind L, Ponelle M, Schilling W, Wioland C. J Antibiot. 1996;49:230–233. doi: 10.7164/antibiotics.49.230.
- 2.Total synthesis: Brittain DEA, Griffiths-Jones CM, Linder MR, Smith MD, McCusker C, Barlow JS, Akiyama R, Yasuda K, Ley SV. Angew Chem Int Ed. 2005;44:2732–7. doi: 10.1002/anie.200500174.
- 3.(a) Qi W, McIntosh MC. Tetrahedron. 2008;64:7021–7025. doi: 10.1016/j.tet.2008.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hutchison JM, Gibson AS, Williams DT, McIntosh MC. Tetrahedron Lett. 2011;52:6349–6351. doi: 10.1016/j.tetlet.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Clay DR, Rosenberg AG, McIntosh MC. Tetrahedron: Asymmetry. 2011;22:713–6. doi: 10.1016/j.tetasy.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.See, for example: Miyagi M, Takehara J, Collet S, Okano K. Org Process Res Dev. 2000;4:346–348.Hansen KB, Chilenski JR, Desmond R, Devine PN, Grabowski EJJ, Heid R, Kubryk M, Mathrea DJ, Varsolonab R. Tetrahedron: Asymmetry. 2003;14:3581–3587.Tanis SP, Evans BR, Nieman JA, Parke TT, Taylor WD, Heasley SE, Herrinton PM, Perrault WR, Hohler RA, Dolak LA, Hesterf MR, Seestg EP. Tetrahedron: Asymmetry. 2006;17:2154–2182.Zhang J, Blazecka PG, Braendl MM, Huang Y. J Org Chem. 2009;74:1411–1414. doi: 10.1021/jo802380j.
- 5.For reviews, see: Ohkuma T, Noyori R. Enantioselective Ketone and -Keto Ester Hydrogenations (Including Mechanisms) In: de Vries JG, Elsevier CJ, editors. Handbook of Homogeneous Hydrogenation. Vol. 3. WILEY-VCH; Weinheim: 2007. pp. 1105–1163.Blacker AL. Enantioselective Transfer Hydrogenation. ibid. :1215–1244.Ikariya T, Blacker AJ. Acc Chem Res. 2007;40:1300–1308. doi: 10.1021/ar700134q.Gladiali S, Alberico E. Chem Soc Rev. 2006;35:226–236. doi: 10.1039/b513396c.Ikariya T, Murataa K, Noyori R. Org Biomol Chem. 2006;4:393–406. doi: 10.1039/b513564h.Noyori R, Ohkuma T. Angew Chem Int Ed. 2001;40:40–73.
- 6.(a) Albrecht W. Justus Liebigs Ann Chem. 1906;343:31–49. [Google Scholar]; (b) Oda M, Kawase T, Okada T, Enomoto T. Org Syn. 1996;73:253–257. [Google Scholar]
- 7.O'Brien DF, Gates JW., Jr J Org Chem. 1965;30:2593–2601. [Google Scholar]
- 8.(a) Hannedouche J, Kenny JA, Walsgrove T, Wills M. Synlett. 2002:263–266. [Google Scholar]; Peach P, Cross DJ, Kenny JA, Mann L, Houson I, Campbell L, Walsgrove T, Wills M. Tetrahedron. 2006;62:1864–1876. [Google Scholar]; (b) Kosjek B, Tellers DM, Biba M, Farr R, Moore JC. Tetrahedron: Asymmetry. 2006;17:2798–2803. [Google Scholar]
- 9.Hennig M, Püntener K, Scalone M. Tetrahedron: Asymmetry. 2000;11:1849–1858. [Google Scholar]
- 10.Porter RF, Rees WW, Frauenglass E, Wilgus HS, III, Nawn GH, Chiesa PP, Gates JW., Jr J Org Chem. 1964;29:588–594. [Google Scholar]
- 11.Experimental procedure: Formic acid (0.6 mL, 16 mmol) was added dropwise to triethylamine (1.18 mL, 16 mmol) at −10 °C. The mixture was diluted with acetonitrile (100 mL) and Ru(p-cymene)(S,S-TsDPEN) (0.04 g, 0.067 mmol, 0.67 mol %) was added, followed by enedione 2 (1.74 g, 10 mmol, 0.1 M). The reaction mixture was allowed to slowly warm to rt and stirred for ca. 6 h. The mixture was concentrated in vacuo and then stirred for ca. 16 h in 30/70 ethyl acetate/hexanes (50 mL) with activated charcoal (2 g), then filtered through a plug of Celite with 30/70 ethyl acetate/hexanes and concentrated in vacuo. Flash chromatography (20/80 ethyl acetate/hexanes) of the residue gave diketone 4 (0.88 g, 50% yield) and hydroxy phenol 5 (0.22 g, 13% yield). All 1H NMR spectra matched reported data.
