Abstract
Introduction:
To evaluate the reliability, validity and feasibility of the Persian version of the Pediatric Quality of Life inventory (PedsQL™ 4.0™ 4.0) Generic Core Scales in Iranian healthy students ages 7-15 and chronically ill children ages 2-18.
Methods:
We followed the translation methodology proposed by developer to validate Persian version of PedsQL™ 4.0™ 4.0 Generic Core Scales for children. Six hundred and sixty children and adolescents and their parents were enrolled. Sample of 160 healthy students were chosen by random cluster method between 4 regions of Isfahan education offices and 60 chronically ill children were recruited from St. Alzahra hospital private clinics. The questionnaires were fulfilled by the participants.
Results:
The Persian version of PedsQL™ 4.0™ 4.0 Generic Core Scales discriminated between healthy and chronically ill children (healthy students mean score was 12.3 better than chronically ill children, P<0.001). Cronbachs’ alpha internal consistency values exceeded 0.7 for children self reports and proxy reports of children 5-7 years old and 13-18 years old. Reliability of proxy reports for 2-4 years old was much lower than 0.7. Although, proxy reports for chronically ill children 8-12 years old was more than 0.7, these reports for healthy children with same age group was slightly lower than 0.7. Constructive, criterion face and content validity were acceptable. In addition, the Persian version of PedsQL™ 4.0™ 4.0 Generic Core Scales was feasible and easy to complete.
Conclusion:
Results showed that Persian version of PedsQL™ 4.0™ 4.0 Generic Core Scales is valid and acceptable for pediatric health researches. It is necessary to alternate scoring for 2-4 years old questionnaire and to find a way to increase reliability for healthy children aged 8-12 years especially, according to Iranian culture.
Keywords: Children, early adolescents, Iran, PedsQL™ 4.0™, Persian, quality of life, toddlers
INTRODUCTION
Prevention of chronic and disabling diseases is a result of new medical researches. One of the most important aspects of preventive medicine is improving quality of life. Health related quality of life (HRQOL) includes not only physical functioning but also emotional and social functioning dimensions.[1] To assess effectiveness of clinical trials, HRQOL measuring instruments are strongly recommended by World Health Organization (WHO). HRQOL instruments assist healthcare services in creating better relationship between patients and their physicians and improving patient's outcome. In addition, the obtained results help the health system to distribute their assets and facilitate risk assessment.[2,3] In pediatric field, an instrument to measure HRQOL must be able to evaluate cognitive development and viewpoints of both child and parents. It has been shown that using instrument appropriate to age development would make HRQOL reports more reliable.[4–7] The Pediatric Quality of Life Inventory™ (PedsQL™ 4.0™) that has been invented to determine HRQOL is one of the most competent measures in this field.[8] The PedsQL™ 4.0 is designed to integrate the relative merits of generic and disease specific HRQOL measuring instruments. Adding some constructs and items to the previous PedsQL™ 2.0 and 3.0 matches the PedsQL™ 4.0 Generic Core Scales to dimensions described by WHO.[1] The PedsQL™ 4.0 Generic Core Scales have been designed to assess child self report for ages 5–18 and parent proxy report for ages 2–18. Furthermore, disease-specific modules have been invented for chronic conditions and children with cancers by Varni.[4,5,9]
The feasibility, reliability and validity of the original version of the PedsQL™ 4.0™ (American English) have been widely demonstrated.[3–7,9–12] Considering the capability of PedsQL™ 4.0 in assessing child and parents and its simplicity in applying and scoring, various translations of different languages has been validated.[13–21] Iran has one of the youngest populations in the world. Therefore, using Persian version of the PedsQL™ 4.0 in estimating HRQOL is mandatory. We were the first Iranian group obtaining the inventor's approval (Dr. James Varni). Even though, a Persian version of the PedsQL™ 4.0 has been published before our study, according to our best knowledge we carried out translation and validation of the PedsQL™ 4.0 into Persian language for children less than 13 years old as the first group.[22]
METHODS
Participants
The survey was performed in accordance with the ethical standards of the Helsinki Declaration. After obtaining the agreement of the main inventor (Dr. James Varni), approval of Isfahan Education Office license and Ethics Committee of the Research Department of Isfahan University of Medical Sciences, 660 children and adolescents and their parents were enrolled. Approximately, 84% of the children were healthy and 16% had chronic diseases. The healthy students aged between 7-18 years were chosen from 4 girl and 4 boy-schools among primary and middle schools in Isfahan by random cluster method. The 60 children and adolescents with chronic diseases were chosen from patients with malignancy, asthma, beta thalassemia, rheumatologic disorders, immune deficiency disorders and chronic hepatitis referred to private clinics of St. Alzahra hospital, Isfahan. After taking participants’ consents and providing enough explanation by the project's staffs, the child and self proxy reports questionnaires were distributed. Five hundred and thirty seven participants out of 660 returned the questionnaires. However, 11 questionnaires were excluded, since they contained more than 50% missing data. Five hundred and twenty six questionnaires were eligible and had children and parents proxy reports. The total response rate was 81.3%. Out of 526 questionnaires, 239 forms were fully filled by children and 287 forms were completed by the parents. The distribution of the participants according to the age was as follows: (2-4 years; n=30), (5-7 years; n=43), (8-12 years; n=253), (13-18 years; n=180).
