Abstract
CSF-1, a key regulator of mononuclear phagocyte production, is highly expressed in the skeleton by osteoblasts/osteocytes and in a number of nonskeletal tissues such as uterus, kidney and brain. The spontaneous mutant op/op mouse has been the conventional model of CSF-1 deficiency and exhibits a pleiotropic phenotype characterized by osteopetrosis, and defects in hematopoiesis, fertility and neural function. Studies to further delineate the biologic effect of CSF-1 within various tissues have been hampered by the lack of suitable models. To address this issue, we generated CSF-1 floxed/floxed mice and demonstrate that Cre-mediated recombination using Meox2Cre, a Cre line expressed in epiblast during early embryogenesis, results in mice with ubiquitous CSF-1 deficiency (CSF-1KO). Homozygous CSF-1KO mice lacked CSF-1 in all tissues and displayed, in part, a similar phenotype to op/op mice that included: failure of tooth eruption, osteopetrosis, reduced macrophage densities in reproductive and other organs and altered hematopoiesis with decreased marrow cellularity, circulating monocytes and B cell lymphopoiesis. In contrast to op/op mice, CSF-1KO mice showed elevated circulating and splenic T cells. A striking feature in CSF-1KO mice was defective osteocyte maturation, bone mineralization and osteocyte-lacunar system that was associated with reduced dentin matrix protein 1 (DMP1) expression in osteocytes. CSF-1KO mice also showed a dramatic reduction in osteomacs along the endosteal surface that may have contributed to the hematopoietic and cortical bone defects. Thus, our findings show that ubiquitous CSF-1 gene deletion using a Cre-based system recapitulates the expected osteopetrotic phenotype. Moreover, results point to a novel link between CSF-1 and osteocyte survival/function that is essential for maintaining bone mass and strength during skeletal development.
Keywords: colony stimulating factor-1 (CSF-1), osteocytes, osteoblasts, dentin matrix protein 1 (DMP1), knockout mouse
Introduction
CSF-1 is essential for regulating the proliferation, differentiation and survival of mononuclear phagocytes in tissues undergoing active morphogenesis and remodeling. The effect of CSF-1 is mediated via its high-affinity c-fms tyrosine kinase receptor (CSF-1R) [1]. Full-length CSF-1 cDNA encodes for the secreted glycoprotein and proteoglycan forms of CSF-1, whereas a truncated mRNA transcript, resulting from alternative splicing in exon 6, encodes for the cell-surface form of CSF-1 [2,3]. CSF-1 is synthesized by many cell types including mesenchymal and epithelial-derived cells. Within bone, release of CSF-1 by osteoblasts stimulates the proliferation of osteoclast progenitors via the CSF-1R and, in combination with RANKL, leads to the formation of osteoclasts [4]. Osteocytes, cells derived from osteoblasts that form the lacunar-canalicular system, also secrete CSF-1 that stimulates osteoclastic repair of damaged bone [5].
The importance of CSF-1 in bone and other tissues has been shown in the spontaneous mutant op/op mouse [6]. These mice have a mutation in the coding sequence of the CSF-1 gene that results in CSF-1 deficiency, leading to a pleiotropic phenotype characterized by osteopetrosis, failure of tooth eruption, reduced growth rate and defects in fertility, neural function, dermal thickness and hematopoiesis. These pleiotropic effects may be due, in part, to a reduction in the number and function of macrophages that are present in most tissues [7]. However, some of these effects may also be due to loss of function of other cell types such as neuronal cells, muscle progenitors, osteoblasts and osteocytes that express CSF-1 and CSF-1R.
A major challenge has been to dissect out the function of CSF-1 within specific tissues. To overcome this limitation, we generated mice harboring conditional alleles of CSF-1. Crossing this floxed line with transgenic mice expressing Cre-recombinase in a cell-specific manner would allow us to determine the relative contribution of specific cell types and/or combinations of these cells in mediating the effect of CSF-1 in different tissues. For example, using this approach to selectively delete CSF-1 in bone cells would provide a means of circumventing fertility, mammary and neuronal defects observed in op/op mice. The rationale for establishing mice with a Cre dependent deletion of CSF-1 in all cells is, first, to validate that the CSF-1 floxed allele can generate a complete CSF-1 deletion. Secondly, it is important to determine whether the phenotype resembles that of op/op mice, since previous studies suggested in vivo biological activity of a truncated form of CSF-1 in op/op mice [8]. Differences between CSF-1KO and op/op mice may also point to novel biologic effects of CSF-1. Thus, in the present study, we took advantage of CSF-1 floxed mice to first generate mice with ubiquitous CSF-1 gene knockout.
The bone phenotype in op/op mice with respect to osteoclasts has been extensively studied; however, few studies have examined the effect of CSF-1 on osteoblasts and osteocytes [9,10]. In op/op and CSF-1R null mice, alterations in osteoblasts and bone formation were attributed to the lack of osteoclastic bone resorption. Interestingly, osteocytes orchestrate bone remodeling through dendritic processes that contact adjacent osteocytes, osteoblasts along bone surfaces and marrow cells including preosteoclasts [11]. Dentin matrix protein 1 (DMP1) is highly expressed in osteocytes and required for proper osteocyte formation and bone mineralization [12,13]. We have previously shown that DMP1 is down-regulated in the teeth of op/op mice, suggesting that CSF-1 may be a positive regulator of DMP1 [14].
To elucidate the biologic effects of CSF-1 in skeletal and nonskeletal tissues, we conditionally ablated CSF-1 by crossing CSF-1 floxed/floxed mice with Meox2Cre mice. The Meox2 promoter drives Cre expression throughout the epiblast [15]. At 3 weeks, CSF-1KO mice showed osteopetrosis, reduced tissue macrophages and B lymphopoiesis that resembled the phenotype of op/op mice. However, in contrast to op/op mice, CSF-1KO mice showed distinct alterations in T cells. Moreover, CSF-1KO mice showed dramatic defects in osteocytes, DMP1 expression and matrix mineralization. These findings provide the first evidence that ubiquitous CSF-1KO can be achieved using a Meox2Cre-based system. More importantly, our findings extend the role of CSF-1 as a key molecule in regulating DMP1 in osteocytes and maintaining the lacunar-canalicular network required for proper bone remodeling and mechanical strength.
