Abstract
Objectives
Early detection of ovarian cancer should improve overall survival. Multiple serum markers have been evaluated as possible tests to detect early stage disease, but few urine markers have been studied. Mesothelin has been detected in serum from patients with ovarian cancer, but has not been previously reported in urine.
Methods
Mesothelin was assayed in the serum and in the urine from 28 patients with early stage (I/II) invasive epithelial ovarian cancers, 111 with advanced stage (III/IV) invasive disease and 19 with tumors of low malignant potential. Marker values have been compared to those in healthy controls and 115 patients with benign pelvic masses. Thresholds were set to include 95% of mesothelin values for 127 sera and 89 urines from healthy women. Urine values were considered: 1) as assayed; 2) normalized using the ratio of serum to urine creatinine; and 3) normalized using the Cockroft-Gault formula for glomerular filtration rate (GFR). Urines were also assayed for human chorionic gonadotropin (hCG) free beta subunit and beta subunit core fragment and similarly normalized.
Results
Optimal sensitivity for early stage disease was obtained when urine mesothelin was normalized using GFR. A greater fraction of patients with early stage disease was detected with the mesothelin urine assay (42%) than with the serum assay (12%). Similarly, 75% of patients with advanced ovarian cancer had elevated mesothelin in urine compared to 48% in serum. Serum and urine levels of mesothelin correlated for early (P=0.02) and late (P<0.001) disease. Urine mesothelin exhibited greater sensitivity for early stage ovarian cancer than did hCG free beta subunit or beta subunit core fragment and complementarity was not observed.
Conclusion
Urine mesothelin deserves further evaluation as a biomarker for detection of early stage ovarian cancer in combination with other urinary markers.
INTRODUCTION
When ovarian cancer is diagnosed in stage I, up to 90% of patients can be cured with currently available therapy. Unfortunately, only 15 to 20% of patients with advanced stage ovarian cancer will be cured of their disease, despite current advances in the surgical and chemotherapeutic management of ovarian cancer. At present, only 25% of ovarian cancers are detected in stage I. Detection of a larger fraction of ovarian cancers in early stage might significantly improve overall survival. The premise that early detection will have a significant impact on patient survival depends on the assumption that advanced metastatic disease arises from clinically detectable stage I lesions. If advanced stage ovarian cancer arises from early stage disease, we would expect to see similar patterns of oncogene activation and tumor suppressor gene loss in all stages of the disease, leading to similar patterns of gene transcription and expression. Results of expression array analysis lend support to the possibility that early stage ovarian cancer is indeed a precursor of late stage disease, at least for the more frequently occurring high grade cancers. Using cDNA microarray analysis, Shridhar, et al, showed that the majority of genes differentially expressed in ovarian cancer when compared to normal ovarian epithelial cell brushings were differentially expressed in both early and late stage high grade tumors. These findings are consistent with the possibility that stage I ovarian cancer is, in fact, the precursor of advanced disease. In the same study, comparative genomic hybridization revealed that gene amplification was more common in late-stage cancers compared to early stage tumors, consistent with substantial evidence that gene amplification is a late event in carcinogenesis (1).
Over the last two decades, attempts to develop effective methods for screening have focused on ultrasonography and serum markers. Given the prevalence of ovarian cancer in the postmenopausal population (1:2,500), an effective strategy must have high sensitivity (>75%) for early stage disease, but even higher specificity (>99.6%) to achieve a positive predictive value of 10%, i.e., 10 laparotomies for each case of ovarian cancer detected. One of the most promising approaches utilizes a two stage strategy, where rising values of serum CA125 trigger transvaginal sonography (TVS) (2). When 6,532 women >50 years of age were screened in the United Kingdom, 16 operations were performed to detect three invasive and one borderline ovarian cancer, providing a specificity of 99.8% and a positive predictive value of 19% (2). This study has encouraged the accrual of 200,000 women into the UKCTOCS trial that will compare conventional diagnosis (100,000) to annual TVS (50,000) to annual CA125 followed by TVS if CA125 values are rising (50,000).
In a two stage strategy, CA125 is not likely to provide an optimally sensitive initial step in that marker levels are elevated in only 50–60% of women with stage I disease at the time of conventional diagnosis(3). Consequently, investigators have sought other markers that might be used in combination with CA125 to detect a greater fraction of patients with early stage disease, while maintaining the requisite specificity to prompt an acceptable fraction of TVS examinations.
Mesothelin (formerly known as soluble mesothelin-related protein) is a serum biomarker that has been shown to complement serum CA125 and to detect a greater fraction of ovarian cancers (4). Mesothelin is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein. The soluble form of mesothelin is thought by some investigators to arise through alternative splicing of the mesothelin gene that disrupts the GPI-anchor motif (5). Others have hypothesized that soluble mesothelin may be a cleavage product of the membrane-bound mesothelin (6). The function of mesothelin is not fully understood. Mesothelin-deficient mice are fertile and exhibit no anatomical abnormalities (7). Recent studies have provided evidence that mesothelin may play a role in cell adhesion, binding to CA125 (8).
