Abstract
The rate of RNA elongation by RNA polymerase II (pol II) is affected by DNA sequences called intrinsic arrest sites. Efficient transcription through these sites requires elongation factor SII. In addition to the sequence-specific features of the DNA, we show that the acquisition of SII-dependence is a function of its “dwell-time” at an arrest site. This temperature-dependent decay in elongation potential appears irreversible, implying that factor-dependent and factor-independent elongation complexes are not mutually interconvertible at this position. TFIIF and NH4Cl are known to increase the elongation rate of pol II. Both agents preempt arrest, consistent with the idea that elongation dwell time influences the process. TFIIF and SII act upon different steps in a complementary way to prevent or resolve arrest, respectively. They are probably instrumental in facilitating the efficient transcription of large eukaryotic genes in vivo.
RNA polymerases are processive enzymes. In general, they cannot extend pre-existing RNA chains, hence RNA release from the template is a terminal event in RNA synthesis (reviewed in Kane, 1994). It is especially detrimental for RNA polymerase II (pol II)1 to terminate prematurely since it transcribes the longest nuclear transcription units, some of which can exceed millions of base pairs. Furthermore, chain elongation is a regulated process that controls gene expression (Kane, 1994) and embryonic development (Thummel, 1992). In vivo, processing of the primary transcript for polyadenylation is coupled to termination by pol II (Logan et al. 1987; Connelly and Manley, 1988). Thus, pol II has evolved to be extremely processive once it becomes template engaged, and a specialized release signal is required for termination.
At specific sites within genes, pol II becomes unable to continue chain elongation but does not release its nascent RNA. This loss of elongation competence is referred to as arrest, the molecular mechanism of which is poorly understood. Same arrest sequences are coincident with a bend in the DNA (Kerppola and Kane, 1990). Template sequence and structure is an important component of the arrest signal since randomly halting transcription by nucleotide starvation does not typically result in arrest. Arrested complexes are not inactive; they resume transcription through the action of elongation factor SII (also known as TFIIS).
SII, a pol II-binding protein, associates with the elongation complex and activates nascent RNA cleavage (Reines, 1992; Izban and Luse, 1992b; Wang and Hawley, 1993; reviewed in Reines, 1994). Transcript shortening precedes, and appears required for, SII-mediated readthrough (Reines et al., 1992; Izban and Luse, 1992b). Multiple rounds of nascent RNA cleavage and re-extension are required to achieve full readthrough (Gu et al., 1993; Guo and Price, 1993). Although the exact means by which arrested complexes are reactivated are not understood, recent models postulate that cleavage restores the proximity of the enzyme’s catalytic site to the 3′-end of the nascent RNA, the topology of which is altered during arrest (Chamberlin, 1994; Izban and Luse, 1993b; Borukhov et al., 1993; Nudler et al., 1994). SII is expressed in a number of species and tissue types (Hirashima et al., 1988; Marshall et al., 1990; Yoo et al., 1991; Kanai et al., 1991; Nakanishi et al., 1992; Chen et al. 1992; Xu et al., 1994) and allows readthrough of other types of blacks to pol II elongation including DNA-bound proteins and drugs (Reines and Mote, 1993; Mote et al., 1994).
We have measured here the process by which pol II becomes arrested on a portion of a human gene in vitro. The acquisition of factor dependence is a first-order decay of the elongation potential of pol II that is temperature dependent and irreversible in the absence of RNA cleavage. We have analyzed the effect of NH4Cl and elongation factor TFIIF, which are known to reduce the average time pol II spends between nucleotide incorporation events, an the arrest process. Although mechanistically distinct, SII and TFIIF probably cooperate in enabling pol II to transcribe lengthy eukaryotic genes.
MATERIALS AND METHODS
Proteins and Reagents
RNA polymerase II, SII, and initiation factors were isolated from rat liver or recombinant sources as described (Gu et al., 1993). Recombinant TFIIF (RAP30/74) was purified from Escherichia coli as described (Tan et al. 1994). Fast protein liquid chromatography-purified nucleotides were purchased from Pharmacia-LKB Biotechnology. Formalin-fixed Staphylococcus aureus was purchased from Life Technologies, Inc. [α-32P]CTP was obtained from Amersham Corp.