Materials and Methods
Construction of the CSF-1 gene targeting vector and generation of CSF-1KO mice
The structure of the WT allele and the targeting vector for generating a conditional KO allele for CSF-1 (deleting exons 4, 5 and 6) is shown in Figure 1A. A floxed construct containing a 5′ loxP site in intron 3 of the CSF-1 gene and a 3′ loxP site with a FRT-polII-neo-FRT-loxP cassette in intron 6 was prepared. Briefly, a 12.5kb Nhe1 BAC fragment was cloned into the ΔLacZ-MHΔXhoI plasmid (Dr. Harris) with the 5′ loxP cassette inserted into the EcoRI site and the FRT-polII-neo-FRT-loxP cassette was inserted into the XhoI-Spe1 site, resulting in a NheI/SpeI 19.1kb fragment. The construct was linearized with SalI and electroporated into C57BL/6 ES cells. Genomic DNA, extracted from G418-resistant clones, was digested with either BglII or SpeI and subjected to Southern blotting using a 3′ probe or 5′ probe directed at the flanking sequences outside the targeting vector. The correctly targeted alleles yielded a 9.9kb BglII or 7.2kb SpeI band that was distinct from the 6.6kb BglII or 16.8kb band derived from the wild-type allele (Figure 1B). Selected clones with a properly targeted allele were injected into C57BL/6 blastocysts to generate chimeric mice, which were then mated with wild-type C57BL/6 mice to establish germline transmission. Heterozygous CSF-1flox mice then were crossed with beta-actin-Flip recombinase mice to remove the neo cassette. Homozygous CSF-1flox-neo/CSF-1flox-neo mice were then bred with C57BL/6 Meox2Cre mice (Jackson Laboratory, Bar Harbor, MA) to generate mice with conditional knock out of CSF-1 in all tissues (designated CSF-1KO). As controls, CSF-1flox-neo/CSF-1flox-neo and CSF-1flox-neo/CSF-1+ were used (designated WT). At 3 weeks of age, CSF-1KO and WT were analyzed for CSF-1 expression, bone phenotype, tissue macrophage numbers and hematopoiesis. Animal protocols were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee.
Fig. 1.
Targeted disruption of the mouse CSF-1 gene: absent circulating CSF-1, decreased growth rate, failure of tooth eruption. A) CSF-1 fx/fx targeting vector and WT allele showing exons 1 to 9, restriction enzyme sites, loxP sites, neomycin resistance cassette and probes used for Southern blot (5′ Spe1 and 3′ BglII probes). B) Southern blot analysis of genomic DNA from G418-resistant ES cell clones using the probes shown in A. Asterisks indicate clones with correctly targeted allele. C) Serum CSF-1 concentration of WT and CSF-1KO mice measured by ELISA. Mean ± SE (n = 3 mice for each genotype) ***p<0.001 WT vs CSF-1KO. D) CSF-1 mRNA expression in primary calvarial osteoblasts by RT-PCR. WT, but not CSF-1KO, mice show the 381 kDa CSF-1 band. The 18S control is shown in lower panel. E) Growth curves for mice weighed at weekly intervals from 1 to 3 weeks of age. Mean ± SE (n = 16 mice for each genotype) ***p<0.001 WT vs CSF-1KO. F) Tooth phenotype: incisors failed to erupt in CSF-1KO mice.
CSF-1 protein and mRNA expression
Bone and tissue lysates and serum samples were assayed for CSF-1 using the Quantikine enzyme linked immunoassay kit (R&D Systems, Inc., Minneapolis, MN) that specifically detects mouse CSF-1. For CSF-1 mRNA analysis, total RNA was isolated from primary calvarial osteoblasts with TRI Reagent (Sigma-Aldrich, Milwaukee, WI) and subjected to RT-PCR using specific primers as previously described [16].
Radiographic and microCT analysis
Skeletal radiographs were performed using an MX-20 Faxitron unit equipped with a digital camera (Faxitron X-ray Corp., Lincolnshire, IL). Tibias were examined by microCT using a high-resolution scanner (SkyScan 1172, Skyscan, Kontich, Belgium) at 5.75 um nominal resolution. The volume of interest (VOI) was 1.15 mm from the proximal growth plate. After thresholding, trabecular bone 3-dimensional (3-D) parameters were calculated within a 1.8 mm long VOI contoured to the endosteal boundary. Cortical bone thickness from the outer to inner surfaces was measured in the diaphysis.
Tissue Preparation and Histological analysis
Skeletal tissues were excised, fixed in 10% formalin, decalcified in 12% EDTA and embedded in paraffin. Five-um thick sections were stained with H&E to evaluate morphology, tartrate resistant acid phosphatase (TRAP) to identify osteoclasts and TUNEL to detect apoptosis as previously described [17,18]. The presence of unmineralized bone matrix was examined using an Alcian blue stain. Immunohistochemical staining was performed for CD31 to localize endothelial-lined vessels in bone using a rabbit anti-CD31 antibody (1:500, sc-1506, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) [18]. Expression of DMP1 protein in osteocytes was evaluated using a rabbit anti-mouse DMP1 antibody (1:1000, 3844-100, Biovision Research Products, Mountain View, CA). To assess macrophages in bone and soft tissues, immunostaining for F4/80, a marker of macrophages, was performed using a rat anti-mouse F4/80 antibody (1:100, MCA497, Serotec, Raleigh, NC). Following incubation with each primary antibody, sections were incubated with a standard HRP-polymer detection kit (Biocare Medical, Concord, CA) and 3,3′-diaminobenzidine substrate was used to visualize immunoreaction sites. In situ hybridization for DMP1 was performed using a digoxigenin-labeled antisense RNA (cRNA) probe as previously described [19]. Histomorphometric measurements to quantitate the number of osteoclasts, apoptotic cells and F4/80-positive macrophages were performed in at least three mice of each genotype using a Bioquant image analysis system and Bioquant Osteo II software (Bioquant Image Analysis Corp., Nashville, TN). In bone, data are presented as the number of osteoclasts or macrophages per mm/bone surface (BS). Macrophages in soft tissues were enumerated in 5-10 random fields at 20-40X magnification and the average number per mm2 was calculated.
Transmission (TEM) and Scanning Electron Microscopy (SEM)
The ultrastructure of bone cells and mineralization was examined using TEM and SEM. For TEM, undecalcified bone specimens were fixed in 4% paraformaldehyde/1% glutaraldehyde, embedded in epoxy resin and 5 um-thick sections were stained with Toluidine blue. Ultrathin sections were stained with uranyl acetate and lead citrate and examined using a Joel 1230 transmission electron microscope (Joel Ltd., Tokyo, Japan) [18]. Backscattered SEM was performed according to established protocol [20]. To visualize the osteocyte lacunar-canalicular system, SEM of acid-etched, resin-casted bone samples was performed as previously described [12,13] and images taken using a FEI/Philips XL30 field emission environmental SEM (Phillips, Hillsboro, OR).
Hematologic parameters by Flow Cytometry
Peripheral blood, bone marrow and spleen cells were harvested and analyzed for the relative presence of mature lymphoid and myeloid cell populations by incubating them with antibodies directed against surface markers for B cells (B220-APC), T cells (CD3-PE) and myeloid cells (Gr1-FITC and CD11b-PE) (BD Biosciences, San Diego, CA). 7AAD was added to exclude dead cells and flow cytometric analysis was performed on a FACS Canto (BD Biosciences, San Diego, CA). Total white blood cell counts were determined using a hemocytometer.
Statistical Analysis
The means and standard errors (SE) of all numeric data were calculated. Data were analyzed statistically using the unpaired Student t test. Differences were considered statistically significant for comparisons of data sets yielding p ≤ 0.05.
Results
Targeted disruption of the mouse CSF-1 gene
The targeting vector (Figure 1A) was electroporated into ES cells and of the 372 G418-resistant clones obtained, three were identified as the correct homologous recombinants (Figure 1B). Of 11 chimeras created with these clones, three showed germline transmission. At birth, the frequency of offspring of CSF-1KO heterozygous crosses was as expected for a gene inherited in a Mendelian fashion. CSF-1 protein analysis by ELISA indicated that serum shown in Figure 1C and all tissues including bone (data not shown) in CSF-1KO mice were devoid of detectable CSF-1. Total RNA isolated from calvarial osteoblasts and analyzed by RT-PCR, showed the expected 381-bp CSF-1 transcript in WT mice, whereas no CSF-1 mRNA was detected in CSF-1KO mice (Figure 1D). Mice heterozygous for the KO allele resembled WT controls, whereas homozygous CSF-1KO mice showed an osteopetrotic phenotype characterized by failure of tooth eruption, domed skull, delayed growth rate and decreased body weight (Figure 1E and F).