While multiple markers including mesothelin have been measured in serum of patients with early stage disease, less attention has been given to potential biomarkers in the urine. Identification of urine markers could provide a more convenient and less invasive initial step in a two stage strategy. Urine proteins are generally small and therefore more thermodynamically stable (9). Urine also tends to be less complex when compared to serum (10) which can be an advantage in proteomic analysis. Recently, eosinophil derived neurotoxin (EDN) and a fragment of osteopontin have been detected in the urine of patients with early stage ovarian cancer (8). In earlier studies, Cole, et al, had reported elevation of human chorionic gonadotropin (hCG) free beta subunit and beta subunit core fragment in the urine of women with ovarian cancer (11). The free beta subunit of hCG is a nicked form of the beta subunit that results in its disassociation from the alpha subunit and this form is removed from circulation at a much greater rate than intact hCG. The free beta subunit can be further degraded in the kidney and excreted into the urine as the beta-subunit core fragment.
In the present study, we have measured mesothelin in the serum and urine of patients with invasive ovarian cancers and tumors of low malignant potential. Values have been compared to those from patients with benign pelvic masses and from healthy controls. To permit comparison with previously studied urine markers for ovarian cancer, hCG free beta subunit and beta subunit core fragment were assayed in the same urine specimens.
MATERIALS AND METHODS
Specimens
All samples were obtained with informed consent on protocols approved by the M.D. Anderson Cancer Center Institutional Review Board. Matched serum and urine specimens were obtained preoperatively from 28 women with early stage ovarian cancer (stage I, II), 111 women with late stage disease (stage III, IV), 19 women with tumors of low malignant potential and 115 women with benign pelvic masses. Histologic diagnoses and clinical characteristics are listed in Table 1. All blood and urine samples were collected preoperatively, kept on ice while in transit, centrifuged for 10 minutes, and the supernatants aliquoted and frozen at −80°C. Sera and urine from healthy controls who participated in an ovarian cancer screening study and who had not developed ovarian cancer were processed under identical conditions. Median age for both the cancer group and the normal group was 61.
Table 1.
Histologic Diagnoses and Clinical Characteristics of the Study Population.
| Years;mean+/−SD | Sample number | |
|---|---|---|
| Normal | 61+/− 7.65 | n=127 |
| Benign Disease | 47+/− 13.5 | n=115 |
| Fibroma and/or cystadenoma | 38 | |
| Mature cystic teratoma | 10 | |
| Endometriosis | 12 | |
| Cyst, endometrioma | 22 | |
| Leiomyoma | 9 | |
| Adenomyosis | 8 | |
| Other | 16 | |
| Low malignant potential tumors | 52+/−14.5 | n=19 |
| Histotype | ||
| Mucinous | 10 | |
| Serous | 7 | |
| Mixed | 2 | |
| Invasive Ovarian Cancer | 61+/−12 | n=139 |
| Histotype | ||
| Serous | 85 | |
| Mixed | 34 | |
| Mucinous | 5 | |
| Endometrioid | 9 | |
| Clear cell | 3 | |
| Other | 3 | |
| Stage | ||
| I | 14 | |
| II | 14 | |
| III | 95 | |
| IV | 16 | |
| Grade | ||
| Low | 13 | |
| Intermediate | 6 | |
| High | 119 | |
| Undetermined | 1 | |
Mesothelin assay
Serum and urine levels of soluble mesothelin protein were determined using Mesomark™, an Enzyme-Linked Immunosorbent Assay (ELISA) produced by Fujirebio Diagnostics, Inc. The assay includes two monoclonal antibodies that react with distinct epitopes on mesothelin: 4H3 is used to capture mesothelin and OV569 is used for detection. Assays were conducted according to the manufacturer’s instructions. Serum samples were prepared as 1:100 dilutions. Urine samples were prepared as 1:10 dilutions. All samples were assayed in duplicate. Serum mesothelin concentrations were determined using calibrators provided in the Mesomark™ kit. Mesothelin concentration is directly related to optical density (OD) detected using a spectrophotometric microtiter plate reader (VERSAmax, Molecular Devices).
Variability between Mesomark™ kits was extremely low as demonstrated by Figure 1A. The percent coefficient of variance (%CV) between assays was 2.49 and 2.68 for high and low controls, respectively. Results were considered valid if duplicates had a %CV ≤ 15%.
Figure 1.
