Addition of protease instead of seeds using a robot can be used to optimize the concentration of protease in in situ proteolysis experiments and has been successfully tested using two proteins.
Keywords: in situ proteolysis, optimization
Abstract
In situ proteolysis is one of the most effective rescue strategies for protein crystallization, and optimization of the ratio between the protein and the protease is one of the key steps in the process. Seeding is a very powerful tool to optimize crystallization conditions and can be performed by most crystallization robots. Addition of protease instead of seed stock using a robot can be used to optimize the concentration of protease in in situ proteolysis experiments and has been successfully tested using two proteins.
1. Introduction
The field of protein crystallography has developed very rapidly, and the use of structural genomics in the last twenty years has resulted in a dramatic increase in the number of structures solved (Grabowski et al., 2007 ▶). However, the nucleation or growth of crystals suitable for X-ray diffraction remains a rate-limiting step in the process of structure solution (Deivanayagam et al., 2007 ▶). Several strategies, such as protein engineering (Dale et al., 2003 ▶; Derewenda, 2004 ▶), changing the surface properties (Walter et al., 2006 ▶; Kim et al., 2008 ▶), adding a new partner (Vedadi et al., 2006 ▶), seeding (Bergfors, 2003 ▶) and in situ proteolysis (Pfuetzner et al., 1997 ▶; Gaur et al., 2004 ▶; Dong et al., 2007 ▶; Wernimont & Edwards, 2009 ▶), have been used to increase the success rate of crystallization.
Using a protease to digest the sample during crystallization (in situ proteolysis) is one of the most effective rescue strategies and is becoming a routine method in protein crystallization. Proteases such as trypsin or α-chymotrypsin are very popular and have been used in previous reports (Dong et al., 2007 ▶; Wernimont & Edwards, 2009 ▶). The success rate in obtaining crystals is very high: greater than 12% in experiments using either small proteins (69 proteins; Dong et al., 2007 ▶) or medium-sized protein (270 proteins; Wernimont & Edwards, 2009 ▶). The simplest technique is to add the protease to the sample and to set up the drop without any additional purification or modification. However, the ratio between the protein and the protease needs to be varied to optimize the condition and in some cases the reproducibility is quite low (Dong et al., 2007 ▶).
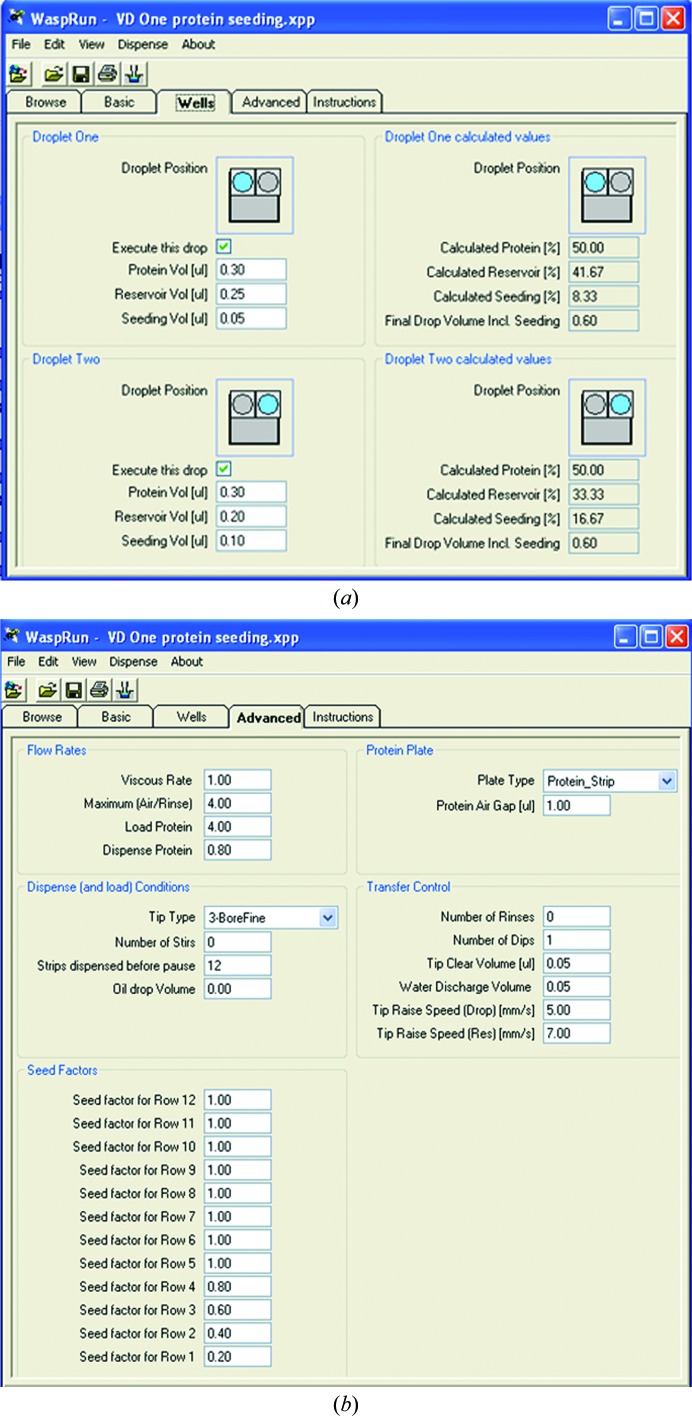