Skeletal phenotype of CSF-1KO mice
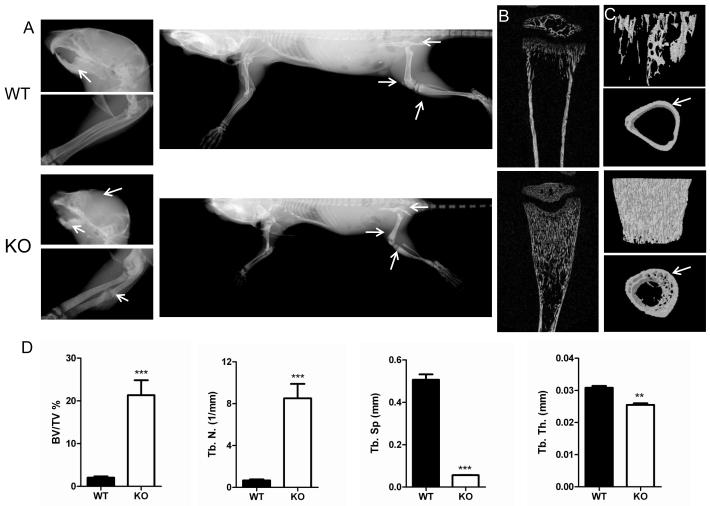
The skeletal phenotype of WT and CSF-1KO mice was then compared. By x-ray, the long bones of CSF-1KO mice were short and showed skeletal sclerosis with dense radiopaque bone in the iliac crest, tibial and femoral metaphysis (Figure 2A). CSF-1KO mice were predisposed to spontaneous fractures, whereas fractures were not identified in WT mice. Two dimensional (2-D) and 3-D microCT images shown in Figures 2B and 2C, respectively, demonstrate increased bone mass in CSF-1KO tibias compared to WT. Quantification of 3-D volumetric microCT trabecular structure parameters calculated in a defined VOI in proximal tibias confirmed increased %BV/TV, greater trabecular number and decreased space between trabeculae in CSF-1KO mice (Figure 2D). The average trabecular thickness in CSF-1KO was slightly decreased compared to WT. The cortical bone in CSF-1KO tibias was substantially reduced in thickness (0.042+/−0.014 vs WT 0.078+/−0.003), contiguous with intramedullary trabeculae and poorly demarcated from the marrow cavity (Figure 2C).
Fig. 2.
Comparative x-ray, microCT and morphometric bone parameters in WT and CSF-1KO mice. A) Representative x-ray images of head (arrows indicate domed skull and sclerotic alveolar bone with unerupted teeth in CSF-1KO), forelimbs (arrows indicate spontaneous fracture in CSF-1KO) and whole body (arrows indicate sclerosis in iliac crest, femoral and tibial metaphysis in CSF-1KO). Representative B) 2-D microCT images of tibias and C) 3-D anterior-posterior microCT views of the tibial metaphyses (upper panel) and cortical bone slices along the diaphysis (lower panel). CSF-1KO mice show dramatically increased trabecular bone and decreased cortical thickness (arrows) compared to WT. D) 3-D trabecular structure parameters calculated in a defined VOI in proximal tibias included: percent bone volume/tissue volume [% BV/TV], trabecular number [Tb.N], trabecular space [Tb. S], trabecular thickness [Tb. Th.]). Bar graphs show Mean ± SE (n = 3-4 mice for each genotype) ***p<0.001, **p<0.01 WT vs CSF-1KO.
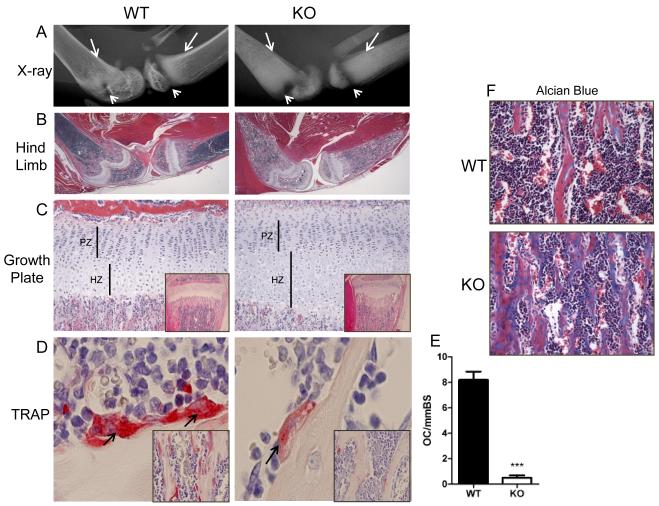
High power radiographs of CSF-1KO hind limbs shown in Figure 3A showed the poorly demarcated cortical bone, metaphyseal sclerosis and a noticeably enlarged radiolucent growth plate. On histological sections, CSF-1KO hind limbs showed osteopetrosis with expansion of the hypertrophic chondrocyte region that correlated with the enlarged radiolucent growth plate on x-ray (Figure 3B and 3C). However, the overall cellular distribution and zonal organization of the growth plate in CSF-1KO was similar to WT. Osteopetrosis was associated with a dramatic reduction in osteoclasts and impaired bone resorption with bone trabeculae containing prominent cartilage cores extending into the marrow cavity (Figure 3B and 3C, inset). TRAP-positive osteoclasts were identified in WT, whereas rare osteoclast cells, mainly weak TRAP-positive and mononucleated, were detected in CSF-1KO bone (Figure 3D and 3E). Alcian blue stain demonstrated the increased unmineralized bone matrix (staining blue) in CSF-1KO mice (Figure 3F).
Fig. 3.
Hind limb x-ray and histology of cartilage and osteoclasts in WT and CSF-1KO mice. A) Representative high power x-ray images of femur and tibia (arrows indicate poor demarcation between cortical bone and marrow cavity in CSF-1KO; arrowheads indicate expanded radiolucent growth plate in CSF-1KO). B) Low power images of H&E stained sections of the same hindlimbs shown in A cut in the midsagittal plane. C) High power images of epiphyseal regions (PZ, proliferating zone; HZ, hypertrophic zone; inset, low power photomicrograph showing expanded growth plate and bone trabeculae obliterating marrow cavity in CSF-1KO). D) TRAP stain (arrows indicate strong TRAP+ multinucleated osteoclasts in WT and weak TRAP+ mononucleated osteoclasts in CSF-1KO; inset shows numerous osteoclasts along bone trabeculae in WT, whereas rare TRAP+ cells are visualized in CSF-1KO). E) Histomorphometric quantification of TRAP+ osteoclasts (OC) per mm bone surface (BS) in WT and CSF-1KO. Mean ± SE (n = 4 mice for each genotype) ***p<0.001 WT vs CSF-1KO. F) Alcian Blue stain demonstrates unmineralized bone trabeculae (stains blue) in CSF-1KO. Original magnification: B: x1.25, C: x10 (inset x4), D: x40 (inset x20), F: x20.