Measures
PedsQL™ 4.0 Generic Core Scales
The forward-backward translation method was applied on 23-items PedsQL™ 4.0 Generic Core Scales, Figure 1. PedsQL™ 4.0 Generic Core scale is a self-administered questionnaire contained 4 subscales including Physical, Emotional, Social and School functions by which different aspects of HRQOL are assessed. Physical function has 8 items and each of the remaining functions contain 5 items. The child self report assesses children at ages 8-12 and 13-18 years. The parent proxy report evaluates children between 5-7 and 2-4 years in addition to the age groups assessed by the child forms. Questions of both reports were scored as follows: 0=never a problem; 1=almost never a problem; 2=sometimes a problem; 3=often a problem; 4=almost always a problem. The items for each of the forms are essentially identical, differing in developmentally appropriate language, or first or third person tense. The instrument took approximately 5 min to complete.[6,12] Validation of the Persian version of PedsQL™ 4.0 Generic Core scale followed the recommended guideline, Figure 1.[23]
Figure 1.
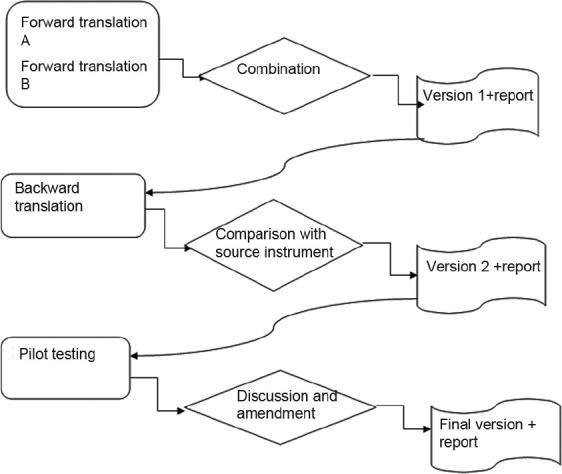
Process, decision and result
Translation process
According to the international guideline for questioner translation (forward-backward translation) two independent Persian versions were made by a general physician fluent in English and an English translator. Then, these two drafts were combined by a pediatrician and a community medicine physician. Next, backward translation was made by a bilingual English native speaker. The product of translation process was reviewed and approved by the principal inventor (Dr. Varni). A pilot study aiming to determine face validity and provide final edition was conducted on 30 students and their parents. Our aim was to find items leading to misunderstanding and to edit final version. Ultimately, the Persian version of PedsQL™ 4.0 Generic Core Scales was the output of the first phase.
Data collection
After obtaining Isfahan Education Office license, a package consisted of a written consent form, a testimonial letter explained how to fill the questioners, contact addresses and phones of main researchers for any possible question or explanation, Persian version of PedsQL™ 4.0 parent proxy form and child form were distributed. The children were recruited from 4 girl-schools and 4 boy-schools among primary and middle schools in Isfahan by random cluster method. Accordingly, four research staffs took students health history and checked their health files. The child forms were filled out by those children whose parents completed the parents’ proxy forms. The research staffs assisted children to complete the questionnaires. All the parents’ packages collected after 10 days. Sixty children with chronic diseases were recruited from patients referred to private clinics of St. Alzahra hospital. The inclusion criteria for these children were the ability of patients and acceptance to fill out the forms.
Statistical analysis
SPSS 18.0 software was used. The following statistical methods were applied:
The face validity of the Persian version of the PedsQL™ 4.0 Generic Core scale was evaluated by asking the children and their parents in a pilot study about the appearance of the text (its font and size) and the meaning of the sentences.
The feasibility of the questionnaire was evaluated by determining the percentage of missing items for each questionnaire. Ceiling and floor effects were determined to show whether more than 15% of the participants achieved the highest or lowest score, respectively.[24]
The content validity was determined by asking two pediatricians whether the items of the Persian version of PedsQL™ 4.0 assessed HRQOL and any irrelative item in the inventory. Kappa analysis was calculated between two physicians for proxy and self reports.
The discriminant validity of the Persian version of PedsQL™ 4.0 was determined for 4 subclasses of the questionnaire between healthy children and children with chronic diseases.