In Vitro Transcription
In vitro transcription was reconstituted as described (Reines, 1991). Briefly, 100 ng of plasmid pAdTerm-2 (Reines, et al., 1989) cut with EcoRI and PstI were incubated with rat liver RNA polymerase II (0.5 µg) and fraction D (TFIID and TFIIH, 2 µg) in 20 µl of 20 mm HEPES, pH 7.9, 20 mm Tris-HCl, pH 7.9, 2.2% (w/v) polyvinyl alcohol, 0.5 mg/ml acetylated bovine serum albumin, 150 mm KCl, 2 mm dithiothreitol, and 3% (v/v) glycerol for 30 min at 28 °C.The same buffer (lacking KCl) containing liver TFIIE/F (1 µg) and rTFIIB were added and incubation continued for 20 min. A 14-nucleotide transcript was synthesized by incubation at 28 °C for 20 min with MgCl2 (7 mm), ATP, UTP, and CTP (20 µm each). Where indicated, RNA was labeled at the 5′-end with ≈0.6 µm[α-32P]CTP (400 Ci/mmol). Chain elongation followed the addition of heparin to 10 µg/ml and ATP, GTP, UTP, and CTP to 800 µm each. After 15 min, complexes were immunoprecipitated with anti-RNA IgG and fixed S. aureus (Eilat et al., 1982; Reines, 1991). For 3′-end labeling of the RNA and walking experiments complexes were washed three times by microcentrifugation and resuspension in reaction buffer (60 mm KCl, 20 mm Tris-HCl, pH 7.9, 2 mm dithiothreitol, 3 mm HEPES-NaOH, pH 7.9, 0.5 mm EDTA, 0.2 mg/ml bovine serum albumin, 2.2% (w/v) polyvinyl alcohol, 3% (v/v) glycerol). Rat liver SII (phasphocellulose) was added, and the reaction was incubated at 28 °C for 1.5 min in the presence of 7 mm MgCl2 for nascent RNA cleavage. After two washings, RNA was labeled with 20 µCi of [α-32P]CTP (3000 Ci/mmol), 400 µm GTP (unless otherwise indicated) and 7 mm MgCl2 at 28 °C for 10 min. These complexes were washed again and manipulated as described in the text. RNA was prepared for electrophoresis and separated on 5% polyacrylamide gels as described (Reines, 1992). For quantitation, gels were analyzed using a Molecular Dynamics PhosphorImager.
RESULTS
To examine the arrest process under conditions that allow us to control elongation, we employed RNA polymerase “walking” experiments. Elongation is restricted by providing a limited subset of the four nucleoside triphosphates (NTPs), removing them, and repeating the process with the next appropriate NTP(s) to assemble elongation complexes bearing sequentially larger RNA chains. Similar approaches have been exploited effectively in the study of bacterial and viral RNA polymerase elongation and termination (Carpousis and Gralla, 1985; Levin et al., 1987; Metzger et al., 1989; Hagler and Shuman, 1992, Krummel and Chamberlin, 1989, 1992a, 1992b; Nudler et al., 1994). A portion of the human histone H3.3 gene arrest site studied here is shown in Fig. 1. Transcription was initiated from the adenovirus major late promoter with partially purified rat liver initiation factors, enabling us to study SII-dependent transcription (Reines et al., 1989). Upon encounter with a well-characterized site called Ia, pol II becomes arrested with ≈50% efficiency at positions +205, 206, and 207 relative to the transcription start site (+1) (Gu et al., 1993). In the presence of SII, pol II quantitatively resumes elongation from this site after the arrested RNA is shortened at its 3′-end and re-extended (Reines et al., 1989).