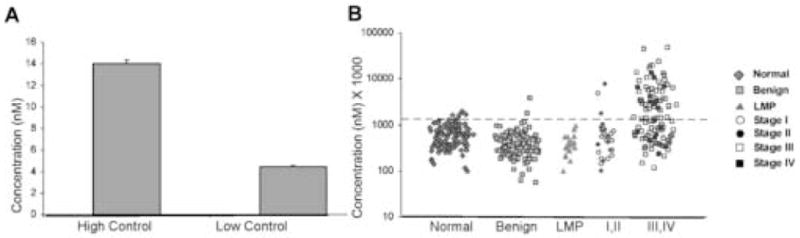
Mesothelin levels are elevated in the serum of patients with ovarian cancer. A) Mean (nM) value for high and low controls over 9 assays. Variablility assessed by calculating the standard deviation. B) Scatterplot of serum mesothelin. Values multiplied by 1,000 and graphed using the logarithmic scale. Serum mesothelin levels in normal controls (n=128), patients with benign pelvic masses (n=115), tumors of low malignant potential (n=19), and patients with early stage (I,II) ovarian cancer (n=28) and late stage (III,IV) ovarian cancer (n=111) are shown.
hCG assays
Free beta subunit and beta subunit core fragment were assayed as previously described at the hCG Reference Laboratory of the University of New Mexico (12). Briefly, beta subunit core fragment concentration was determined using an enzyme-linked immunometric assay. This assay is a microtiter plate “in-house” assay that uses the B210 anti-beta core fragment as the coating antibody and an anti-beta subunit peroxidase as the tracer. Free beta subunit and total hCG were determined using the robotic chemiluminescence DPC Immulite test (DPC Inc.).
Creatinine assays
Serum and urine creatinine values were determined using the Vitros Crea slide method (Vitros Chemistry Products). Patient samples are placed on the multilayered VITROS CREA slide. The final result of a series of enzymatic reactions is read using a leuco dye which generates a blue product. Reflection density is measured at 670nm and is proportional to the concentration of creatinine in the sample.
Data analysis
Mesothelin levels were considered elevated when they exceeded the 95th percentile of normal samples. Urine values of mesothelin (MU) were initially normalized using the ratio of serum creatinine to urine creatinine values:
Urine samples were then normalized using the Cockroft-Gault formula for glomerular filtration rate:
GFR= X 0.85 (female patients).
Similar corrections were calculated for hCG free beta subunit and beta core subunit fragment. Correlations between urine and serum values were tested using the Spearman Rho coefficient.
Receiver Operator Characteristic (ROC) curves
ROC curves were drawn using STATA software.
RESULTS
Mesothelin levels are elevated in the serum of patients with early and late stage ovarian cancer
As a standard cutoff value for mesothelin has not been established, we used the 95th percentile of mesothelin values for healthy controls as a threshold. Mesothelin was elevated in sera from 48% of late stage ovarian cancer patients and 12% of early stage patients at 95% specificity. Importantly, only 1.7% of serum taken from patients with benign masses and 0% of patients with LMP tumors had elevated mesothelin. (Fig. 1B)
Levels of mesothelin are elevated in the urine more frequently than in serum from ovarian cancer patients
When urine was assayed from the same donors, mesothelin was elevated in 23% of early cancers and 68% of late cancers (Fig. 2A and Table 2). As physiologic variation in renal tubular resorption of water can affect the concentration of all analytes in individual urine samples taken at different times, we examined two methods of normalization that would take renal function into account. In the first, urine mesothelin values were normalized using the ratio of serum and urine creatinine levels. With this method, sensitivity for early stage disease was increased minimally to 25%, whereas sensitivity for late stage disease remained at 68% (Fig. 2B). Normalization using this method improved specificity slightly as demonstrated by the decrease from 13% to 11% for benign samples identified with elevated mesothelin values. In the second method, urine mesothelin values were normalized using the glomerular filtration rate calculated with the Cockroft-Gault formula that takes into account the weight and age of each donor, as well as the serum and urine creatinine. Normalization by this second method increased sensitivity for early stage disease to 42% and sensitivity for late stage cancers to 75%. (Fig. 2C and Table 2) Some specificity was lost with the fraction of elevated mesothelin levels in patients with benign pelvic masses increasing to 20%. Similar sensitivity (16–17%) for tumors of low malignant potential was observed with all three methods of analysis.
Fig. 2.