In addition to osteoclasts, significant defects in other bone cells, vascularity and mineral deposition were identified in CSF-1KO bone. In WT mice, osteoblasts were elongated with one side adjacent to the bone surface and the other side facing the marrow cavity indicating their polarity. In contrast, osteoblasts in CSF-1KO mice were disorganized with loss of polarity and displayed abnormal entrapment in matrix (Figure 4A and 4B). Loss of directional matrix deposition toward the bone surface by CSF-1KO osteoblasts likely contributed to their entrapment. The transition of osteoblasts to form osteocytes was impaired in CSF-1KO mice. A striking feature in CSF-1KO mice were irregularly shaped and abnormal clusters of osteocytes within lacunar spaces, whereas only single osteocytes were seen in lacunar spaces in WT (Figure 4C, left panels). To determine whether these defects were associated with abnormal DMP1 protein and mRNA expression levels, we performed DMP1 immunostaining (Figure 4C, right upper panels) and in situ hybridization (Figure 4C, right lower panels). Figure 4C (right panels) shows that DMP1 protein and mRNA are highly expressed in WT osteocytes, whereas DMP1 was significantly reduced in CSF-KO osteocytes. DMP1 protein was also present along WT, but not CSF-1KO, lacunar walls. Increased alterations in CSF-1KO osteoblasts were noted from the metaphysis to the diaphysis, with osteoblasts becoming progressively flattened and showing foci of detachment and loss from trabeculae (Figure 4D).
Fig. 4.
Osteoblast and osteocyte defects in CSF-1KO mice. Comparative histology of WT and CSF-1KO metaphysis. A) WT is composed of organized trabeculae parallel to the longitudinal axis of the tibia, whereas metaphyseal bone in CSF-1KO shows a meshwork of irregularly shaped trabeculae with transverse connections between trabeculae. Osteoblast and osteocyte morphology. B) Osteoblasts in CSF-1KO are abnormally clustered, disorganized and lack the normal polarity of WT osteoblasts (circles). C, left panels) WT osteoblasts (asterisks) show directional matrix deposition and normal differentiation into osteocytes; a single osteocyte is identified in lacunae (arrows). CSF-1KO osteoblasts (asterisks) show impaired transition to osteocytes; clusters of abnormally shaped osteocytes are identified in lacunae (arrows). C, right panels) DMP1 immunohistochemistry (upper panel) and in situ hybridization (lower panel): Compared to WT, DMP1 protein (brown color) and mRNA expression (dark blue color) in CSF-1KO osteocytes are dramatically reduced (arrows). D) CSF-1KO osteoblasts (arrow) become more flattened and focally detached in the diaphysis. E) In some foci, bone marrow cells and endothelial cells of vessels in CSF-1KO directly contact bone surfaces (arrows). Original magnification: A: x10, B: x40, C: x40, D: x10, F: x20. BM, bone marrow; EC, endothelial cell.
Alterations in the vascular architecture were identified in CSF-1KO mice. Due to the focal loss of osteoblasts along trabeculae in CSF-1KO mice, vascular sinusoids and marrow cells abutted directly on unmineralized matrix (Figure 4E). Sinusoids lined by CD31 positive endothelial cells were also smaller with narrow lumens in CSF-1KO compared to WT (Figure 5A). Histology of cortical bone correlated with x-ray and microCT images. The cortical bone in WT mice was clearly demarcated from bone marrow, dense and well-organized with regularly interspersed osteocytes; a uniform layer of osteoblasts lined the endosteal surface. In contrast, cortical bone in CSF-1KO mice was thin, interrupted by marrow elements, and showed variable matrix density and randomly scattered osteocytes; irregularly distributed osteoblasts lined the endosteal surface (Figure 5B). Under polarized light, WT cortical bone contained long, dense collagen fibrils that were well-oriented in a parallel fashion to form lamellar bone, whereas in CSF-1KO bone, collagen fibrils were decreased, short and in loose clusters that lacked lamellar structure (Figure 5C).
Fig. 5.
Altered vascularity, cortical bone and osteocyte viability in CSF-1KO mice. A) CD31+ immunostain of tibial metaphysis. Vascular sinusoids are narrowed in CSF-1KO compared to widely patent vessels in WT (brown color, arrows). B) H&E sections of cortical bone. WT cortical bone is dense with regularly interspersed osteocytes and the endosteal surface is lined by a uniform layer of osteoblasts (arrow). CSF-1KO cortical bone is thinner (bar), less dense than WT, contains few randomly interspersed osteocytes and the endosteal surface is lined by disorganized osteoblasts (arrow). C) Collagen fibrils visualized under polarized light. WT cortical bone shows dense, parallel arrangement of collagen fibrils, whereas in CSF-1KO, collagen fibrils are decreased, shortened and disorganized. D) TUNEL analysis for apoptosis. WT bone showed rare apoptotic osteocytes whereas CSF-1KO showed increased osteocyte apoptosis (brown stain, arrows). Inset shows karyorrhexis of apoptotic nuclei. E) Histomorphometric quantification of TUNEL. Apoptotic osteocytes were significantly increased in CSF-1KO bone. Mean ± SE (n = 4 mice for each genotype) ***p<0.001 WT vs CSF-1KO.
As CSF-1KO osteocytes were embedded in abnormal matrix, we examined their viability using TUNEL stain and subjected bone samples to ultrastructural analysis. Little apoptosis was identified in WT mice, whereas increased numbers of TUNEL positive osteocytes were observed in CSF-1KO bone (Figure 5D). Quantification of TUNEL by histomorphometry showed significantly increased apoptotic osteocytes in CSF-1KO compared to WT bone (Figure 5E). Plastic embedded Toluidine blue stained sections confirmed osteocyte loss and empty lacunae in CSF-1KO but not in WT bone (Figure 6A). By TEM, WT bone showed polarized osteoblasts along bone surfaces and osteocytes with well-developed dendritic processes surrounded by homogenous dense mineralized matrix (black color) (Figure 6B). In contrast, CSF-1KO osteoblasts were disorganized and osteocytes were irregular in contour with poorly defined dendrites and resided in patchy mineralized matrix (Figure 6B and 6C). Micropetrosis, representing lacunar spaces filled with mineral after osteocyte death, was also present in CSF-1KO (Figure 6B).
Fig. 6.
Impaired osteocyte maturation and lacunar-canalicular system in CSF-1KO mice. Toluidine blue stained sections (A), TEM (B,C) and SEM (D,E) images of WT and CSF-1KO bone. A) WT shows well-organized trabecular and cortical bone with osteocytes in lacunae, whereas CSF-1KO shows poor demarcation between trabecular and cortical bone and several empty lacunae (arrows). B,C) WT osteoblasts are layered along bone surfaces with one side adjacent to the bone surface indicating their polarity, whereas CSF-1KO osteoblasts are irregularly shaped and disorganized. Micropetrosis was identified in CSF-1KO by mineralization of lacunar spaces (arrow). WT osteocytes were embedded in well-mineralized matrix and showed well-developed dendritic processes (arrows). Osteocytes in CSF-1KO were surrounded by patchy mineralized matrix (arrowhead) and showed poorly formed dendrites (arrows). D) Backscattered SEM and E) SEM of acid-etched, resin-casted osteocyte lacunar-canalicular system. Note that CSF-1KO osteocyte lacunae are disorganized, decreased in number and have rough walls. There is also a large amount of osteoid present in the matrix. TB, trabecular bone; CB, cortical bone, OB, osteoblast; OC, osteocyte.