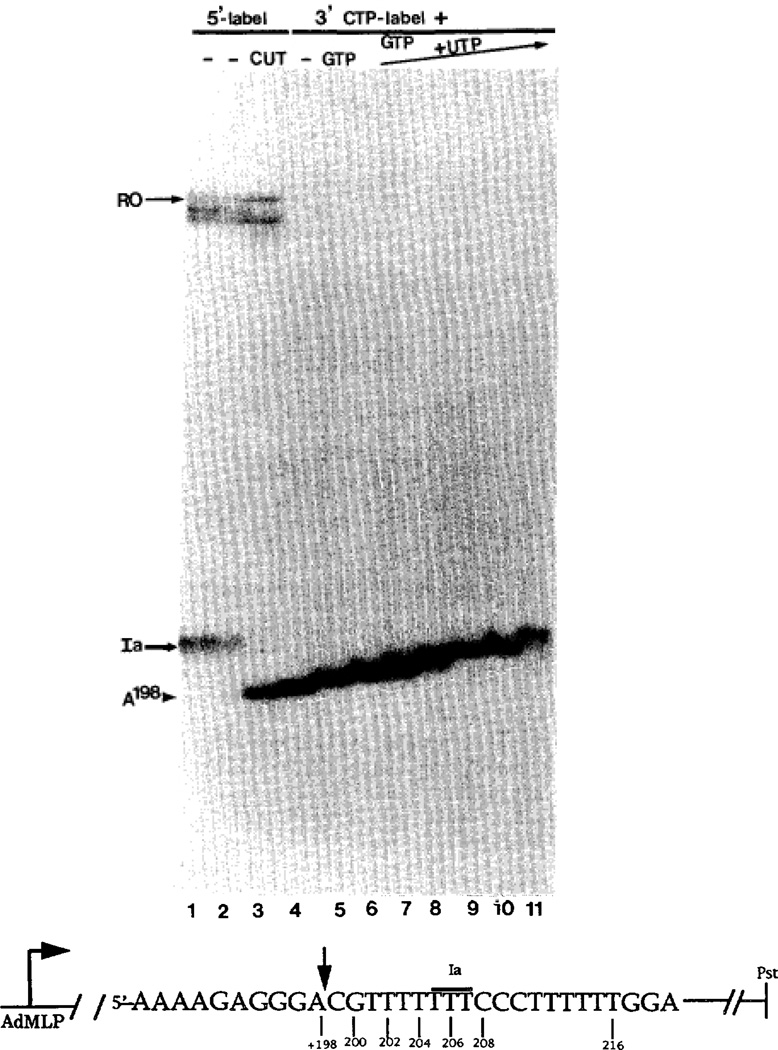
Fig. 1. Assembly and “walking” of pol II elongation complexes.
Arrested complexes were washed free of NTPs and treated with SII to yield a 198-nucleotide RNA (A198; vertical arrow on sequence). Uncleaved (duplicate lanes 1 and 2) and cleaved (lane 3) RNAs labeled at their 5′-ends are shown. Unlabeled A198 complexes were independently 3′-end labeled with [α-32P]CTP only (C199; lane 4), or [α-32P]CTP and GTP (G200 complexes; lane 5). G200 complexes were washed and extended with varying amounts of UTP (lane 6, 1 µm; lane 7, 2 µm, lane 5, 4 µm; lane 9, 16 µm; lane 10, 40 µm; lane 11, 400 µm) for 10 min. Non-template DNA sequence around site Ia is shown. Arrested Ia-RNA is overlined.
Arrested complexes were precipitated with a monoclonal antibody against RNA and washed free of NTPs (Reines, 1992). After the addition of MgCl2 and SII, the nascent transcripts (205–207 nt) were cleaved to a specific 198-nucleotide transcript terminating in an A residue (Fig. 1, lane 2 versus lane 3; Gu et al., 1993). We refer to this as an A198 complex using the conventional notation citing the identity of the last base and its position in the RNA chain. Unlabeled A198-RNA was extended and uniquely labeled at position 199 with [α-32P]CTP (C199 Fig. 1, lane 4). This RNA could be extended by a single base when GTP was added (G200 lane 5). Addition of varying concentrations of UTP to G200 complexes after removal of CTP and GTP by another round of washing resulted in extension of the chain by an additional 1–7 bases through a tract of seven template A residues (U201–U207 lanes 6–11).
Complexes A198 through U204 were stable and each remained elongation competent, i.e. they did not require SII for the resumption of elongation when UTP was restored (Fig. 2, lane 1 versus lane 8, lane 2 versus lane 3, lane 4 versus lane 5, and lane 6 versus lane 7). Since CTP (required at position 208) was not added during this chase period, RNAs were extended no further than position 207 (Ia; Fig. 2, lanes 3, 5, 7, and 8). If CTP was supplied at the same time as UTP, half of these complexes readthrough site Ia in a factor-independent manner and stop at position 216 due to the lack of GTP (Fig. 3A, lane 6). This confirmed prior work demonstrating that the readthrough efficiency in the absence of SII is ≈50% for both the first and second encounter of pol II with site Ia (Fig. 1A. lane 1 and Fig. 3B, lane 1; Gu et al., 1993). The complexes arrested at site Ia can be quantitatively chased to runoff length by the addition of SII (Gu et al., 1993). Hence, two types of complexes bearing a 207-base transcript can be identified after transcript cleavage and walk-back; one group that can extend the 207-base RNA when CTP is present (U216; Fig. 3A, lane 6), and another that requires CTP and the assistance of SII (Ia; Fig. 3A, lane 6). We will show below that this former class can be converted rapidly into the latter class. The reciprocal conversion has not been observed.
