Abstract
The aim of the present study was to determine whether Clostridium difficile was present in uncooked retail ground beef and ground pork products sold in Winnipeg, Manitoba. Using an alcohol treatment protocol and inoculation of cultures on C difficile Moxalactam Norfloxacin (CDMN), toxigenic C difficile was found in 6.3% of 48 meat samples. The C difficile isolates belonged to different pulsotypes, all of which had been previously isolated from the stool of Manitoba patients with C difficile disease. Because cooking of meat will not eradicate C difficile spores, this raises a concern regarding potential foodborne transmissibility of this organism.
Keywords: C difficile, Food-borne disease, Zoonotic disease
Abstract
La présente étude visait à déterminer si on décelait du Clostridium difficile dans les produits de viande hachée et de porc haché non cuits vendus au détail à Winnipeg, au Manitoba. Au moyen d’un protocole de traitement par l’alcool et d’inoculation de cultures sur la C difficile Moxalactam Norfloxacin (CDMN), les chercheurs ont trouvé du C difficile toxicogénique dans 6,3 % des 48 échantillons de viande. Les isolats de C difficile appartenaient à divers pulsotypes, tous déjà isolés dans les selles de patients manitobains atteints d’une maladie à C difficile. Puisque la cuisson de la viande n’éradique pas les spores C difficile, ce phénomène soulève de l’inquiétude au sujet de la transmissibilité potentielle de cet organisme par voie alimentaire.
Clostridium difficile is an anaerobic spore-forming human pathogen associated with serious enteric diseases such as antibiotic-associated diarrhea and pseudomembranous colitis (1). Although C difficile infection (CDI) occurs mostly in health care facilities and is considered to be a nosocomial infection, the rate of community-acquired C difficile infection (CA-CDI) appears to be increasing (2–3) and may now account for 20% to 45% of all CDI cases (4–5). The recent increase in mortality, morbidity and relapse rates of CDI has largely been associated with one strain of C difficile, ribotype 027/ North American pulsotype 1 (NAP1)/toxinotype III (4–9). However, C difficile isolated from CA-CDI belong to a range of different C difficile types.
Several studies that identified C difficile contamination in retail meat have reported a predominance of ribotype 027/NAP1/toxinotype III and ribotype 078/NAP7-8/toxinotype V strains (1,4,6–7,10–11). Although previous Canadian studies have described the presence of C difficile spores in meat products purchased in Ontario, Quebec and Saskatchewan (6,10), no Manitoba data have yet been available. Here, we report the occurrence and characteristics of C difficile in retail meat sold in Winnipeg, Manitoba.
METHODS
Ground beef and ground pork were purchased from three major food chains and three local meat shops in Winnipeg over a four-week period in January 2007. Every week, one beef and one pork sample were collected from each store. In total, 48 meat samples were tested for the presence of C difficile spores. The meat was purchased in the smallest available volume and was stored at refrigeration temperature (2°C to 8°C) until processed. For each meat sample, 20 g were placed into a sterile 50 mL tube and processed using an alcohol meat treatment protocol to eliminate vegetative bacteria. Briefly, 5 mL of sterile reverse osmosis (RO) water and 20 mL of 95% ethanol were added to the meat. The blend was mixed and kept at room temperature for 40 min, followed by centrifugation at 3000 rpm for 15 min at 4°C. The supernatant was discarded and the pellet was resuspended in 30 mL of sterile RO water. The mixture was then filtered using a coffee filter placed inside a sterile funnel to remove gross particles. The filtrate was centrifuged at 3000 rpm for 15 min at 4°C and the pellet was suspended in 100 μL of sterile RO water. This entire sample was then inoculated onto C difficile Moxalactam Norfloxacin (CDMN) agar medium. The plates were examined for growth after 72 h of anaerobic incubation at 35°C to 37°C. Suspected colonies (grey to pinkish-white in colouration with a distinct ‘horse manure’ odour) were enumerated and the presence of C difficile was confirmed with Gram staining, latex agglutination and a fluorescent appearance under ultraviolet light. The identity of C difficile isolates were also confirmed by the presence of the triose phosphate isomerase (tpi) housekeeping gene (12). The isolates were further tested for the presence of tcdA (enterotoxin gene), tcdB (cytotoxin gene), cdtB (binary toxin), and for the 18 bp deletion in tcdC (negative regulatory gene). Pulsed-field gel electrophoresis (PFGE)-SmaI was performed at the National Microbiology Laboratory (NML) (Winnipeg, Manitoba) to determine the pulsotype of C difficile isolates. The susceptibility of these three C difficile isolates to metronidazole, vancomycin, clindamycin, rifampicin, moxifloxacin and tigecycline was also tested at NML by E-test. Resistance to antimicrobials was determined based on the Clinical and Laboratory Standards Institute (Wayne, USA) (13–14) and United States Food and Drug Administration guidelines (15).