The criterion validity was evaluated by Spearman Coefficient test.
Cronbach's alpha (α) coefficient was calculated to determine the internal consistency. Alpha coefficients equal or more than 0.7 were regarded as acceptable. The intraclass correlation coefficient (ICC) was measured to show the agreement between child self-report and parent proxy-report on the PedsQL™ 4.0 subscales: ICCs ≤0.4 as poor to fair agreement; 0.41-0.60 as moderate agreement; 0.61-0.8 as good agreement, and >0.8 as excellent agreement.[22,25]
Kaiser-Meyer-Olkin test revealed the adequacy of the sample size. In addition, Bartlett's test of sphericity showed that assumption of sphericity was met. [Table 1] Therefore, applying factor analysis to the results was suitable. Exploratory factor analysis (EFA), operating principal component analysis and the Rotation method (Varimax with Kaiser Normalization) was used to extract factor structure of the PedsQL™ 4.0. The Extraction method (Principal Component Analysis) and were utilized. Rotation was converged in 8 iterations. Confirmatory factor analysis (CFA) was applied to evaluate the fitness of EFA extraction model the observed data. The reasonable values of Fit indices for CFA were considered as X2/df <5 and root mean square error approximation (RMSEA) <0.08. Furthermore, values more than 0.9 were accepted appropriate for comparative fit index (CFI), goodness of fit index (GFI) and adjusted goodness of fit index (AGFI). In addition, split half method was applied to assess internal consistency.
Table 1.
Kaiser-Meyer-Olkin shows the adequacy of sample size and Bartlett's test of sphericity indicates the assumption of sphericity was met

RESULTS
Sample characteristics
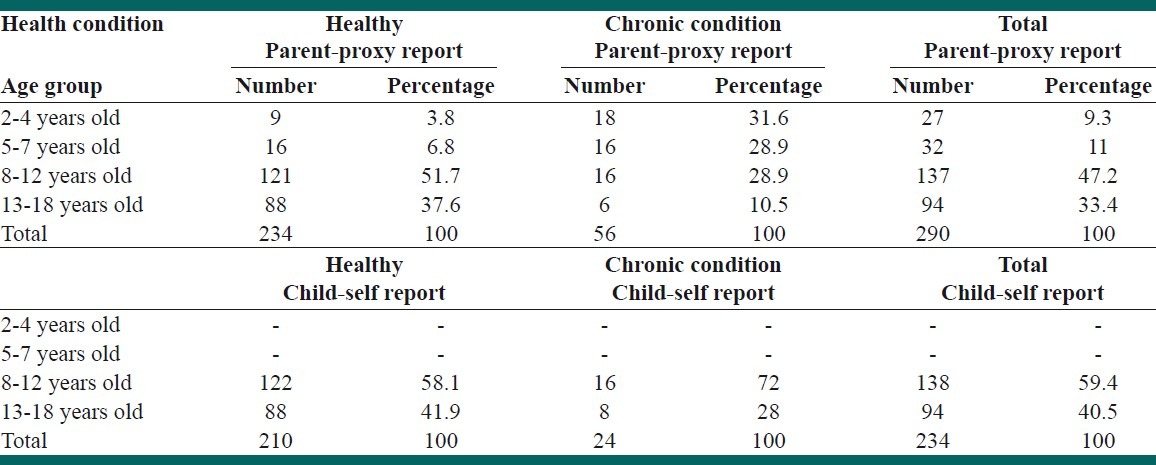
Five hundred and thirty seven participants out of 660 (81%) returned the questionnaires. The characteristics of the participants are depicted in Table 2. Eleven out of 537 questionnaires (2%) were omitted because of having more than 50% missing data. Five hundred and twenty six questionnaires were eligible and had children and parents proxy reports. Out of 526 questionnaires, 236 forms were full filled by children and 290 forms were completed by the parents. Forty six percent of healthy children and 52% of children with chronic condition were female.
Table 2.
Sample characteristics: Parent-proxy and child self reports according to health condition
To evaluate face validity, 29 children (15 healthy children and 14 children with chronic diseases) and 25 parents (10 parents of healthy children and 15 parents of sick children) were enrolled in a pilot study. Twelve healthy children (80%) out of 15 and 57.1% of children with chronic diseases found the meaning of the questions as the researchers expected. Fisher Exact test did not show any significant difference, P=0.26. Eleven healthy participants out of 15 (73.3%) and 71.4% of the children with chronic diseases replied that the appearances of the forms are attractive enough, P=0.99. The P value for the first and second questions between parents groups were 0.18 and 0.13, respectively. These results proved the acceptable face validity of the children self-report and parent proxy-report forms.