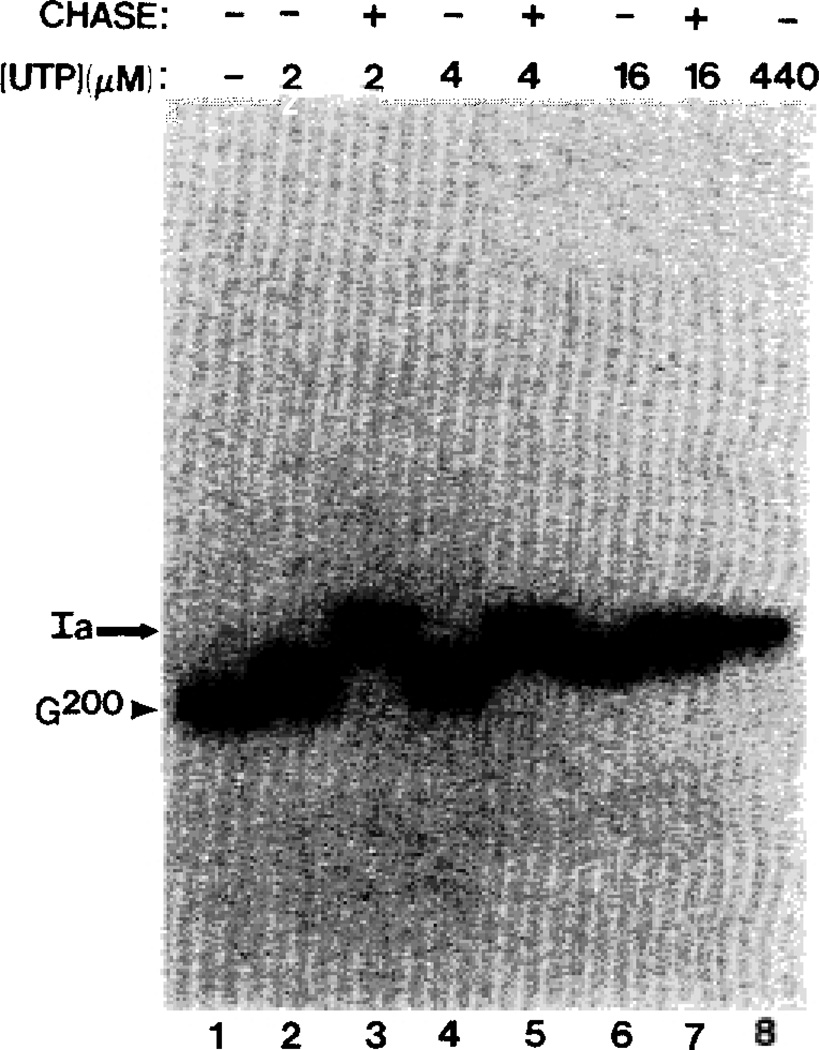
Fig. 2. Elongation from G200 to site Ia is SII-independent.
Labeled G200 complexes were washed (lane 1) and extended with the indicated concentrations of UTP for 10 min. A sample at each UTP concentration was stopped (−) or chased with 400 µm UTP (+) for 10 min in the presence of 0.25% Sarkosyl (lanes 3, 5, and 7).