To determine how many spores needed to be present in 20 g of ground meat such that at least a single C difficile colony could be detected on CDMN plates, the limit of detection for the alcohol meat treatment protocol was determined. A known number of spores (2×107 obtained from C difficile strain 765 clinical isolate) were added to 20 g of a meat sample that was culture negative for C difficile. The meat sample was then processed using the alcohol treatment protocol. The final alcohol-treated sample was used to prepare serial 1:10 dilutions. One hundred microlitres of each dilution was inoculated and spread over the surface of a CDMN plate. After 72 h of anaerobic incubation at 35°C to 37°C, C difficile colonies were counted and the limit of detection was calculated.
RESULTS
C difficile was isolated from 6.3% (three of 48) of retail meat samples. C difficile isolates were detected in two ground beef samples (two of 24, [8.3%]) and one ground pork sample (one of 24, [4.2%]). Each C difficile isolate was from meat purchased at different stores, but the two beef isolates were from meat that was purchased during the same week. The limit of detection for the alcohol treatment protocol was 4 spores/g of ground meat.
All three C difficile isolates were positive for toxin A (tcdA) and toxin B (tcdB). Only one of the beef isolates (EH-4) was positive for binary toxin and had an 18 bp deletion in tcdC. This isolate belonged to NML PFGE type 0088 (Figure 1). The other beef isolate (FH-4) was NML PFGE type 0348. Both beef-isolated C difficile organisms were sensitive to all antimicrobial agents tested (Table 1). The only C difficile strain isolated from ground pork (CP-1) belonged to NML PFGE type 0139. This pork-isolated C difficile was resistant to both clindamycin (resistant at ≥8 μg/mL) (13–14) and moxifloxacin (resistant at ≥8 μg/mL) (13–14) (Table 1).
Figure 1).

Pulsed-field gel electrophoresis (PFGE)-SmaI fingerprints of three Clostridium difficile strains that were isolated from retail ground meat in Manitoba and other characteristics of the isolates. The PFGE banding patterns have been aligned with the results of other genetic analyses including: Polymerase chain reaction (PCR) detection of tpi (housekeeping gene), tcdA (toxin A gene), tcdB (toxin B gene), tcdC (negative regulatory gene) and the binary toxin gene. The sample designation is the strain stock number. The National Microbiology Laboratory (NML) pulsotype is also indicated.
TABLE 1.
Antimicrobial susceptibility of three Clostridium difficile strains isolated from retail meat sold in Manitoba
| C difficile isolate |
Minimum inhibitory concentrations (MIC) μg/mL* |
|||||
|---|---|---|---|---|---|---|
| Metronidazole | Vancomycin | Clindamycin | Rifampicin | Moxifloxacin | Tigecycline | |
| EH-4 | 0.125 | 0.5 | 0.75 | <0.002 | 1 | 0.064 |
| FH-4 | 0.125 | 0.5 | 0.75 | <0.002 | 1 | 0.064 |
| CP-1 | 0.125 | 0.5 | >256 | <0.002 | >32 | 0.094 |
Only the >256 μg/mL for clindamycin (resistant at ≥8 μg/mL) and >32 μg/mL for moxifloxacin (resistant at ≥8 μg/mL) are considered resistant; all other minimum inhibitory concentrations are considered sensitive
In total, approximately 4800 C difficile strains have been typed at NML, of these, 588 were from Manitoba. Of the 588 Manitoba isolates, 0.51%, 0.34% and 3.74% were pulsotypes 0088, 0348 and 0139, respectively (Figure 2). The 588 Manitoba isolates represent 140 unique PFGE types, 62 of which were exclusive to this province. Overall, 4.6% of total Manitoba pulsotypes had pulsotypes similar to C difficile isolated from retail meat in the present study. Pulsotype 0088 has been previously detected in two isolates from Manitoba, as well as five isolates from other provinces (Ontario and Alberta), whereas pulsotypes 0348 and 0139 have only been isolated in Manitoba.