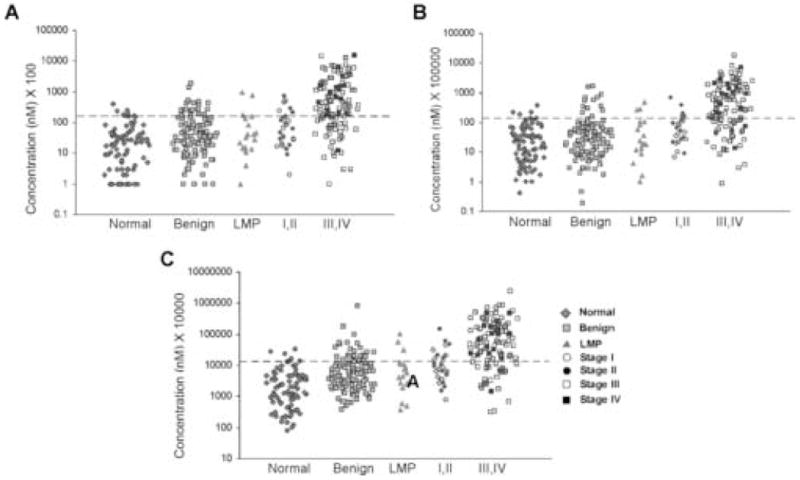
Mesothelin levels are elevated in the urine of patients with ovarian cancer. A) Scatterplot of unadjusted urine mesothelin. Values multiplied by 1,000 and graphed using a logarithmic scale. Urine mesothelin levels in normal controls (n=89), patients with benign pelvic masses (n=115), tumors of low malignant potential (n=19), and patients with early stage (I,II) ovarian cancer (n=28) and late stage (III,IV) ovarian cancer (n=111). B) Scatterplot of creatinine normalized urine mesothelin values. Values multiplied by 100,000 and graphed using a logarithmic scale. Urine mesothelin levels in normal controls (n=86), patients with benign pelvic masses (n=114), tumors of low malignant potential (n=19), and patients with early stage (I,II) ovarian cancer (n=28) and late stage (III,IV) ovarian cancer (n=108). C) Scatterplot of GFR normalized urine mesothelin values. Values multiplied by 10,000 and graphed using the logarithmic scale. Urine mesothelin levels in normal controls (n=86), patients with benign pelvic masses (n=114), tumors of low malignant potential (n=18), and patients with early stage (I,II) ovarian cancer (n=28) and late stage (III,IV) ovarian cancer (n=108).
Table 2.
Elevation of mesothelin in serum (MS) and in urine (MU) from healthy women and from patients with benign lesions, tumors of low malignant potential, early stage ovarian cancer (EOC) and late stage ovarian cancer (LOC). Urine values are presented as assayed or based on the ratio of urine creatinine to GFR (MGFR).
| MS | MU | MGFR | ||||
|---|---|---|---|---|---|---|
| ROC-AUC | 95% | ROC-AUC | 95% | ROC-AUC | 95% | |
| Normal vs Benign | 74 | 2% | 63 | 13% | 71 | 20% |
| Normal vs All Cancer | 68 | 40% | 88 | 59% | 91 | 68% |
| Normal vs EOC | 41 | 12% | 75 | 23% | 85 | 42% |
| Normal vs LOC | 75 | 48% | 91 | 68% | 93 | 75% |
| Benign vs All Cancer | 81 | 55% | 81 | 43% | 81 | 49% |
| Benign vs EOC | 62 | 23% | 63 | 12% | 67 | 6% |
| Benign vs LOC | 86 | 64% | 85 | 52% | 84 | 58% |
When serum mesothelin values were compared to urine values corrected for GFR from the same donors, a greater fraction of patients with early stage disease was detected with the mesothelin urine assay (42%) than with the serum assay (12%) (p< 0.0001). Similarly, 75% of patients with advanced ovarian cancer had elevated mesothelin in urine compared to 48% in serum.
A significant correlation is observed between serum and normalized urine SMRP values
A possible correlation between matched serum and urine specimens was tested using the Spearman rho coefficient. A significant correlation between serum and urine mesothelin levels was observed in patients with benign pelvic masses, early stage and late stage ovarian cancer (Table 3). The most significant association was observed in late stage ovarian cancer patient samples (p<0.001).
Table 3.
Correlation between serum (MS) and urine mesothelin values using the Spearman rho correlation coefficient for specimens from healthy women and from patients with benign lesions, tumors of low malignant potential, early stage ovarian cancer and late stage ovarian cancer.
| MS vs MU | MS vs MGFR | |
|---|---|---|
| Normal | 0.22 (p=0.04) | 0.16 (p=0.14) |
| Benign | 0.44 (p<0.001) | 0.45 (p<0.001) |
| LMP | −0.07 (p=0.77) | 0.29 (p=0.24) |
| EOC | 0.39 (p=0.04) | 0.43 (p=0.02) |
| LOC | 0.62 (p<0.001) | 0.66 (p<0.001) |
Urinary hCG free subunit and beta core fragment are elevated in early stage ovarian cancer, but their sensitivity is lower than mesothelin
Levels of the hCG free beta subunit and the beta subunit core fragment were analyzed in the same set of urines (Fig. 3). A cutoff value of 0.04ng/mg creatinine for hCG and its subunits or degradation products was established in the early 1990’s by Laurence Cole and colleagues (personal communication). Using this cutoff value for free beta subunit, we were able to detect 15% and 16% of early and late cancers, respectively (Table 4). Sensitivity using the beta subunit core fragment was better, where levels were elevated in 31% of early cancers and 67% of late cancers. Using this cutoff value, however, produced a prohibitive number of false positives with 12% of normal samples positive for free beta subunit and 24% of normal samples positive for beta subunit core fragment.
Fig. 3.