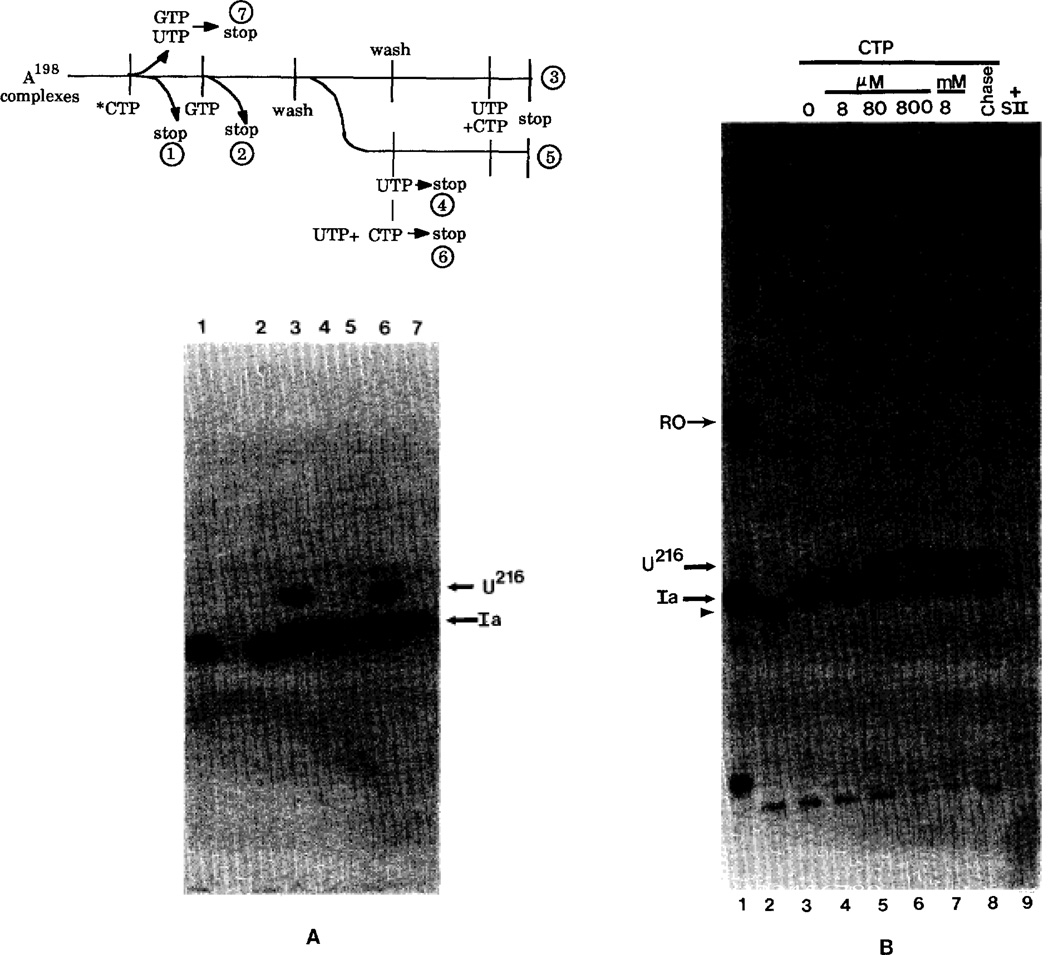
Fig. 3. Effect of the next nucleotide upon transcription arrest.
A, A198 complexes were labeled and extended with [α-32P]CTP alone (lane 1), with GTP (lanes 2–6), or with GTP and UTP (lane 7). Complexes extended with [α-32P]CTP and GTP were either stopped (lane 2) or washed to remove NTPs (lanes 3–6). Aliquots were incubated with 800 µm UTP (lane 4) or 400 µm each of UTP and CTP (lane 6) for 10 min and stopped. One aliquot (lane 5) was incubated with 800 µm UTP for 10 min then washed and incubated with 400 µm UTP and 400 µm CTP for 10 min. Some G200 complexes (lane 3) were washed a second time and provided with 400 µm each of UTP and CTP for 10 min. Elongation took place in the presence of 0.25% Sarkosyl and 7 mm MgCl2. Readthrough is monitored by accumulation of RNA at position U216. B, labeled G200 complexes (arrowhead; lane 2) were washed and provided with 7 mm MgCl2, 0.25% Sarkosyl, 800 µm UTP, and the indicated concentration of CTP for 15 min. One sample receiving 8 µm CTP (as in lane 4) was chased with 800 µm CTP for an additional 10 min (lane 8). Washed G200 complexes incubated with SII and MgCl3 lose their label (lane 9). Lane 1, 5′-end labeled Ia and runoff RNA markers.