Figure 2).
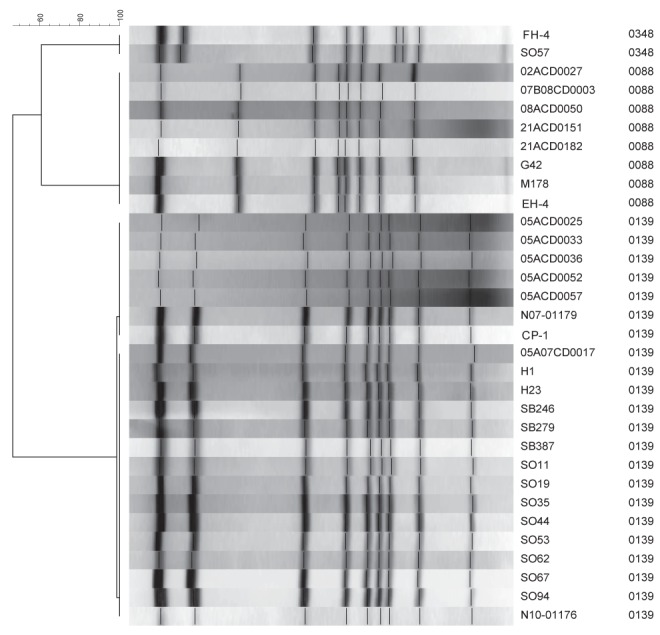
Pulsed-field gel electrophoresis-SmaI fingerprints of clinical Clostridium difficile isolates typed at the National Microbiology Laboratory (Winnipeg, Manitoba) that had the same pulsotypes as C difficile isolated from meat in the present study. Three C difficile isolates from ground beef and ground pork purchased in Manitoba (FH-4, EH-4 and CP-1) are also included in this figure. The isolates from patients in Alberta and Ontaro are 07B08CD0003 and 02ACD0027, 08ACD0050, 21ACD0151 and 21ACD0182, respectively. All other clinical isolates are from Manitoba patients
DISCUSSION
In the present study, we found C difficile in 6.3% of ground meat products in Winnipeg. Despite the fact that we analyzed only 48 meat specimens, our results were similar to those reported by other groups. Rodriguez-Palacios et al (10) conducted an across-Canada meat study and reported a C difficile prevalence of 6.1%. Molecular typing of our C difficile isolates from retail meat in Manitoba revealed that all of these strains have pulsotypes identical to strains previously isolated from patients with CDI in Manitoba. Finding strains of C difficile in meat with identical pulsotypes to those isolated from humans (9) suggests that meat could be a source of transient but frequent ingestion of C difficile and raises a concern regarding food as a source for C difficile acquisition that may lead to CDI (ie, CDI as a zoonosis).
C difficile resides in the digestive tract and can be isolated from several food animals such as pigs, cattle and chicken (1,3,6,11). Although C difficile can cause enteric disease in animals, the major public health concern is the shedding of C difficile by clinically healthy animals that may result in contamination of retail meat (1,3).
Different sources have been suggested for C difficile contamination in retail meat: deposition of spores in the animal’s muscle or other tissues; fecal or environmental contamination of carcasses; and contamination during meat processing (3–4,11). C difficile spores can survive the routine cleaning and disinfection procedures used in slaughter facilities and for meat processing equipment, which may promote their survival and proliferation (1) .
Meat products are not expected to be sterile, and contamination with Salmonella and Campylobacter is common (6). However, such bacteria are killed if the contaminated meat is thoroughly cooked at the recommended temperature (7). C difficile spores, however, are heat tolerant and can survive at a temperature of 71°C for 120 min (7) and 85°C for 5 min (16). This raises concerns regarding the persistence of C difficile in cooked meat and supports a potential zoonotic route of infection for this organism (2,6).