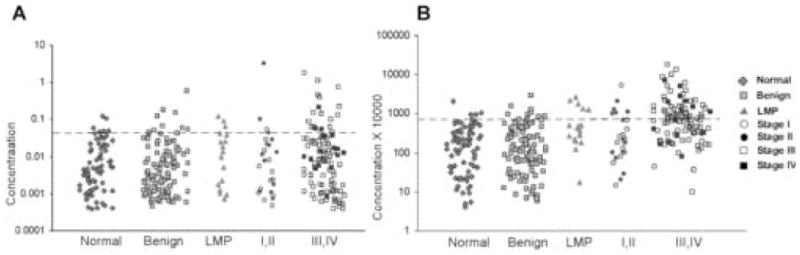
hCG free beta subunit and hCG beta subunit core fragment levels are elevated in a limited number of urines from patients with early stage ovarian cancer. A) Scatterplot of GFR normalized urinary free beta subunit values. Urine hCG free beta subunit levels in normal controls (n=84), patients with benign pelvic masses (n=106), tumors of low malignant potential (n=18), and patients with early stage (I,II) ovarian cancer (n=26) and late stage (III,IV) ovarian cancer (n=105) are shown. B) Scatterplot of GFR normalized urinary hCG beta subunit core fragment values. Urine hCG free beta subunit levels in normal controls (n=84), patients with benign pelvic masses (n=106), tumors of low malignant potential (n=18), and patients with early stage (I,II) ovarian cancer (n=26) and late stage (III,IV) ovarian cancer (n=105) are shown.
Table 4.
Elevation of hCG free beta subunit and hCG beta subunit core fragment using a threshold of 0.04ng/mg urinary creatinine to define healthy donors.
| hCG Free Beta Subunit | hCG Beta Subunit Core Fragment | |
|---|---|---|
| Normal | 12% (10/84) | 24% (20/80) |
| Benign Disease | 4% (5/112) | 17% (19/112) |
| LMP | 16% (3/19) | 37% (7/19) |
| Early Ovarian Cancer | 15% (4/26) | 31% (8/26) |
| Late Ovarian Cancer | 16% (17/106) | 67% (71/106) |
Urine values of the two moieties were analyzed after setting a threshold to exclude 95% of healthy donors from our own study. Using the 95th percentile of normal as a cutoff, both markers exhibited dramatically reduced sensitivity. Normalization of hCG values was performed as described for mesothelin. Little improvement was observed with the two different methods of normalization. Results of urinary mesothelin values as assayed and values normalized using the glomerular filtration rate are presented in Table 5. In general, hCG beta core fragment was a more sensitive marker than free beta subunit for both early and late stage ovarian cancer. When values were normalized to GFR, sensitivity for early stage disease was 13% for the free beta subunit and 23% for the beta core fragment. Values for advanced disease were 18% and 49%, respectively. When hCG beta core fragment or free beta subunit were normalized to GFR and compared to MGFR, greater sensitivity (p<0.001) was observed with mesothelin (Table 6). Inspection of individual values indicated that MGFR was elevated in all of the early stage cases where elevation of GFR normalized beta core fragment was found. Among the 28 early stage cancers studied, free beta subunit detected only 1 of the 15 cases with normal levels of MGFR. Consequently, significant complementarity between the hCG subunits and mesothelin was not observed (p= 0.3755).
Table 5.
Elevation of hCG free beta subunit (FBS) and hCG beta subunit core fragment (BCF) using values as assayed or Glomerular Filtration Rate (GFR)-normalized values for healthy individuals (N), benign disease (B), early ovarian cancer (EOC) and late ovarian cancer (LOC). Thresholds were set to exclude values from urines of 95% of healthy women.
| hCG FBSU | hCG FBSGFR | hCG BCFU | hCGBCFGFR | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | 95% | AUC | 95% | AUC | 95% | AUC | 95% | |
| N vs B | 59 | 0% | 43 | 6% | 55 | 0% | 47 | 9% |
| N vs C | 56 | 15% | 55 | 16% | 76 | 26% | 77 | 44% |
| N vs EOC | 48 | 8% | 51 | 13% | 54 | 12% | 58 | 23% |
| N vs LOC | 58 | 18% | 56 | 18% | 81 | 29% | 82 | 49% |
| B vs C | 63 | 23% | 61 | 16% | 78 | 32% | 77 | 30% |
| B vs EOC | 56 | 8% | 58 | 8% | 58 | 15% | 60 | 23% |
| B vs LOC | 65 | 27% | 62 | 18% | 84 | 37% | 81 | 32% |
Table 6.