The feasibility of the forms was evaluated by determining the percentage of missing data and ceiling and floor effects. In healthy children, questions number 18 and 11 with 8 missing, had the highest and questions number 2 and 10 with no missing, had the lowest missing data. Total missing data in children self-report forms was 3.6% (7 items in healthy children and 1 item in diseased children; totally less than 5%). The result was 2.2% in parents-proxy reports (5 items in healthy group and 1 item in diseased group; totally less than 5%). It took 5-7 min to complete each questionnaire. Children and their parents left 0.5% and 2.2% of questions unanswered, respectively. Total missing item was less than 5% that showed satisfactory feasibility.
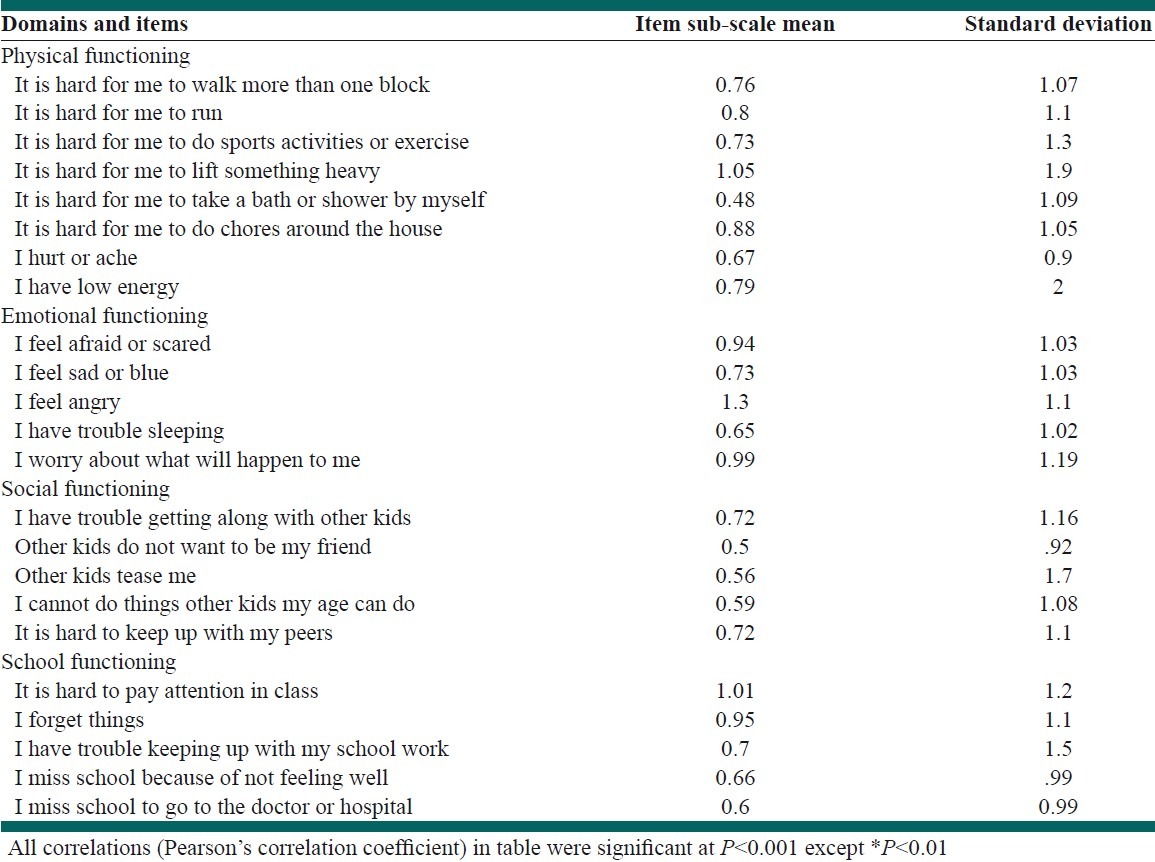
We did not show any floor effects while ceiling effects were observed from a minimum of 4% in child self-report social functioning to a maximum of 10% in parent proxy-report physical functioning [Table 3]. The Cronbach's alpha for total scale scores of child-self and parent-proxy reports by applying ceiling and floor effects were 0.73 and 0.9, respectively. The intraclass correlation coefficients (ICC) were high for most scales indicating excellent agreement for social functioning and school functioning, good agreement for total score and physical functioning. Nonetheless the lowest agreement was identified for emotional functioning scale (ICC=0.32, 95% CI: 0.2-0.4). Item-subscale, item-total and subscale-total score correlations were significant for all items [Table 4].
Table 3.