Analysis by SEM further documented the abnormalities in the distribution and organization of the CSF-1KO osteocyte lacunar-canalicular system. On backscatter SEM, lacunae in WT mice were well-organized and showed uniform distribution and shape, whereas in CSF-1KO mice, lacunae were disorganized, decreased in number, randomly oriented, irregularly contoured and surrounded by hypomineralized bone (Figure 6D). Resin-casted SEM revealed WT osteocytes with dendrites surrounded by lacunae with smooth walls. In contrast, CSF-1KO bone showed lacunae with rough surfaces, markedly deformed osteocytes with very few dendrites and a significant reduction in the overall density of the dendrite network. Taken together, these findings indicate that CSF-1 is crucial for maintaining osteocyte viability, dendrite contacts, matrix mineralization and vascularity.
Tooth defects in CSF-1KO mice
Since failure of tooth eruption as well as tooth matrix defects occur with CSF-1 deficiency [14], mandibles of CSF-1KO mice were analyzed. Similar to CSF-1KO hind limbs (Figure 3D), rare TRAP positive osteoclasts were identified in the alveolar bone (data not shown). Unerupted teeth in CSF-1KO mice resembled those reported in op/op mice. Failure of bone resorption in CSF-1KO mice led to impaired root formation and a narrow dental follicle invaded by bone (Figure 7A and 7B). Tooth defects in CSF-1KO mice included a widened predentin layer, an irregular mineralization front and unevenly mineralized dentin (note alternating light and dark dentin staining) (Figure 7B).
Fig. 7.
Dental defects and deficiency of tissue macrophages in CSF-1KO mice. A,B) H&E stained sections of first molars. CSF-1KO mice show impaired root formation (arrows) compared to WT and a narrow dental follicle (bars) invaded by bone (arrows). B) Dentin morphology. CSF-1KO shows a widened predentin layer (bar) with an irregular mineralization front and unevenly mineralized dentin (irregular light and dark staining). C,D) Immunostain and quantification of F4/80+ macrophages in dental pulp. Macrophages are dramatically reduced in CSF-1KO pulp compared to WT (brown color). Mean ± SE (n = 4 mice for each genotype) ***p<0.001. E-I) Quantification of F4/80 cell densities in tissues (bar graphs) and representative F4/80 immunostained sections of lung, liver, skin, testes, uterus, synovium and spleen are shown. CSF-1KO mice show decreased macrophages in: lung alveoli (arrow), Kuppfer cells in liver surrounding central vein (arrow), skin dermis associated with reduced dermal thickness (bar), testes adjacent to Leydig cells (arrow), uterus (arrow), synovial membrane (arrow) and spleen. Original magnification A, x4, B, C, F, G, x20. Bar graph shows mean macrophages/mm2 ± SE (n = 3-5 mice for each genotype) *p<0.05, **p<0.001, ***p<0.001 WT vs CSF-1KO. DF, dental follicle; PD, predentin; D, dentin.
Reduced mononuclear phagocyte production in CSF-1KO mice
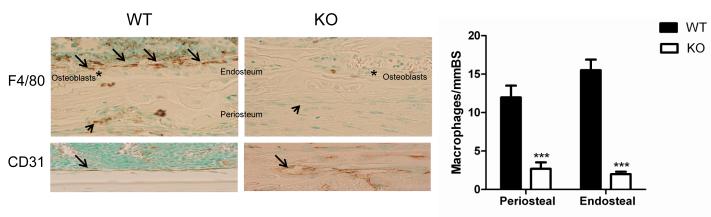
Previous studies showed reduced F4/80+ macrophages in many tissues of op/op mice [21-25]. We therefore assessed the number and distribution of macrophages in multiple organs of CSF-1KO mice by determining the F4/80+ cell density. Similar to op/op mice, macrophages in CSF-1KO were significantly decreased in the dental pulp (Figure 7C and 7D) and other tissues including liver, spleen, lung, skin (both dermal macrophages and epidermal Langerhans cells), colon, kidney, testes, uterus, adipose tissue, synovium, tendon and thymus (Figure 7E-I). The pattern of macrophage deficiency in these tissues resembled that in op/op mice. For example, in the liver, Kupffer cells were preferentially reduced in the centrilobular region; in the testes, macrophages were decreased adjacent to Leydig cells; in the kidney, macrophages were decreased in cortex and medulla; and in the synovium, macrophages were decreased along the synovial lining. Epidermal Langerhans cells were somewhat lower than previously reported in 2 week op/op mice, but correlate with the decline in this population with age [23,26]. Macrophages within bone were also evaluated: periosteal macrophages as well as macrophages adjacent to the osteoblasts lining the endosteal surface (so called osteomacs) were decreased in CSF-1KO mice (Figure 8, upper panel) [27]. Interestingly, WT endosteum was lined by a uniform layer of CD31 positive endothelial cells, whereas this layer was interrupted by bone trabeculae along the endosteum in CSF-1KO mice (Figure 8, lower panel).
Fig. 8.
Endosteal and periosteal F4/80 macrophages in CSF-1KO and WT mice. Immunostained cortical bone sections and quantification of F4/80+ macrophages (bar graph) are shown. Upper panel: Osteomacs in WT mice are identified as a canopy of cells covering osteoblasts along the endosteal surface (arrows); F4/80+ macrophages are also present along the periosteum (arrowhead). Osteomacs and periosteal macrophages are decreased in CSF-1KO mice. Original magnification x20. Bar graph shows mean macrophages/mm bone surface (BS) ± SE (n = 5 mice for each genotype) ***p<0.001 WT vs CSF-1KO. Lower panel: CD31 immunostain of WT and CSF-1KO endosteal surface shows abnormal vascular pattern in CSF-1KO. Asterisks indicate osteoblasts.
Altered hematopoietic parameters in CSF-1KO mice
Since osteoblasts and osteoclasts play important regulatory roles in the hematopoietic system, alterations in these populations may affect hematopoiesis. We therefore examined the hematopoietic status of CSF-1KO mice (Table 1, Figure 9). First, we observed a 15-fold reduction in bone marrow cellularity of CSF-1KO mice compared to WT (Table 1, Figure 9A), which was most likely due to the decreased marrow space associated with osteopetrosis (Figure 3B). In addition to a significant overall decrease in the number of both myeloid and lymphoid cells in CSF-1KO mice (Figure 9A), the ratio between these lineages was reversed (Table1, Figure 9B and C). We then analyzed the spleen to determine if it was serving as a compensatory site of hematopoiesis. When corrected for body weight, the spleen size in CSF-1KO mice was significantly less than WT (~1.4-fold) [28]. The frequency of myeloid cells was increased ~2-fold, which is likely sufficient to compensate for the loss of bone marrow myelopoiesis (Table 1, Figure 9D). This was reflected in the peripheral blood, where the total number of CD11b+ myeloid cells was similar between both genotypes, although Gr1-/lo subpopulations of monocytes were significantly decreased and the Gr1-hi subpopulation of granulocytes was increased in CSF-1KO compared to WT (Table 1, Figure 9E). However, B cell lymphopoiesis was reduced in all three hematopoietic tissues analyzed (Table 1, Figures 9B and F). Interestingly, the T cell lineage seems to be increased in both spleen and peripheral blood, indicative of the fact that T cell lymphopoiesis at this stage of development predominantly occurs outside the bone marrow (Table 1, Figure 9F).