Seeding is another very powerful tool for optimizing crystallization conditions and helps in finding more hits during screening (Bergfors, 2003 ▶). Crystals, precipitate or polymers can be used as seeds. The new development of microseed matrix screening (MMS) has been automated to dispense small volumes and achieve high throughput (D’Arcy et al., 2007 ▶). Crystallization robots such as Phoenix (Art Robbins Instruments), Mosquito (TTP LabTech) and Oryx (Douglas Instruments) are able to dispense drops for seeding experiments. For example, the script used by Oryx allows the ratio between the seeds and protein to be varied either by changing their amounts in different drops in the same well (Fig. 1 ▶ a) or by using ‘seed factors’ for different drops in each row (Fig. 1 ▶ b).
Figure 1.
The protease can be added to the drop instead of seed and the concentration can be varied by changing of the volume of the ‘seed’ in drops in the same well (a) or the ‘seed factor’ of the drops in different rows (b). When the factors were set to 0.2–1.0 in steps of 0.2 in rows 1–5, the volume of seed was 0.01 µl in the drops in row 1 and 0.05 µl in the drops in row 5.
Here, we present a method for adding protease instead of seed in order to simplify the process of optimizing the ratio between the protein and the protease and to increase the possibility of obtaining better crystals.
2. Materials and methods
GroEL from Escherichia coli was purified as a contaminant during the purification of another protein, MoPrp17. The MGG00780 gene from rice blast fungus was cloned into pHAT2 (kindly supplied by Dr Arie Geerlof from EMBO) and expressed in E. coli, and the recombinant protein was purified by affinity, ion-exchange and gel-filtration chromatography using an AKTApurifier 10 system (GE Healthcare). Trypsin was purchased from Sigma (catalogue No. T8003; 0.5 g) and α-chymotrypsin was purchased from Ameresco (catalogue No. 0164; 1 g). Stock solutions of the proteases were prepared at 0.1 mg ml−1 in 10 mM Tris–HCl pH 8.0.
Crystallization experiments were performed in two-drop MRC plates (Hampton Research) using an Oryx 4 robot with a three-bore tip. Typically, 0.3 µl protein solution (8–10 mg ml−1 in 20 mM Tris–HCl pH 8.0, 50 mM NaCl) was mixed with 0.25 µl reservoir solution. Protease was simultaneously added to the drop (0.01–0.05 µl in 0.01 µl steps). Trypsin or α-chymotrypsin was used for MGG00780. In the case of GroEL, 0.04–0.05 µl trypsin was added to the crystallization drop. The concentration of the protease was changed from 1.8 to 8.3 µg ml−1 in different drops by varying the ‘seed factors’ in the experimental script, while the concentration of protein remained nearly constant in all drops (Table 1 ▶).