Floor and ceiling effects of child-self and parent proxy reports
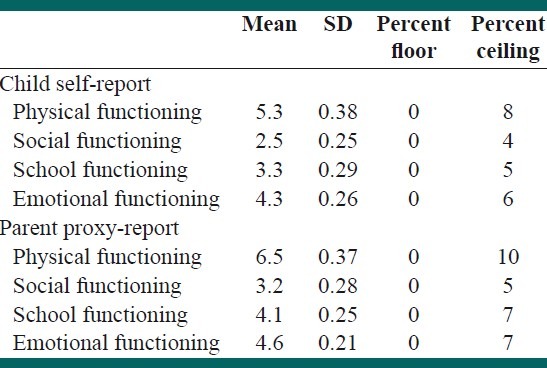
Table 4.
Item-subscale, item-total, and subscale-total score correlations
Factor analysis with varimax rotation extracted 7 factors from the PedsQL™ 4.0 self- and parent-proxy reports are shown in Table 5. The results of the CFA for 7-factor models showed appropriate fitness of the model for self- and parent-proxy reports [Table 6].
Table 5.
Factor analysis results for child-self reports and parent proxy reports
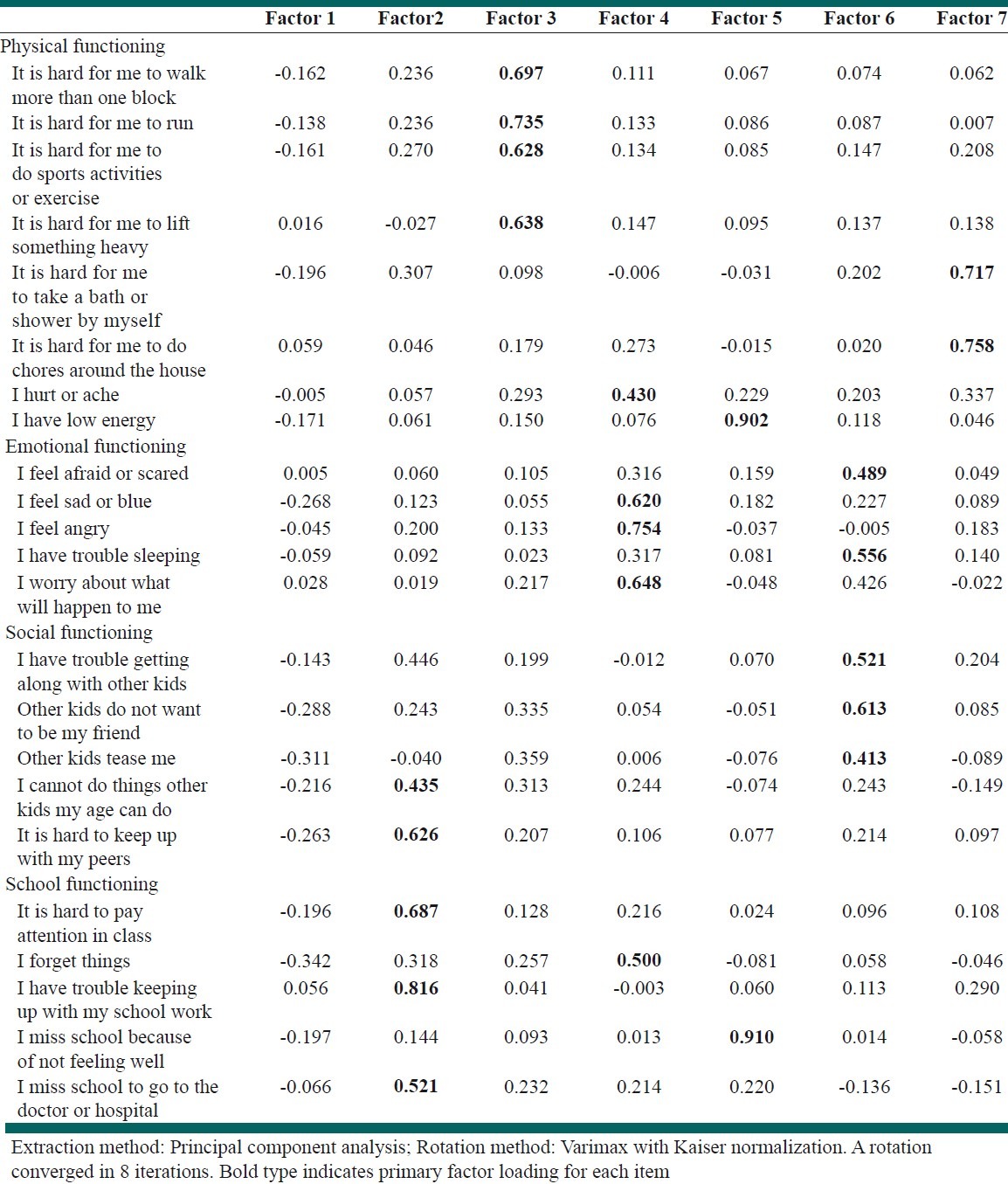
Table 6.