Elevation of urinary hCG free beta subunit (FBSGFR), hCG beta subunit core fragment (BCFGFR) and urinary mesothelin (MGFR) from healthy women (N) and from patients with benign lesions (B), patients with early stage ovarian cancer (EOC) and late stage ovarian cancer (LOC). Thresholds were set to include 95% of urine values from healthy women.
| hCG FBSGFR | hCG BCFGFR | MGFR | M+FBS+BCF | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | 95% | AUC | 95% | AUC | 95% | AUC | 95% | |
| N vs B | 43 | 6% | 47 | 9% | 71 | 20% | 72 | 21% |
| N vs C | 55 | 16% | 77 | 44% | 91 | 68% | 92 | 73% |
| N vs EOC | 51 | 13% | 58 | 23% | 85 | 42% | 85 | 42% |
| N vs LOC | 56 | 18% | 82 | 49% | 93 | 75% | 95 | 78% |
| B vs C | 61 | 16% | 77 | 30% | 81 | 49% | 84 | 49% |
| B vs EOC | 58 | 8% | 60 | 23% | 67 | 6% | 59 | 12% |
| B vs LOC | 62 | 18% | 81 | 32% | 84 | 58% | 90 | 54% |
ROC curves illustrate the benefit of GFR corrected urine mesothelin in the detection of early stage ovarian cancer
When Receiver Operator Characteristic (ROC) curves were compared, GFR corrected urine mesothelin was a significantly better marker than serum mesothelin (Figs. 4 and 5). ROC curves clearly show a greater sensitivity for early stage disease using GFR-adjusted urinary mesothelin (AUC=85%) compared to serum mesothelin (AUC=41%) (Fig. 5). Urinary HCG beta core fragment and free beta subunit did not improve upon the ROC curve for urine mesothelin alone for all patients or early stage patients (Fig. 5). Patients with tumors of low malignant potential were not included in this analysis.
Fig. 4.
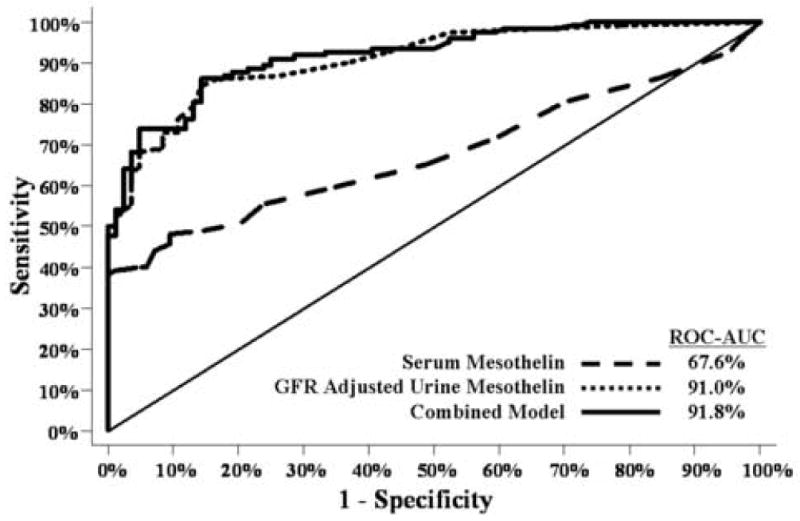
Ability of serum mesothelin, GFR-adjusted urine mesothelin and a combination of GFR-adjusted urine mesothelin, hCG free beta and hCG beta core to differentiate between normals and all stage (I–IV) ovarian cancer patients (n=207), (p<0.0001).
Fig. 5.
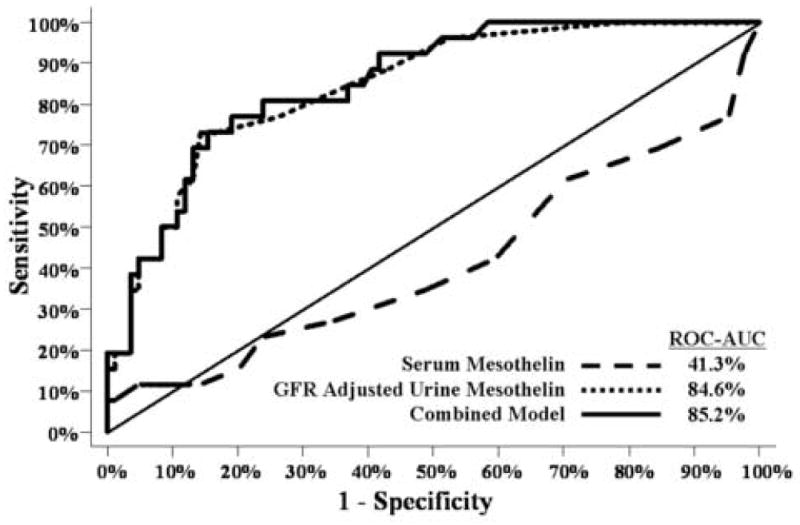
Ability of serum mesothelin, GFR-adjusted urine mesothelin and a combination of GFR-adjusted urine mesothelin, hCG free beta and hCG beta core to differentiate between normals and early stage (I–II) ovarian cancer patients (n=110), (p<0.0001).