Table 1.
Hematopoietic Parameters
| Parameter | WT (± SE) | CSF-1KO (± SE) |
|---|---|---|
| Bone Marrow | ||
| Cells (× 10−6 /femur)*** | 17.6 ± 0.8 | 1.2 ± 0.3 |
| B220+ (%)*** | 41.4 ± 0.9 | 18.6 ± 2.5 |
| CD3+ (%) | 0.9 ± 0.0 | 1.4 ± 0.3 |
| CD11b+Gr1− (%) | 1.0 ± 0.1 | 2.1 ± 0.3 |
| CD11b+Gr1+ (%)*** | 20.0 ± 0.9 | 45.4 ± 3.6 |
| Spleen | ||
| Wgt/body wgt (mg/g)** | 6.3 ± 0.4 | 4.6 ± 0.3 |
| B220+ (%)*** | 74.8 ± 1.9 | 44.7 ± 3.1 |
| CD3+ (%)*** | 11.2 ± 0.8 | 23.5 ± 1.5 |
| CD11b+Gr1− (%) | 3.1 ± 0.5 | 2.9 ± 0.4 |
| CD11b+Gr1+ (%)** | 6.5 ± 0.6 | 15.7 ± 2.3 |
| Blood | ||
| WBC (× 10−6/ml) | 4.0 ± 0.4 | 4.2 ± 0.7 |
| B220+ (%)** | 52.5 ± 2.7 | 26.1 ± 7.2 |
| CD3+ (%)** | 14.2 ± 1.2 | 32.0 ± 4.1 |
| CD11b+Gr1− (%)* | 8.0 ± 1.2 | 3.4 ± 0.7 |
| CD11b+Gr1lo (%)* | 5.9 ± 0.8 | 3.1 ± 0.4 |
| CD11b+Gr1hi (%) | 12.0 ± 2.7 | 19.8 ± 5.3 |
Data expressed as Means ± SE (n = 6 mice per genotype)
Significant difference between WT and CSF-1KO,
p<0.001,
p<0.01,
p<0.05.
Fig. 9.
Myeloid and lymphoid lineage parameters in WT and CSF-1KO mice. A) Average number ± SE of mononuclear cells (MNC), B220+ and CD11b+Gr1+ cells in the bone marrow. Representative FACS profiles of B) B cells (B220+) and C) myeloid cells (CD11b+Gr1+) in the bone marrow, D) myeloid cells (CD11b+Gr1+) in the spleen, E) CD11b+Gr1−, CD11b+Gr1lo, CD11b+Gr1hi myeloid subpopulations in the peripheral blood and F) B cells (B220+) and T cells (CD3+) in the spleen. The percentage of positive cells is shown within each FACS profile. The means and percentages from FACS analysis for 6 mice of each genotype are presented in Table 1.
Discussion
In this study, we have shown that ubiquitous CSF-1KO can be achieved using a Meox2Cre-based system and results in viable offspring with an osteopetrotic phenotype. Serum and tissues from C57BL/6 CSF-1KO mice in which both CSF-1 alleles were targeted by the insertion of a loxP cassette to delete exons 4, 5 and 6 failed to express CSF-1 indicating that they are truly nullizygous. CSF-1KO mice showed impaired bone resorption due to a dramatic reduction in osteoclasts that led to failure of tooth eruption and delayed growth that resembled the phenotype of 3 week old B6C3 op/op mice [6,7]. There was a similar depletion in circulating monocytes and F4/80+ tissue macrophages that would contribute to pleiotropic effects including altered fertility. These findings validate our CSF-1KO model; however, CSF-1KO mice also showed distinct alterations in T cells and osteocytes not previously reported in op/op mice. Thus, the described phenotype of CSF-1KO mice establishes a different and more appropriate CSF-1 null control model which will be important for our future studies to delineate tissue-specific effects of CSF-1 using the CSF-1 floxed mice.
The interplay between osteoclasts, osteoblasts and osteocytes is essential for proper bone modeling and remodeling. Studies in op/op mice have focused on the lack of osteoclast-mediated bone resorption that presumably leads to defects in bone formation. In op/op mice, diaphyseal osteoblasts were reported to be flatter compared WT osteoblasts and osteocytes were embedded in poorly mineralized matrix [9]. Similar features including loss of osteoblast polarity were described in CSF-1R null mice and, due to the lack of CSF-1R expression in osteoblasts, the authors indicated that impaired osteoblast function was due to failure of bone resorption and release of osteogenic factors from the bone matrix or directly from osteoclasts [26]. Abnormal bone formation in op/op mice may be due, in part, to the lack of bone resorption; however, recent studies indicate that CSF-1R is expressed in osteoblasts and osteocytes [16,29]. Our findings in CSF-1KO mice suggest a potential role for CSF-1 in regulating osteoblasts/osteocytes directly and/or indirectly via DMP1.
Osteocytes are the main mechanosensory cells in bone and have long dendritic processes encased in a lacunar-canalicular network that enables contact with adjacent osteocytes, osteoblast lining cells and marrow cells [11,30]. Osteocytes direct osteoblasts and osteoclasts to sites of remodeling. DMP1 is highly expressed in osteocytes, lacunar walls and matrix and, to a lesser extent in osteoblasts. It is essential for osteocyte formation and mineralization as shown in Dmp1 null mice, where absence of DMP1 leads to a hypomineralized bone phenotype and defective osteocyte maturation [12]. A striking feature in CSF-1KO mice, not previously reported in op/op mice, was the dramatic reduction in DMP1 mRNA and protein levels in osteocytes that likely contributed to the altered osteoblast polarity and their ability to differentiate into osteocytes. Interestingly, CSF-1KO mice showed several morphologic abnormalities with SEM that resembled Dmp1 null mice including reduced/uneven mineralization, poorly formed lacunar-canalicular system with rough lacunar walls, reduced number of canaliculi and osteocytes with abnormal dendrites [12,13]. The growth plate in 10 day old Dmp1 null mice also showed expansion of the hypertrophic zone and whether expansion of this zone in CSF-1KO mice is related to decreased DMP1 expression would be of interest [13].
In addition to DMP1, absence of CSF-1 itself in CSF-1KO mice likely contributed to matrix defects by altering collagen fibrils. Collagen fibrils act as scaffolds for growth of mineral crystals and are a major determinant of matrix mineralization and the extent of collagen matrix [31]. The proteoglycan form of CSF-1 binds to extracellular matrix proteins and has been identified in pepsin extracted bone collagen [32]. The ability of CSF-1 to normalize collagen cross-links and mineral crystallinity has been shown in CSF-1 deficient tl/tl rats injected with CSF-1 [33]. Matrix integrity is also influenced by hydration that is dependent on the vascular bed [34]. CSF-1 has a potent stimulatory effect on angiogenesis, probably through monocyte-derived angiogenic factors [35]. Our findings in CSF-1KO mice showing disturbed vascular channels similar to that observed in op/op mice could compromise the mineralization process.