The result of confirmatory factor analysis for the 7 factors model

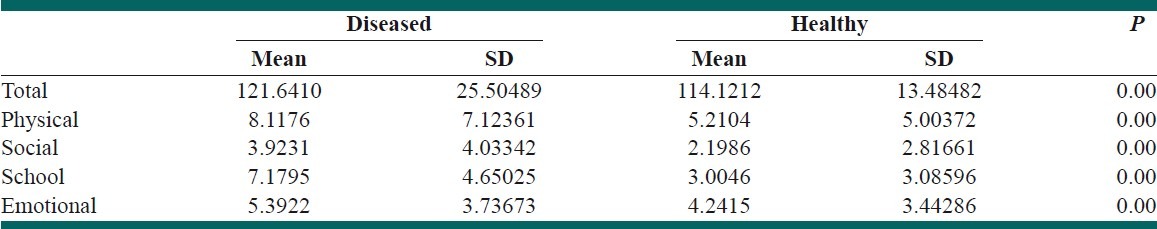
To evaluate discriminant validity, the mean score of each function was compared between two groups separately [Table 7]. The score for each function was significantly lower in healthy children comparing with children with chronic diseases. In addition, the mean total score for healthy children and children with chronic diseases were 114.12±13.48 and 121.6±25.5; respectively, P=0.001. Therefore, the PedsQL™ 4.0™ Persian version has the acceptable discriminant validity for each function and for total score.
Table 7.
Discriminant validity of the PedsQL™ 4.0™ according to health status
To evaluate content validity, a pediatrician and a pediatric nephrologist were asked if the items of Persian version of PedsQL™ 4.0 assessed HRQOL and if there was any irrelative item in the inventory. An appropriate content validity was achieved by Kappa analysis that showed 95% and 91% agreement between two physicians for parent-proxy and child-self reports, respectively.
Criterion validity was assessed by Spearman coefficient test. Spearman coefficient values for parent proxy reports for children 2-4 years and 5-7 years were 0.3 and 0.73, respectively. The results did not support criterion validity of parent-proxy forms for children 2-4 years. The values for children self- and parent-proxy forms of children 8-12 years and adolescents 13-18 years were more than 0.7.
Covariance matrices between each function and other functions were less than 0.7 (the least was between social and school functions=0.39 and the greatest value was between physical and social functions=0.58).
DISCUSSION
The present study is the first to explain and validate the different aspects and properties of the Persian version of PedsQL™ 4.0 Generic Core Scale in children. The results of the study provided enough primary supports for reliability, validity and feasibility of Persian version of PedsQL™ 4.0 in toddlers, children and early adolescents’ self- reports and parent proxy-reports. Our results provided enough evidences to support validity, feasibility and reliability of the Persian version of PedsQL™ 4.0 Generic Core Scale. At the time of approving the final edition of the manuscript, we realized that another Iranian research team[22] has obtained the approval of Varni to translate PedsQL™ 4.0 into Persian (adolescent 13-18 years). However, an important contribution of our paper compared to their work is that, we enrolled a greater sample of chronically ill patients. In addition, the participants of our study were chosen by cluster sampling from different divisions of the city (Isfahan). Another major contribution of this paper is the fact that, we have studied toddlers and children.
We confirmed the face validity of the Persian version of PedsQL™ 4.0. We did not find the same results for face validity in similar studies. The feasibility of our study was approved by missing data analysis (3.6% of children self and 2.2% of parent proxy reports). Roizen et al. demonstrated suitable feasibility by only 9 missing data in 5-7 years old range.[20] The participants of Roizen et al. study spent 5-6 min to complete the forms comparing with an average of 5 min in the Brazilian study and 5-7 min for our participants.[20,21]
Reliability was assessed by Cronbach's alpha.
The main inventor (Dr. Varni) achieved Cronbach's alpha=0.89 for child and 0.92 for parent proxy reports with minimal missing data.[26]
In our study, the Cronbach's alpha for total scale scores of child-self and parent-proxy reports by applying ceiling and floor effects were 0.73 and 0.9 respectively, the lowest agreement was identified for emotional functioning scale (ICC=0.32, 95% CI: 0.2-0.4). Klatchoian et al. reported Cronbach's alpha between 0.6 and 0.9 for all dimensions, showing appropriate internal consistency.[21]
Laaksonen et al. demonstrated that the Finnish version had excellent Cronbach΄s alpha values for the total scale score (0.91 and 0.88 for child self and parent proxy report, respectively).[17] Reinfjell et al. reported Chronbach's alpha equaled to 0.77 for self and 0.88 for proxy reports.[16] Construct validity was assessed using exploratory factor analysis and by exploring the inter-correlations between and among the 4 PedsQL subscales for adolescents and their parents.