Serum CA125 values provide some complementarity to urinary mesothelin values in the detection of early stage ovarian cancer
Matching serum from these patients was analyzed for CA125 expression levels. CA125 levels were elevated (values greater than 35 units/ml) in 21/28 (75%) of early cancer patient samples. When individual samples were analyzed for serum CA125 and GFR-normalized mesothelin urine levels, 23/28 (82%) early cancer samples could be detected using the criteria that one or both markers were elevated.
DISCUSSION
Other investigators have reported elevated levels of mesothelin in sera from 60–77% of women with ovarian cancer (Table 7). In these three studies, very few samples from women with early stage disease were evaluated and thresholds for normal values varied from study to study. In our present report, a sensitivity of 48% was observed in patients with advanced disease using a cutoff that would exclude 95% of healthy women. Consequently, mesothelin exhibited a somewhat lower sensitivity in our patient population. Data from the larger number of patients evaluated in this study may be more representative of the potential of mesothelin as a stand-alone marker. As mesothelin is, however, elevated in a fraction of patients with normal serum CA125 (4), the most useful application of this marker may be in combination with CA125 to detect early stage disease.
Table 7.
Elevation of mesothelin in sera from patients with epithelial ovarian cancer in three published studies.
| Sensitivity | Specificity | Stage, sample# | Reference | Comments |
|---|---|---|---|---|
| 77% | 100% | Stages III, IV n=68 (normal) n=30 (cancer) | Scholler, 1999 | Most cancer patients undergoing chemo, measured O.D. above normal |
| 60% | 98% | n=42 (late cancer) n=10 (early cancer) n=220 (normal) |
McIntosh, 2004 | 80% cases were late stage, measured sensitivity at 98% specificity of ROC curve |
| 67% | 100% | Stages III, IV n=21 | Hassan, 2006 | Prior surgical debulking, chemo, used 9ng/ml as cutoff |
Our study documents for the first time that urinary levels of mesothelin provide a more sensitive marker than serum levels of mesothelin for distinguishing patients with early and late stage ovarian cancer from healthy controls. Normalization of urinary mesothelin using the ratio of urine creatinine to GFR improved sensitivity for detecting early stage disease at a constant specificity of 95%. Although investigators have utilized urine creatinine to compensate for more or less dilute urine in the past, so far as we know, this is one of the first instances where correction for GFR has improved the sensitivity of a urinary biomarker. When specificity was set at 95%, early stage disease was detected using urinary mesothelin with a sensitivity of 42%, compared to a sensitivity of 23% with hCG beta core fragment, one of the best studied urinary markers for ovarian cancer. Had we used the lower standard cutoff for hCG beta core fragment, higher sensitivity could have been obtained, but at the cost of a substantial reduction in specificity. The lower specificity of hCG beta core fragment in our study population may reflect the predominance of postmenopausal women among our ovarian cancer patients and among the healthy controls from our screening study. Levels of hCG beta core fragment have been shown to increase with age in normal women (11).
When we examined the relationship between histology and urinary mesothelin levels, we found that none of the five mucinous tumors (0%) and only one of the nine endometrioid tumors (11%) had elevated mesothelin in the urine whereas 77.6% of serous and 71% of mixed tumors had elevated levels. One of the three clear cell tumors (33%) was detected using the urine mesothelin assay. While the low detection rate of mucinous and endometrioid tumors in this study is interesting, the number of tumors included in the study is too small to allow any conclusions to be made from these results. Studies including larger numbers of mucinous and endometrioid tumors will be required to clarify this result. Importantly, serous tumors are the most frequently occurring histotype among the invasive ovarian cancers and observation that a large fraction of serous cancers can be detected using elevated urinary mesothelin is encouraging.
Detection of mesothelin in urine from a significant fraction of women with early stage ovarian cancer is encouraging, but a sensitivity of 42% for early stage disease falls far short of the sensitivity required for an effective screening strategy. Given the prevalence of ovarian cancer in the postmenopausal population (1:2500), even at a specificity of 99.6%, a sensitivity of 75% would be required to attain a positive predictive value of 10%. Ideally, the sensitivity of an initial step, in a two stage screening strategy would exceed 90%. Although urinary mesothelin cannot serve as a stand alone marker for detection of ovarian cancer, it might, as in the case of serum mesothelin, be used in combination with other analytes to achieve an appropriate sensitivity. Data presented above suggest that use in combination with hCG free beta subunit or hCG beta core fragment would not improve sensitivity, as significant complementary was not observed. Recently, Kruk et al, have reported that elevated levels of Bcl-2 could be found in the urine from more than 92% of women with ovarian cancers (13). It will be of great interest to determine whether mesothelin and Bcl-2 prove complementary for early stage disease.