Transcript cleavage restores factor-independence to ≈50% of the elongation complexes (Reines et al., 1992; Gu et al., 1993). This alteration is stable for complexes at position 200 since they could be washed repeatedly by immunoprecipitation and resuspension and upon providing them with CTP and UTP, their RNAs were elongated through site Ia to the same extent as unwashed (Gu et al., 1993) or doubly washed complexes (Fig. 3A, lanes 3 and 6). These RNAs remained associated with pol II since they could undergo cleavage resulting in a loss of 32P-label (Fig. 3B, lane 9) and extend their RNA chains in an SII-dependent manner.2 If G200 complexes were incubated with UTP but not CTP, all RNA chains could be extended back to the arrest site (Fig. 3A, lane 4) but none could respond to CTP added at a later time (Fig. 3A, lane 5), i.e. they had all become arrested. This suggested that poor availability of the next nucleotide could convert complexes that were elongation competent into an SII-dependent farm as reported for a viral arrest site (Wiest and Hawley, 1990; Wiest et al., 1992). This was only the case for complexes walked downstream to the arrest site since upstream complexes (A198 to U204) did not display the arrest phenotype when their next nucleotide was absent (Figs. 2 and 3A).
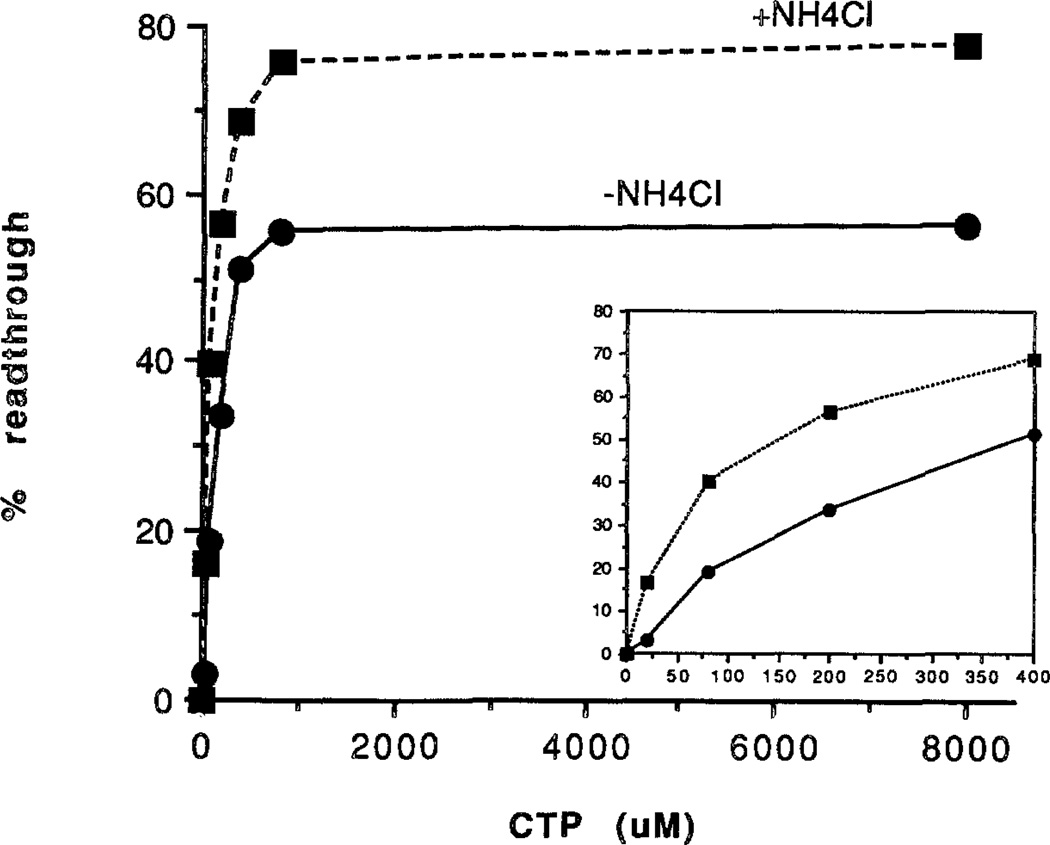
These results indicated that we were observing pol II molecules in the process of becoming arrested and that we should be able to track the time course of this event. A simple way to alter the complex’s dwell time at the arrest site was to limit the concentration of the next nucleotide. Indeed, there was a clear relationship between the CTP concentration and transcription through site Ia (Fig. 3B, lanes 3–7). Readthrough was half-maximal at ≈160 µm CTP and reached a plateau at 800 µm CTP (lower carve, Fig. 4). Complexes that were unable to pass site Ia at law CTP (Fig. 3B, lane 4) were refractory to a high CTP chase (Fig. 3B, lane 8) showing that they had become arrested. This suggested that the length of time pol II spent between incorporation events or its “dwell” time (von Hippel and Yager, 1991) was a critical factor in attaining the arrested phenotype.