In general, C difficile can be found relatively commonly in various meat products, and strains isolated from retail meat share similar PFGE fingerprints with those isolated from CDI patients (1,3,11). Different sampling and isolation methods could account for differences in C difficile prevalence rates in meat, ranging from 2.4% (8) to 47.8% (4). However, these studies demonstrate that the presence of low levels of C difficile in retail meat is a common occurence throughout many different geographical regions (7,10). Differences in meat handling in various regions may also contribute to variations in spore detection rates. Our data support the assessment reported by Weese (1) that because the prevalence of CA-CDI is not as common as the prevalence of C difficile in retail meat, the existence of an obligatory association between ingestion of C difficile and CA-CDI is unlikely. In other words, ingestion of C difficile does not automatically lead to CDI and, in the majority of cases, it is likely that the presence of C difficile spores in the gastrointestinal tract of humans is transient. However, it is difficult to overlook the fact that strains of C difficile with the same pulsotypes are found in meat and patients with CDI. It is likely that additional factors (eg, concurrent antibiotics and lack of immune protection) are necessary before the C difficile spores present in meat can cause infection. The possibility of foodborne transmission of C difficile and conditions under which such C difficile ingested from meat develops into CDI warrants further investigation.
Acknowledgments
Monique Visser performed this study as part of her academic studies at Fort Richmond Collegiate in 2007.
REFERENCES
- 1.Weese JS. Clostridium difficile in food – innocent bystander or serious threat? Clin Microbiol Infect. 2010;16:3–10. doi: 10.1111/j.1469-0691.2009.03108.x. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin Microbiol Infect. 2007;13:457–9. doi: 10.1111/j.1469-0691.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Gould LH, Limbago B. Clostridium difficile in food and domestic animals: A new foodborne pathogen? Clin Infect Dis. 2010;51:577–82. doi: 10.1086/655692. [DOI] [PubMed] [Google Scholar]
- 4.Songer JG, Trinh HT, Killgore GE, Thompson AD, McDonald LC, Limbago BM. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis. 2009;15:819–21. doi: 10.3201/eid1505.081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloud J, Kelly CP. Update on Clostridium difficile associated disease. Curr Opin Gastroenterol. 2007;23:4–9. doi: 10.1097/MOG.0b013e32801184ac. [DOI] [PubMed] [Google Scholar]
- 6.Weese JS, Reid-Smith RJ, Avery BP, Rousseau J. Detection and characterization of Clostridium difficile in retail chicken. Lett Appl Microbiol. 2010;50:362–5. doi: 10.1111/j.1472-765X.2010.02802.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Palacios A, Staempfli HR, Duffield T, Weese JS. Clostridium difficile in retail ground meat, Canada. Emerg Infect Dis. 2007;13:485–7. doi: 10.3201/eid1303.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Abercron SM, Karlsson F, Wigh GT, Wierup M, Krovacek K. Low occurrence of Clostridium difficile in retail ground meat in Sweden. J Food Prot. 2009;72:1732–4. doi: 10.4315/0362-028x-72.8.1732. [DOI] [PubMed] [Google Scholar]
- 9.Songer JG. Clostridia as agents of zoonotic disease. Vet Microbiol. 2010;140:399–404. doi: 10.1016/j.vetmic.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Palacios A, Reid-Smith RJ, Staempfli HR, et al. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg Infect Dis. 2009;15:802–5. doi: 10.3201/eid1505.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupnik M, Songer JG. Clostridium difficile its potential as a source of foodborne disease. Adv Food Nutr Res. 2010;60:53–66. doi: 10.1016/S1043-4526(10)60003-4. [DOI] [PubMed] [Google Scholar]
- 12.Lemee L, Dhalluin A, Testelin S, et al. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (Toxin A), and tcdB (Toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol. 2004;42:5710–4. doi: 10.1128/JCM.42.12.5710-5714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; approved standard – Seventh edn. CLSI document M11-A7. Wayne: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 14.Bourgault AM, Lamothe F, Loo VG, Poirier L. In vitro susceptibility of Clostridium difficile clinical isolates from a multi-institutional outbreak in Southern Quebec, Canada. Antimicrob Agents Chemother. 2006;50:3473–5. doi: 10.1128/AAC.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein GE, Craig WA. Tigecycline: A critical analysis. Clin Infect Dis. 2006;43:518–24. doi: 10.1086/505494. [DOI] [PubMed] [Google Scholar]
- 16.Alfa MJ, Olson N, Buelow-Smith L. Simulated-use testing of bedpan and urinal washer disinfectors: Evaluation of Clostridium difficile spore survival and cleaning efficacy. Am J Infect Control. 2008;36:5–11. doi: 10.1016/j.ajic.2007.04.277. [DOI] [PubMed] [Google Scholar]