Table 1. Volume and concentration of protease in the drops (total volume of 0.56–0.6 µl).
| Drop 1 | Drop 2 | Drop 3 | Drop 4 | Drop 5 | |
|---|---|---|---|---|---|
| Volume (µl) | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 |
| Concentration (µg ml−1) | 1.8 | 3.5 | 5.2 | 6.8 | 8.3 |
| No. of crystals | 4–14 | 3–10 | 16–18 | 1–4 | 5–13 |
| Crystal dimensions (µm) | 25 × 25 × 31 | 25 × 31 × 37 | 12 × 19× 25 | 44 × 50 × 56 | 12 × 19 × 31 |
3. Results and discussion
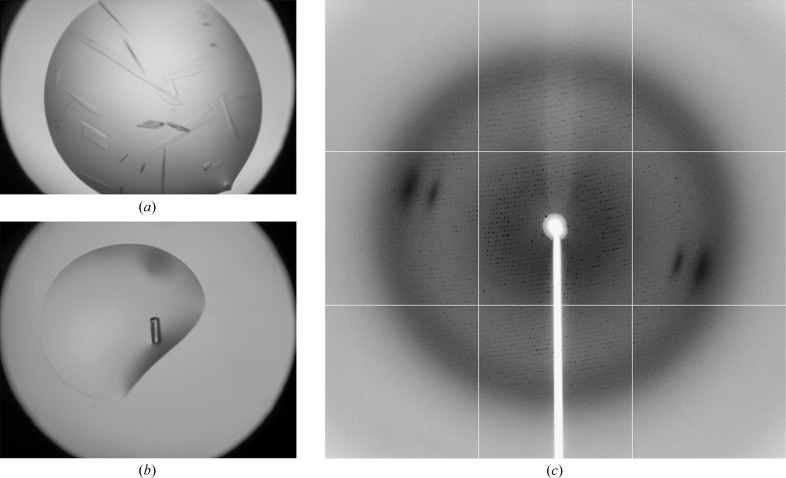
E. coli GroEL crystallized as thin plates from 12–15% PEG 3350, 0.15 M potassium fluoride (Fig. 2 ▶ a). Changing the pH or the use of additives did not improve the quality of the crystals. Fortunately, the addition of trypsin increased the thickness of the GroEL crystals (Fig. 2 ▶ b). These crystals diffracted to about 3.0 Å resolution at the synchrotron (beamline BL-17U at Shanghai Synchrotron Research Facility or beamline I02 at Diamond Light Source; Fig. 2 ▶ c), while unoptimized thin crystals gave no diffraction.
Figure 2.
Crystals of GroEL digested with (a) or without (b) trypsin; (c) the diffraction image obtained on beamline BL-17U at Shanghai Synchrotron Research Facility (SSRF) from a crystal of GroEL digested with trypsin.
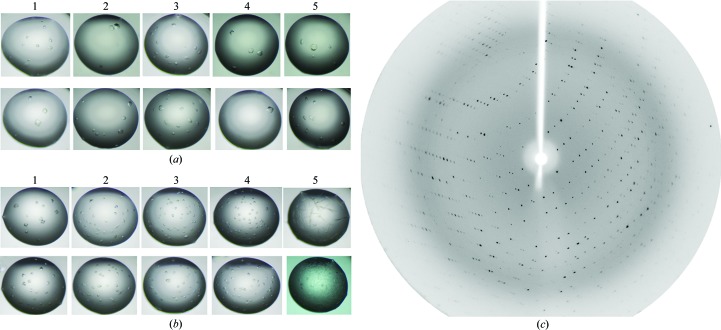
The MGG00780 sample was used in an additional experiment to optimize the in situ proteolysis by changing the ‘seed factors’. MGG00780 was crystallized using 22–25% PEG 3350, 0.2 M calcium acetate, 0.1 M Tris–HCl pH 8.0. Crystals appeared after one month and could not easily be reproduced. Protease (either trypsin or α-chymotrypsin) was added to the drops to test its effect on the reproducibility and the speed of crystallization. Crystals could be obtained easily within 10–13 d of addition of trypsin or α-chymotrypsin. The number and the size of the crystals changed on varying the concentration of protease: trypsin worked better than α-chymotrypsin (Figs. 3a ▶ and 3b ▶, Table 1 ▶). The largest crystals were obtained using a concentration of 6.7 µg ml−1 trypsin (Fig. 3 ▶ a). These crystals diffracted to better than 2.5 Å resolution at the 3W1A station of Beijing Synchrotron Research Facility (Fig. 3 ▶ c).