To obtain adequate numbers of urines for analysis, patients have been grouped as early stage (I–II) or late stage (III–IV). Detection of disease in stage I is likely to have a greater impact than detection in stage II, although the latter clearly has a survival advantage when compared to stage III and IV. Patients with Stage I and Stage II disease were detected equally well using mesothelin urine values in the early stage group. As additional samples are accrued, we will be able to define the sensitivity for stage I with greater precision. Identification of markers such as mesothelin, Bcl-2, EDN and osteopontin (13), (10) underlines the need for banking of urine as well as serum from ovarian cancer patients to permit rapid assessment of new biomarkers.
Remarkably, urinary mesothelin detected 17% of LMP tumors. Gilks, et al, using tissue arrays found higher levels of mesothelin protein in LMP tumors than in late stage serous carcinomas (14). Our group found several biomarkers, including mesothelin, that complement CA125 at the level of tissue. In our study, 34% of tumor tissues that lacked CA125 staining expressed mesothelin (15). Although mortality from LMP tumors is often delayed, their detection would not be a disadvantage.
The specificity of urinary mesothelin is limited. Some 21% of benign pelvic lesions were associated with elevated levels. Mesothelin could, however, still prove useful in a two stage screening strategy where elevated levels of urine markers triggered transvaginal sonography (TVS) that could distinguish many benign lesions from malignant disease. In the context of a two stage strategy, the choice of a 95% cutoff for healthy individuals would mandate TVS in 5% of the screened population which should still be feasible.
Development of a panel of urinary biomarkers for early detection of ovarian cancer could provide a more convenient and potentially more cost-effective screening strategy that did not require the discomfort and expense of phlebotomy. One might even imagine the development of self-administered colorimetric assays that would be used more widely. Realization of this vision will require the development of a panel of urinary markers that, in aggregate, can achieve greater than 90% sensitivity at 95% specificity. Proteomic techniques might aid in discovery of such markers.
Acknowledgments
Dr. Bast receives royalties for CA125 and the Bast lab receives research support for this project from Fujirebio Diagnostics, Inc.
This work has been funded by the M.D. Anderson SPORE in Ovarian Cancer (P50 CA083639) from the National Cancer Institute, Department of Health and Human Services; by a contract from Fujirebio Diagnostics, Inc., and by philanthropic support from the Mossy Foundation, the Golfers Against Cancer and the Tracy Jo Wilson Ovarian Cancer Foundation. D.B. is the recipient of a fellowship from the Ovarian Cancer Research Fund. We thank Joseph Celestino, Mary A. Hernandez, R.N. and Sabrina Morales for their help in obtaining serum and urine specimens. We thank Craig Miller for his statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, et al. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res. 2001 Aug 1;61(15):5895–904. [PubMed] [Google Scholar]
- 2.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005 Nov 1;23(31):7919–26. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC., Jr Status of tumor markers in ovarian cancer screening. J Clin Oncol. 2003 May 15;21(10 Suppl):200–5. doi: 10.1200/JCO.2003.01.068. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh MW, Drescher C, Karlan B, Scholler N, Urban N, Hellstrom KE, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004 Oct;95(1):9–15. doi: 10.1016/j.ygyno.2004.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11531–6. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muminova ZE, Strong TV, Shaw DR. Characterization of human mesothelin transcripts in ovarian and pancreatic cancer. BMC Cancer. 2004 May 12;4:19. doi: 10.1186/1471-2407-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000 Apr;20(8):2902–6. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004 Mar 5;279(10):9190–8. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Tsai CJ, Nussinov R. Temperature range of thermodynamic stability for the native state of reversible two-state proteins. Biochemistry. 2003 May 6;42(17):4864–73. doi: 10.1021/bi027184+. [DOI] [PubMed] [Google Scholar]
- 10.Ye B, Skates S, Mok SC, Horick NK, Rosenberg HF, Vitonis A, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006 Jan 15;12(2):432–41. doi: 10.1158/1078-0432.CCR-05-0461. [DOI] [PubMed] [Google Scholar]
- 11.Cole LA, Nam JH. Urinary gonadotropin fragment (UGF) measurements in the diagnosis and management of ovarian cancer. Yale J Biol Med. 1989 Jul-Aug;62(4):367–78. [PMC free article] [PubMed] [Google Scholar]
- 12.Cole LA, Isozaki T, Jones EE. Urine beta-core fragment, a potential screening test for ectopic pregnancy and spontaneous abortion. Fetal Diagn Ther. 1997 Nov-Dec;12(6):336–9. doi: 10.1159/000264500. [DOI] [PubMed] [Google Scholar]
- 13.Kruk PABY, Saunders BO, Wilbanks GD, Cantor A, Nicosia SV. Detection of ovarian cancer by elevated urinary levels of Bcl-2. Proc Amer Assoc Cancer Res. 2006;47:572–3. [Google Scholar]
- 14.Gilks CB, Vanderhyden BC, Zhu S, van de Rijn M, Longacre TA. Distinction between serous tumors of low malignant potential and serous carcinomas based on global mRNA expression profiling. Gynecol Oncol. 2005 Mar;96(3):684–94. doi: 10.1016/j.ygyno.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005 Nov;99(2):267–77. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]