- 12.Hiroya K, Kurihara Y, Ogasawara K. Angew Chem Int Ed. 1995;34:2287–2289. [Google Scholar]
- 13.Hoye TR, Eklov BM, Ryba TD, Vologshin M, Yao LJ. Org Lett. 2004;6:953–956. doi: 10.1021/ol049979+. [DOI] [PubMed] [Google Scholar]
- 14.Experimental procedure: Enedione 2 (87 mg, 0.5 mmol, 0.1 M) was added to a solution of Ru(p-cymene)(S,S-TsDPEN) (4 mg, 0.0067 mmol, 1.3 mol %) in i-PrOH (5 mL). An aliquot was removed from the reaction mixture and placed directly into an NMR tube. The progress of the reaction in the NMR tube was monitored by 1H NMR spectrometry.
- 15.Xue D, Chen YC, Cui X, Wang QW, Zhu J, Deng JG. J Org Chem. 2005;70:3584–3591. doi: 10.1021/jo0478205. [DOI] [PubMed] [Google Scholar]
- 16.Marchand AP, LaRoe WD, Sharma GVM, Suri SC, Reddy DS. J Org Chem. 1986;51:1622–1625. [Google Scholar]
- 17.Hashiguchi S, Fujii A, Haack KJ, Matsumura K, Ikariya T, Noyori R. Angew Chem Int Ed. 1997;36:288–291. [Google Scholar]
- 18.Each reduction was performed at least 3 times. Product yields shown are representative.
- 19.Experimental procedures: (a) p-Cymene catalyst: Diol 6 (0.45 g, 2.5 mmol, 0.1 M) was added to a solution of Ru(p-cymene)(S,S-TsDPEN) (0.075 g, 0.125 mmol, 5 mol %) in acetone (25 mL). The reaction mixture was allowed to stir for ca. 24 h, then concentrated in vacuo. Activated charcoal (2 g). was added, and the mixture stirred for ca. 16 h in 30/70 ethyl acetate/hexanes (25 mL). After filtration through a plug of Celite with 30/70 ethyl acetate/hexanes and concentration in vacuo, the residue was purified by flash chromatography (20/80 ethyl acetate/hexanes) to give enedione 2 (0.13 g, 30%), diketone 4 (0.11 g, 25%), hydroquinone 5 (0.013 g, 3%), and recovered diol (20%). (b) Mesitylene catalyst: diol 6 (65 mg, 0.36 mmol, 0.1M) was added to a solution of Ru(mesitylene)S,S-TsDPEN (3 mg, 0.0049 mmol, 1.3 mol %) in acetone (3.6 ml). The reaction mixture was allowed to stir for ca. 16 h, then concentrated in vacuo. Activated charcoal (0.5 g) was added and the mixture stirred for ca. 16 h in 30/70 ethyl acetate/hexanes (5 mL), then was filtered through a plug of Celite with 30/70 ethyl acetate/hexanes and concentrated in vacuo. Flash chromatography (20/80 ethyl acetate/hexanes) of the residue gave enedione 2 (24 mg, 38% yield), dihydro diketone 4 (5.8 mg, 9% yield), hydroquinone 5 (0.317 mg, 0.5% yield), hydroxyl ketone 7 (27 mg, 42% yield), and recovered starting material (6.8 mg, 10%)
- 20.Experimental procedure: Diol 6 (0.087 g, 0.5 mmol, 0.1 M) was added to a solution of Ru(p-cymene)(S,S-TsDPEN) (4 mg, 0.0067 mmol, 1.34 mol %) in acetone (5 mL). An aliquot was removed from the reaction mixture and added directly to an NMR tube. The progress of the reaction in the NMR tube was monitored by 1H NMR spectrometry. The oxidation with the mesitylene catalyst was performed similarly.
- 21.These results collectively suggest that ATH with these catalysts is not a feasible approach to hydroxy enone 7 on preparative scale. While the Noyori report (ref 17) of this transformation has been cited multiple times in reviews of ATH,5 we can find no reports of its use in synthesis.
- 22.Experimental procedure: Epoxy diketone 3 (190 mg, 1 mmol, 0.1 M) was added to a solution of Ru(p-cymene)(S,S-TsDPEN) (0.04 g, 0.067 mmol, 0.67 mol %) in i-PrOH (10 mL) and allowed to stir until no starting material remained by TLC analysis (ca. 16 h). The mixture was concentrated in vacuo, then stirred for ca 16 h in 30/70 ethyl acetate/hexanes (5 mL) with activated charcoal (1 g). The mixture was filtered through a plug of Celite with 30/70 ethyl acetate/hexanes, concentrated in cacuo, and the residue purified by flash chromatography (30/70 ethyl acetate/hexanes) to give keto alcohol 8 (154 mg, 80% yield). Alternatively, formic acid (3.62 mL, 96 mmol) was added dropwise to triethylamine (13.5 mL, 96 mmol) at −10 °C. The mixture was diluted with acetonitrile (500 mL) and Ru(p-cymene)(S,S-TsDPEN) (0.24 g, 0.4 mmol, 0.67 mol %) was added, followed by epoxy diketone 3 (11.4 g, 60 mmol). The reaction mixture was allowed to slowly warm to rt and stirred for ca. 16 h. The mixture was concentrated in vacuo and then stirred for ca. 16 h in 30/70 ethyl acetate/hexanes (200 mL) with activated charcoal (10 g). The mixture was filtered through a plug of Celite with 30/70 ethyl acetate/hexanes, concentrated in vacuo, and the residue purified by flash chromatography (30/70 ethyl acetate/hexanes) to give keto alcohol 8 (9.8 g, 85% yield, 82:18 er).