Figure 3.
Crystals of MGG00780 obtained by adding (a) trypsin or (b) α-chymotrypsin; (c) the diffraction image obtained at station 3W1A at Beijing Synchrotron Research Facility (BSRF) from a crystal of MGG00780 obtained by seeding with trypsin. The concentrations and volumes of protease and the numbers and approximate sizes of the crystals in each drop in (a) are listed in Table 1 ▶.
In conclusion, proteases can be added to crystallization drops as ‘seed’ and the volume dispensed can be varied during an experiment. The number and the size of the crystals is affected by the volume of protease added. Most crystallization robots such as Phoenix, Mosquito and Oryx can add protease to the drop instead of seed (or additive) and optimize the concentration by varying the volume of protease in the drop. One possible advantage of this strategy is that the process of screening the effectiveness of different proteases and their optimal concentration may be carried out in situ without the necessity of making up mixtures of protein and protease beforehand.
Acknowledgments
We thank the staff of Shanghai Synchrotron Research Facility (SSRF) beamline BL-17U, Beijing Synchrotron Research Facility (BSRF) stations 3W1A and 1W2B and Diamond Light Source beamlines I02 and I04 for help with crystal screening and data collection. This work was supported by National Natural Science Foundation of China (NSFC) grant No. 31171800 and 973 Project grant No. 2012CB114000 from the Ministry of Science and Technology of China (MOST), as well as by funding from the China Agricultural University (CAU) and the Chinese Universities Scientific Fund (Project No. 2011JS071).
References
- Bergfors, T. (2003). J. Struct. Biol. 142, 66–76. [DOI] [PubMed]
- Dale, G. E., Oefner, C. & D’Arcy, A. (2003). J. Struct. Biol. 142, 88–97. [DOI] [PubMed]
- D’Arcy, A., Villard, F. & Marsh, M. (2007). Acta Cryst. D63, 550–554. [DOI] [PubMed]
- Deivanayagam, C., Cook, W. J. & Walter, M. R. (2007). Methods Mol. Biol. 383, 337–349. [DOI] [PubMed]
- Derewenda, Z. S. (2004). Methods, 34, 354–363. [DOI] [PubMed]
- Dong, A. et al. (2007). Nature Methods, 4, 1019–1021. [DOI] [PMC free article] [PubMed]
- Gaur, R. K., Kupper, M. B., Fischer, R. & Hoffmann, K. M. V. (2004). Acta Cryst. D60, 965–967. [DOI] [PubMed]
- Grabowski, M., Joachimiak, A., Otwinowski, Z. & Minor, W. (2007). Curr. Opin. Struct. Biol. 17, 347–353. [DOI] [PMC free article] [PubMed]
- Kim, Y. et al. (2008). Nature Methods, 5, 853–854. [DOI] [PMC free article] [PubMed]
- Pfuetzner, R. A., Bochkarev, A., Frappier, L. & Edwards, A. M. (1997). J. Biol. Chem. 272, 430–434. [DOI] [PubMed]
- Vedadi, M. et al. (2006). Proc. Natl Acad. Sci. USA, 103, 15835–15840.
- Walter, T. S., Meier, C., Assenberg, R., Au, K. F., Ren, J., Verma, A., Nettleship, J. E., Owens, R. J., Stuart, D. I. & Grimes, J. M. (2006). Structure, 14, 1617–1622. [DOI] [PMC free article] [PubMed]
- Wernimont, A. & Edwards, A. (2009). PLoS One, 4, e5094. [DOI] [PMC free article] [PubMed]