The reliability coefficient for the Persian questionnaire was comparable with other studies[6,7,15–19,22,27] and more than Argentinean-Spanish version.[20]
However, Persian version of PedsQL™ 4.0 did not show acceptable reliability for assessing healthy 8-12 years old children. It might be due to the fact that children aged 8-12 years face physical and psychological aspects of puberty. Therefore, they may become introverted. This might make them difficult to understand for their parents.
In contrast, chronically ill children encounter many challenges in fulfilling their daily needs. This makes them more dependent and often closer to their parents. This strong family relationship might capable their parents of better assessment.
In Iranian society pediatric health assessment is often based on physical and school function. Therefore, more studies are required to assess discriminant validity of each aspect of HRQOL.
Similar to the Argentinean-Spanish version,[20] the Persian version did not show acceptable reliability for 2-4 years old toddlers’ proxy-report. This can be associated to the following reasons:
Poor communication skills of toddlers results in poor assessment of children for parents.
None of school items were completed for toddlers.
The physical function plays a more important role in predicting HRQOL. Other studies also confirm this observation.[6,7,15–19,22] Probably because physical function is more objective than other items and it is easier to assess. However, utilizing HRQOL measurement in pediatric healthcare settings facilitates patient-physician communication, improves patient/parent satisfaction, identifies hidden morbidities, and assists in clinical decision-making.
CONCLUSION
We confirmed that Persian version of PedsQL™ 4.0 Generic Core Scales is valid and reliable.
This paper has two major contributions. Firstly, we enrolled a greater sample of chronically ill patients compared to similar studies. Secondly, we are the first to validate PedsQL™ 4.0 on Iranian toddlers and children.
However, another study is yet to be conducted to address cultural specifications of Iranian society, especially for pubertal age. We also suggest another study with larger sample size for toddlers while omitting school items.
ACKNOWLEDGMENT
This school-based nationwide health survey was conducted in Iran with corporation of the Ministry of Education and Training.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Upton P, Eiser C, Cheung I, Hutchings HA, Jenny M, Maddocks A, et al. Measurement properties of the UK English version of the pediatric quality of life inventory 4.0 (PedsQL™ 4.0) generic core scales. Health Qual Life Outcomes. 2005;3:22. doi: 10.1186/1477-7525-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis E, Hynan S, Limbers A, Andersen C, Greene C, Varni JW, et al. The PedsQL™ 4.0_ in Pediatric Patients with Duchenne Muscular Dystrophy: Feasibility, Reliability, and Validity of the Pediatric Quality of Life Inventory Neuromuscular Module and Generic Core Scales. J Clin Neuro Mascul Dis. 2010;11:97–109. doi: 10.1097/CND.0b013e3181c5053b. [DOI] [PubMed] [Google Scholar]
- 3.Varni JW, Burnwinkle TM, Lane MM. Health related quality of life measurement in pediatric clinical practice: An appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varni JW, Burnwinkle TM, Jacobs JR, Gootschalk M, Kaufman F, Jones KL. The Peds QL in type 1 and type 2 diabetes: Reliability and validity of pediatric quality of life inventory generic core scale and type 1 diabetes module. Diabetes Care. 2003;26:631–7. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 5.Varni JW, Burnwinkle TM, Rapoff MA, Kamps JL, Alson N. The Peds QL in pediatric asthma: Reliability and validity of pediatric quality of life inventory generic core scale and asthma module. J Behav Med. 2004;27:297–318. doi: 10.1023/b:jobm.0000028500.53608.2c. [DOI] [PubMed] [Google Scholar]
- 6.Varni JW, Seid M, Rode CA. The PedsQL™ 4.0: Measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0 4.0: Reliability and validity of the pediatric quality of life inventory TM version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Eiser C, Morse R. Quality-of-life measures in chronic diseases in childhood. Health Technol Assess. 2001;5:1–147. doi: 10.3310/hta5040. [DOI] [PubMed] [Google Scholar]