Although the exact mechanism by which osteoblasts transition into osteocytes and incorporate into matrix is not fully understood, osteocyte defects in CSF-1KO suggest that CSF-1 may act in an autocrine and/or paracrine fashion to stimulate DMP1 that, in turn, promotes osteocyte formation and dendrite development. CSF-1 may also directly influence osteoblasts/osteocytes. Our data showing increased TUNEL positive osteocytes in CSF-1KO bone suggests that CSF-1 is a key factor for preventing osteocyte apoptosis and micropetrosis. It is tempting to speculate that loss of dendrite contacts throughout the lacunar-canalicular system in CSF-1KO bone further exacerbates osteoblast and osteoclast defects. The combination of decreased cortical thickness, mineralization, hydration and osteocyte death would be expected to reduce bone quality and strength [36]. This is supported by our findings in CSF-1KO mice and studies in osteopetrotic humans and rodents that show increased susceptibility to fracture [37,38]. Despite increased bone mass in osteopetrosis, material properties and biomechanical testing show increased fragility and reduced strength.
Osteopetrosis also involved the alveolar bone in CSF-1KO mice and led to failure of tooth eruption. CSF-1 also regulates tooth matrix formation including dentin [14]. DMP1, expressed in odontoblasts, is required for dentin mineralization [39]. We previously reported dentin defects in op/op mice including widened predentin and uneven dentin mineralization that was associated with reduced DMP1 expression in odontoblasts [14]. The presence of similar dentin defects in CSF-1KO mice is consistent with down-regulation of DMP1 in CSF-1KO odontoblasts.
F4/80+ macrophages were significantly reduced in CSF-1KO tissues similar to that described in op/op and CSF-1R null mice [21-24]. Of the tissues examined, the testes and uterus showed a profound reduction in macrophages that, in adult mice, would be associated with reduced testosterone in males, altered estrous cycling times in females and impaired fertility in both genders. As in op/op mice, decreased macrophages in the skin and in the adipose tissue of CSF-1KO mice were associated with reduced dermal thickness and body fat, respectively [21,25]. Our findings in CSF-1KO mice, taken together with studies in op/op and CSF-1R null mice indicate that the residual macrophage populations are not due to a mutated partially active CSF-1, but rather are likely due to other growth factors. Such factors include GM-CSF and IL-3 that in combination reverse the osteopetrotic defect in op/op mice and restore macrophages in several tissues [40].
Beside the effect on tissue macrophages, absence of CSF-1 affected other hematopoietic cell populations. Similar to that reported in op/op mice [21,41], CSF-1KO mice displayed decreased circulating monocytes, reduced bone marrow cellularity and decreased B lymphopoiesis. Both CSF-1KO and op/op bone marrow showed an increased percentage of the CD11b+Gr1+ cells. However, this was not accompanied by peripheral granulocytosis in 3 week old CSF-1KO mice as described in 6-8 week old op/op mice, perhaps due to the age of the mice [21]. The percentage of B220+ cells was significantly reduced in CSF-1KO marrow, spleen and peripheral blood. In op/op bone marrow, the frequency of B cell precursors responsive to normal stromal cells and IL-7 was severely reduced; however, progenitor cells that give rise to these precursors were maintained [42,43]. These findings suggest that defects in the bone microenvironment may account for reduced B lymphopoiesis in CSF-1KO mice, although a direct effect via the CSF-1R cannot be excluded [42]. In contrast to B cells, CD3+ mature T cells were increased in the circulation and spleen of CSF-1KO. Indeed, increased numbers of CD3+ T cells have been reported in the thymus of osteopetrotic mice (oc/oc, fos) and may contribute to elevated T cells in blood and spleen of CSF-1KO mice [44,45].
A significant defect in the CSF-1KO microenvironment which could impact hematopoiesis was the striking depletion of osteomacs along the endosteal surface. Bone marrow macrophages are deficient in op/op mice; however, osteomacs were not examined [21-23]. These cells form a canopy covering osteoblasts along the endosteal surface and are critical for maintaining osteoblast function and the endosteal niche for hematopoietic stem cells (HSC) [27]. Ablation of osteomacs in vivo, as demonstrated in macrophage Fas-induced apoptosis (Mafia) transgenic mice or by administration of clodronate-loaded liposomes in wild-type mice results in loss of endosteal osteoblasts, reduced levels of HSC-supportive cytokines at the endosteum and mobilization of HSCs into the circulation [46,47]. It is conceivable that loss of osteomacs in CSF-1KO mice contributed to the patchy distribution of osteoblasts along the endosteum/reduced cortical bone and may affect HCS function. CSF-1KO mice will be useful for analyzing the effect of osteomacs on osteoblasts, HSC niche and HSC function.
In summary, the present analysis of the CSF-1KO mouse contributes significantly to our understanding of CSF-1 in bone formation and suggests that both osteoblasts and osteocytes are targets of CSF-1. CSF-1 is crucial for osteoblast polarity, matrix mineralization and formation of the osteocytes and the lacunar-canalicular system. Importantly, results provide a novel link between CSF-1, DMP1 and osteocyte survival/function that is essential for maintaining bone mass and strength during skeletal development. Deletion of the CSF-1 gene selectively in osteocyte precursors and mature osteocytes using a conditional knockout strategy will further clarify the molecular mechanisms and downstream signaling pathways by which CSF-1 regulates bone turnover. Such an approach may also reveal CSF-1-mediated biologic interactions between bone and non-bone cells that may have therapeutic application for improving fracture healing and preventing bone and/or hematopoietic defects.
Highlights.
Studies to delineate the effect of CSF-1 in various tissues have been hampered by the lack of suitable models.
We generated CSF-1flox/flox mice and demonstrate that Cre-mediated recombination using Meox2Cre results in global CSF-1 deficiency.
CSF-1KO mice displayed, in part, a similar phenotype to op/op mice, including osteopetrosis and decreased macrophages.
CSF-1KO mice showed defects in the osteocyte-lacunar system that was associated with reduced DMP1 in osteocytes.
Results suggest a novel link between CSF-1, DMP1 and osteocyte function that is essential for maintaining bone strength.