- 23.Er's were determined at low (5-10%) conversion to minimize effects of kinetic resolution, and are an average of at least 3 runs.
- 24.Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. J Am Chem Soc. 1996;118:2521–2522. [Google Scholar]; Hamada T, Torii T, Izawa K, Noyori R, Ikariya T. Org Lett. 2002;4:4373–4376. doi: 10.1021/ol020213o. [DOI] [PubMed] [Google Scholar]
- 25.(a) Yamakawa M, Ito H, Noyori R. J Am Chem Soc. 2000;122:1466–1478. [Google Scholar]; (b) Noyori R, Yamakawa Masashi, Hashiguchi S. J Org Chem. 2001;66:7932–7944. doi: 10.1021/jo010721w. [DOI] [PubMed] [Google Scholar]
- 26.(a) Alonso DA, Brandt P, Nordin SJM, Andersson PG. J Am Chem Soc. 1999;121:9580–9588. [Google Scholar]; (b) Brandt P, Roth P, Andersson PG. J Org Chem. 2004;69:4885–4890. doi: 10.1021/jo030378q. [DOI] [PubMed] [Google Scholar]
- 27.Clapham SE, Hadzovic A, Morris RH. Coord Chem Rev. 2004;248:2201–2237. [Google Scholar]
- 28.Casey CP, Johnson JB. J Org Chem. 1998;68:1998–2001. doi: 10.1021/jo0205457. [DOI] [PubMed] [Google Scholar]
- 29.Yamakawa M, Yamada I, Noyori R. Angew Chem Int Ed. 2001;40:2818–2821. [PubMed] [Google Scholar]
- 30.No model has been explicitly proposed for enones, ynones or dialkyl ketones.
- 31.(a) Takehara J, Hashiguchi S, Fujii A, Inoue Si, Ikariya T, Noyori R. J Chem Soc Chem Commun. 1996:233–234. [Google Scholar]; (b) Noyori R, Yamakawa Masashi, Hashiguchi S. J Org Chem. 2001;66:7932–7944. doi: 10.1021/jo010721w. [DOI] [PubMed] [Google Scholar]
- 32.Handgraaf JW, Meijer EJ. J Am Chem Soc. 2007;129:3099–3103. doi: 10.1021/ja062359e. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Liu J, Tommaso DD, Iggo JA, Catlow CRA, Bacsa J, Xiao J. Chem Eur J. 2008;14:7699–7715. doi: 10.1002/chem.200800559. [DOI] [PubMed] [Google Scholar]
- 34.Transition state structures i and ii were adapted from refs 32 and 33, respectively.
- 35.Przybytek JT, Krieger PA. High Purity Solvent Guide. 2. Burdick & Jackson Laboratories, Inc.; 1984. [Google Scholar]
- 36.Pihko PM, editor. Hydrogen Bonding in Organic Synthesis. Wiley-VCH; Weinheim: 2009. [Google Scholar]
- 37.Hiroya K, Kurihara Y, Ogasawara K. Angew Chem Int Ed. 1995;34:2287–2289. [Google Scholar]; Salles RC, Lacerda V, Jr, Beatriz A, Ito FM, dos Santos RB, Greco SJ, de Castro EVR, de Lima DP. Magn Reson Chem. 2010;48:409–415. doi: 10.1002/mrc.2588. [DOI] [PubMed] [Google Scholar]
- 38.Experimental procedure: Diketone 4 (100 mg, 0.57 mmol, 0.1 M) was added to a solution of Ru(p-cymene)(S,S-TsDPEN) (4.8 mg, 0.0067 mmol, 1.34 mol %) in i-PrOH (5.7 mL) and allowed to stir until TLC analysis showed no further consumption of diketone (ca. 48 h). The mixture was concentrated in vacuo and was then passed through a plug of silica gel (30/70 ethyl acetate/hexanes). The filtrate was analyzed by chiral stationary phase gas chromatography. Alternatively, formic acid (0.036 mL, 0.97 mmol) was added dropwise to triethylamine (0.14 mL, 0.97 mmol) at −10 °C. The mixture was diluted with acetonitrile (5.7 mL) and Ru(p-cymene)(S,S-TsDPEN) (2.4 mg, 0.0038 mmol, 0.67 mol %) was added, followed by diketone 4 (100 mg, 0.57 mmol). The reaction mixture was allowed to slowly warm to rt and and allowed to stir until TLC analysis showed no further consumption of diketone (ca. 24 h). The mixture was concentrated in vacuo and then passed through a plug of silica gel (30/70 ethyl acetate/hexanes). The filtrate was analyzed by chiral stationary phase gas chromatography.
- 39.The absolute configurations of the alcohols were not determined, although it seems likely that the major enantiomer is 9 by analogy to the reduction of epoxy diketone 3. The reductions were repeated at least 3 times each; the er's are an average of the runs.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