Fig. 4. Quantitation of CTP dependence of readthrough.
An experiment such as that described in Fig. 3B (–NH4Cl) and one including 0.1 m NH4Cl, were quantitated and expressed as % readthrough = 100 × [U216 RNA/(U216 RNA + Ia RNA)]. Inset, plot with expanded x axis scale.
The time course of acquisition of the arrested phenotype was measured by providing UTP to the G200 complex for varying lengths of time and testing for elongation competence with a challenge of 800 µm CTP. Pol II progressively lost its ability to escape from site Ia as the time interval between UTP addition and CTP addition increased (Fig. 5A, diamond symbols). This decay was rapid and essentially complete by 5 s at 28 °C.
Fig. 5. Time course of acquisition of the arrested phenotype.
A, the time course experiment described in part B was quantitated and the average % readthrough (± standard deviation; n = 3) as a function of time with UTP was determined. An identical experiment at 28 °C is also plotted, Inset, first-order plot of the 15 °C data. The lag indicated by the dotted line is due to the arrival time of pol II at site Ia, hence the zero time value was not included in the calculation of the apparent first-order rate constant. B, labeled G200 complexes were assembled, washed, warmed to 15 °C (lanes 2–12), and adjusted to 7 mm MgCl2, 0.25% Sarkosyl, and 800 µm UTP. At the indicated times, aliquots were brought to 800 µm CTP for 30 min at 15 °C before preparation for gel electrophoresis. An additional aliquot of G200 complexes was adjusted to 7 mm MgCl2, 0.25% Sarkosyl, and 800 µm each CTP and UTP and incubated at 28 °C for 10 min (lane 1).
We repeated this experiment at 15 °C to facilitate a more accurate measurement of the decay and to assess its temperature dependence. A similar profile was observed showing a longer half-life (120 s; Fig. 5, A and B) at 15 °C. The data can be plotted as a first-order process with an apparent rate constant of 0.008 s−1 (Fig. 5A, inset). This measurement includes the time pol II takes to reach Ia from G200 as well as that required to assume the arrested phenotype or achieve readthrough to position 216. An independent measurement revealed that half of the G200 complexes extended their chains to position 207 in 75 s.2 Hence, the decay in elongation potential displayed a half-life of approximately 50 s at 15 °C which was at least 10-fold slower than that observed at 28 °C. Note that maximal readthrough efficiency varied as a function of temperature (Fig. 5A, t = 0: 44% at 15 °C versus 57% at 28 °C). This demonstrated that the relative partitioning of Ia complexes between the alternatives of chain extension and arrest was temperature-dependent.