Acknowledgments
This work has been supported, in part, by funding from the National Institutes of Health (R01 AR042306, Veteran’s Administration Merit grant, S.A-W), National Institute of Health (P01 AR46798, S.E.H. and J.Q.F.) and with funding from the GCCRI/UTHSCSA (VIR). We thank the micro-CT Core Facility at UTHSCSA for their assistance in analyzing the skeletal tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yeung Y-G, Stanley ER. Proteomics approaches to the analysis of early events in colony-stimulating factor-1 signal transduction. Mol Cell Proteomics. 2003;2:1143–1155. doi: 10.1074/mcp.R300009-MCP200. [DOI] [PubMed] [Google Scholar]
- [2].Rettenmier CW, Roussel MF. Differential processing of colony-stimulating factor 1 precursors encoded by two human cDNAs. Mol Cell Biol. 1998;8:5026–5034. doi: 10.1128/mcb.8.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Price LKH, Choi HU, Rosenberg L, Stanley ER. The predominant form of secreted colony stimulating factor is a proteoglycan. J Biol Chem. 1992;267:2190–2199. [PubMed] [Google Scholar]
- [4].Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [5].Heino TJ, Kurata K, Higaki H, Vaananen HK. Evidence of a role of osteocytes in the initiation of targeted remodeling. Technology and Health Care. 2009;17:49–56. doi: 10.3233/THC-2009-0534. [DOI] [PubMed] [Google Scholar]
- [6].Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is a mutation in the coding region of the macrophage colon stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- [7].Pollard JW, Stanley ER. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Adv Dev Biochem. 1996;4:153–193. [Google Scholar]
- [8].Hume DA, Favot P. Is the osteopetrotic (op/op mutant) mouse completely deficient in expression of macrophage colony-stimulating factor? J Interferon and Cytokine Res. 1995;15:279–284. doi: 10.1089/jir.1995.15.279. [DOI] [PubMed] [Google Scholar]
- [9].Sakagumi N, Amizuka N, Li M, Takeuchi K, Hoshino M, Nakamura M, Nozawa-Inoue K, Udagawa N, Maeda T. Reduced osteoblastic population and defective mineralization in osteopetrotic (op/op) mice. Micron. 2005;36:688–695. doi: 10.1016/j.micron.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [10].Dai X-M, Zong X-H, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res. 2004;19:1441–1451. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- [11].Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Feng JQ, Ward LM, Kiu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature Genetics. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, et al. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res. 2011;26:331–340. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abboud-Werner S, Gluhak-Heinrich J, Woodruff K, Wittrant Y, Cardenas L, Roudier M, MacDougall M. Targeted expression of csCSF-1 in op/op mice ameliorates tooth defects. Arch Oral Biol. 2007;52:434–443. doi: 10.1016/j.archoralbio.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [16].Wittrant Y, Gorin Y, Mohan S, Wagner B, Abboud-Werner SL. CSF-1 directly inhibits RANKL expression by osteoblasts. Endocrinology. 2009;150:4977–4988. doi: 10.1210/en.2009-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abboud SL, Woodruff K, Liu C, Shen V, Ghosh-Choudhury N. Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony stimulating factor-1 (CSF-1) Endocrinology. 2002;143:1942–1949. doi: 10.1210/endo.143.5.8775. [DOI] [PubMed] [Google Scholar]
- [18].Mohan S, Reddick RL, Musi N, Horn D, Yan B, Prihoda TJ, Natarajan M, Werner SL Abboud. Diabetic eNOS knockout mice develop distinct macro and micro vascular complications. Lab Invest. 2008;88:515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- [19].Gluhak-Heinrich J, Yang W, Harris, Harris SE. Quantitative in situ hybridization with enhanced sensitivity in soft, bone and tooth tissue using digoxigenin tagged RNA probes. Biochemia Medica. 2008;18:59–80. [Google Scholar]
- [20].Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, et al. Dmp1-deficient mice display severe defects in cartilage formation responsible for chondrodysplasia-like phenotype. J Biol Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ryan GR, Dia X-M, Dominguez MG, Tong W, Chuan F, Chisholm O, et al. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1op/Csf1op) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- [22].Dia X-M, Zong X-H, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient CSF1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- [23].Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- [24].Nagahama SI, Cunningham ML, Lee MY, Byers MR. Normal development of dental innervations and nerve/tissue interactions in the colony-stimulating factor-1 deficient osteopetrotic mouse. Dev Dyn. 1998;211:52–59. doi: 10.1002/(SICI)1097-0177(199801)211:1<52::AID-AJA5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [25].Wei S, Lightwood D, Ladyman H, Cross S, Neale H, Griffiths M, et al. Modulation of CSF-1-regulated post-natal development with anti-CSF-1 antibody. Immunobiology. 2005;210:109–119. doi: 10.1016/j.imbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [26].Dai X-M, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- [27].Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- [28].Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell DW, Ahmed-Ansari A, Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992;20:1004–1010. [PubMed] [Google Scholar]
- [29].Paic F, Igwe JC, Ravi N, Kronenberg MS, Franceschetti T, Harrington P, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao S, Kato Y, Zhang Y, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- [31].Robey PG, Boskey AL. Extracellular matrix and biomineralization of bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed American Society for Bone and Mineral Research; Washington, DC: pp. 38–46. [Google Scholar]
- [32].Ohtsuki T, Suzu S, Hatake K, Nagata N, Miura Y, Motoyoshi K. A proteoglycan form of macrophage colony-stimulating factor that binds to bone-derived collagens can be extracted from bone matrix. Biochem Biophys Res Commun. 1993;190:215–222. doi: 10.1006/bbrc.1993.1033. [DOI] [PubMed] [Google Scholar]
- [33].Wojtowicz A, Dziedzic-Goclawska A, Kaminski A, Stachowicz W, Wojtowicz K, Marks SC, Jr, et al. Alteration of mineral crystallinity and collagen cross-linking of bones in osteopetrotic toothless (tl/tl) rats and their improvement after treatment with colony stimulating factor-1. Bone. 1997;20:127–132. doi: 10.1016/s8756-3282(96)00336-5. [DOI] [PubMed] [Google Scholar]
- [34].Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aharinejad S, Marks SC, Bock P, Mason-Savas A, MacKay CA, Larson EK, et al. CSF-1 treatment promotes angiogenesis in the metaphysis of osteopetrotic (toothless, tl) rats. Bone. 1995;16:315–324. doi: 10.1016/8756-3282(94)00044-1. [DOI] [PubMed] [Google Scholar]
- [36].Manolagas SC, Parfitt AM. What old means to bone. Trends in Endocrinology and Metabolism. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Landa J, Margolis N, Di Cesare P. Orthopaedic management of the patient with osteopetrosis. J Am Acad Orthop Surg. 2007;15:654–662. doi: 10.5435/00124635-200711000-00004. [DOI] [PubMed] [Google Scholar]
- [38].Tuukkanen J, Kivukangas A, Jamsa T, Sundquist K, Mackay CA, Marks SC., Jr Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J Bone Miner Res. 2000;15:1905–1911. doi: 10.1359/jbmr.2000.15.10.1905. [DOI] [PubMed] [Google Scholar]
- [39].Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- [40].Myint YY, Miyakawa K, Naito M, Schultz LT, Oike Y, Yamamura K, et al. Granulocyte/macrophage colony-stimulating factor and interleukin-3 correct osteopetrosis in mice with osteopetrosis mutation. Am J Pathol. 1999;154:553–556. doi: 10.1016/s0002-9440(10)65301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wiktor-Jedrzejczak W, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tagaya H, Kunisada T, Yamazaki H, Yamane T, Tokuhisa T, Wagner EF, et al. Intramedullary and extramedullary B lymphopoiesis in osteopetrotic mice. Blood. 2000;95:3363–3370. [PubMed] [Google Scholar]
- [43].Lu L, Osmond DG. Regulation of cell survival during B lymphopoiesis in mouse bone marrow: Enhanced pre-B-cell apoptosis in CSF-1-deficient op/op mutant mice. Exp Hematol. 2001;29:596–601. doi: 10.1016/s0301-472x(01)00621-x. [DOI] [PubMed] [Google Scholar]
- [44].Blin-Wakkach C, Wakkach A, Sexton PM, Rochet N, Carle GF. Hematological defects in the oc/oc mouse, a model of infantile malignant osteopetrosis. Leukemia. 2004;18:1505–1511. doi: 10.1038/sj.leu.2403449. [DOI] [PubMed] [Google Scholar]
- [45].Wang Z-Q, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- [46].Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- [47].Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSC. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]