- 9.Varni JW, Burnwinkle TM, Katz ER, Measke K, Dickinson P. The Peds QL in pediatric cancer: reliability and validity of pediatric quality of life inventory generic core scale, multi dimensional fatigue and cancer module. Cancer. 2002;94:2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW, Burwinkle TM, Seid M. The Peds QL 4.0 as a school health population measure: Feasibility, reliability and validity. Qual Life Res. 2006;15:203–21. doi: 10.1007/s11136-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 11.Varni JW, Burnwinkle TM, Berrin SJ, Sherman SA, Artavia K, Malcarne VL, et al. The PedsQL™ 4.0 in pediatric cerebral palsy; reliability, validity and sensitivity of the generic core scales and cerebral palsy module. Dev Med Child Neurol. 2006;48:442–9. doi: 10.1017/S001216220600096X. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Limbers CA, Burnwinkle TM. How young can children reliability and validity self-report their health related quality of life?: An analysis of 8591 children across age subgroups with the Peds Ql 4.0 generic core scales. Health Qual Life Outcomes. 2007;3:1. doi: 10.1186/1477-7525-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varni JW, Limbers CA, Burnwinkle TM. Parent proxy-report of their children's health-related quality of life; an analysis of 13,878 parents’ reliability and validity across age sub groups using the Peds QL generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer MG, Whitham JN, Roberton MD, Taplin JE, Varni JW, Baghurst PA. The relationship between health-related quality of life, pain and coping strategies in juvenile idiopathic arthritis -a one year prospective study. Qual Life Res. 2005;14:1585–98. doi: 10.1007/s11136-004-7710-3. [DOI] [PubMed] [Google Scholar]
- 15.Felderpuig R, Frey E, Proksch K, Varni JW, Gadner H, Topf R. Validation of German version of the pediatric quality of life inventory (PedsQL™ 4.0) in childhood cancer patients off treatment and children with epilepsy. Qual Life Res. 2004;13:223–34. doi: 10.1023/B:QURE.0000015305.44181.e3. [DOI] [PubMed] [Google Scholar]
- 16.Reinfjell T, Diseth TH, Veenstra M, Vikan A. measuring health-related quality of life in young adolescent: reliability and validityin the Norwegian version of the Pediatric Quality of Life Inventor 4.0 (PedsQL™ 4.0) generic core scales. Health Qual Life Outcomes. 2006;14:61. doi: 10.1186/1477-7525-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laaksonen C, Aromaa M, Heinonen OJ, Suominen S, Salantera S. Pediatric health-related quality of life instrument for primery school children: Cross-cultural validation. J Adv Nurs. 2007;59:542–50. doi: 10.1111/j.1365-2648.2007.04347.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Origasa H, Ichida F, Kamibeppu K, Varni JW. Reliability and validity of pediatric quality of life inventory (Peds QL) short form generic core scales in Japan. Qual Life Res. 2007;16:1239–49. doi: 10.1007/s11136-007-9230-4. [DOI] [PubMed] [Google Scholar]
- 19.Gkoltsiou K, Dimitrakaki C, Tzavara C, Papaevangelou V, Varni JW, Tountas Y. Measuring health-related quality of life in Greek children: psychometric properties of the Greek version of the Pediatric Quality of Life Inventory (TM) 4.0 Generic Core Scales. Qual Life Res. 2008;17:299–305. doi: 10.1007/s11136-007-9294-1. [DOI] [PubMed] [Google Scholar]
- 20.Roizen M, Rodriguez S, Bauer G, Medin G, Bevilaqua S, Varni JW, et al. initial validation of the Argentinean Spanish version of the PedsQL™ 4.0 TM 4.0 Generic Core scales in children and adolescents with chronic diseases; acceptability and comprehensibility in low income settings. Health Qual Life Outcomes. 2008;6:59. doi: 10.1186/1477-7525-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatchoian DA, Len CA, Terreri MT, Silva M, Itamoto C, Ciconelli RM, et al. Quality of life of children and adolescents from sao Paulo: reliability and validity of the Brazilian version of the Pediatric Quality of Life Inventor 4.0 (PedsQL™ 4.0) generic core scales. J Pediatr (Rio J) 2008;84:308–15. doi: 10.2223/JPED.1788. [DOI] [PubMed] [Google Scholar]
- 22.Amiri P, Ardekani EM, Jalali-Farahani S, Hosseinpanah F, Varni JW, Ghofranipour F, et al. Reliability and validity of the Iranian version of the Pediatric Quality of Life Inventory™ 4.0 Generic Core Scales in adolescents. Qual Life Res. 2010;19:1501–8. doi: 10.1007/s11136-010-9712-7. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW. PedsQL™ 4.0™ translation methodology. [Last accessed on 1998]. Available from: http://www.PedsQL™4.0.org .
- 24.McHorney CA, Tarlov AR. Individual- patient monitoring in clinical practice: Are available health status surveys adequate? Qual Life Res. 1995;4:293–307. doi: 10.1007/BF01593882. [DOI] [PubMed] [Google Scholar]
- 25.Bartko JJ. Intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Burnwinkle TM, Seid M, Skarr D. the PedsQL™ 4.0 4.0as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Jafari P, Ghanizadeh A, Akhondzadeh SH, Mohammadi MR. Health-related quality of life of Iranian children with attention deficit/hyperactivity disorder. Qual Life Res. 2011;20:31–6. doi: 10.1007/s11136-010-9722-5. [DOI] [PubMed] [Google Scholar]