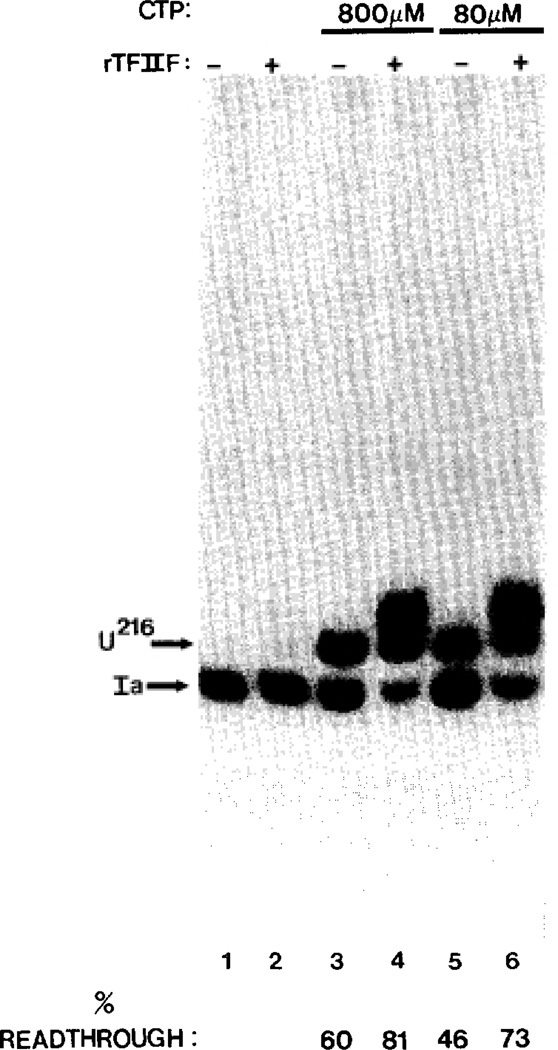
Elongation factor TFIIF (Price et al., 1989; Flores et al. 1989; Bengal et al., 1991; Izban and Luse, 1992a) and the monovalent salt NH4Cl (Sluder et al., 1988; Izban and Luse, 1991, 1992a) are known to increase the average elongation rate and thus reduce the dwell time of pol II. To strengthen the argument that dwell time at site Ia is a determinant of arrest, we measured the effect of TFIIF and NH4Cl on readthrough at different CTP concentrations. Consistent with this idea, both agents increased the efficiency of readthrough at low CTP levels in the absence of added SII (Fig. 4, upper versus lower curve and Fig. 6, lane 5 versus 6). The maximal extent of readthrough at saturating CTP (800 µm) was also higher in the presence of either NH4Cl (Fig. 4, upper versus lower curves) or TFIIF (Fig. 6, lane 3 versus lane 4). Neither NH4Cl2 nor TFIIF (Fig. 6, lane 1 versus lane 2) rescued pol II after it had become arrested, in accordance with the work of others (Bengal et al., 1991; Izban and Luse, 1991, 1992a). We take this as additional evidence that the longer pol II dwells at an arrest site the more likely it is to assume the arrested configuration.
Fig. 6. Effect of TFIIF on arrest at site Ia.
A, G200 complexes were incubated for 10 min at 28 °C with 7 mm MgCl2, 800 µm UTP, and 800 (lanes 3 and 4) or 80 (lanes 5 and 6) µm CTP in the absence (lanes 3 and 5) or presence (lanes 4 and 6) of 0.9 µg of rTFIIF. RNAs were quantitated and percent readthrough determined. Control G200 complexes were incubated at 28 °C with 7 mm MgCl2, 800 µm UTP, for 10 min followed by 10 min with 800 µm CTP alone (lane 1) or with rTFIIF (lane 2).
DISCUSSION
RNA polymerases located at a single template position can assume more than one state due to the partitioning of complexes between different functional pathways (Yager and von Hippel, 1991; von Hippel and Yager, 1992; Krummel and Chamberlin, 1992a, 1992b; Jin et al., 1992; Izban and Luse, 1993b; Erie et al., 1992, 1993; McDowell et al., 1994). The pathway choice can be influenced by transcription elongation or termination factors and is an important point of regulatory control in both eukaryotes and prokaryotes (Roberts, 1992; Kane, 1994; Chan and Landick, 1994; Chamberlin, 1994). The options for an elongation complex include: 1) insert the next nucleotide, 2) terminate transcription via RNA release, or 3) cease elongation without RNA release.
The length of time at which RNA polymerases dwell at a specific template position contributes to the efficiency of termination by E. coli RNA polymerase (reviewed in von Hippel and Yager, 1991; Roberts, 1992; Chan and Landick, 1994; Chamberlin, 1994) and arrest by pol II (Wiest and Hawley, 1990; Wiest et al., 1992). Dwell time can be modulated by template sequence, auxiliary transcription factors, availability of nucleotide substrates, the structure of nascent RNA, translation (in prokaryotes), and perhaps co-transcriptional processing of the primary transcript in eukaryotes. The influence of substrate availability on elongation by pol II was elucidated by experiments where the dependence of arrest was altered from GTP to CTP or UTP when the template base downstream from an arrest site was mutated from C to G or A, respectively (Wiest et al., 1992).
Here, we have observed and measured the rate at which RNA polymerase II assumes the arrested conformation. Arrest efficiency is determined in part by a kinetic competition between the rate at which pol II incorporates the next nucleotide and the rate at which its elongation potential decays. Partitioning is temperature dependent with more arrest at lower temperature. This is congruent with observations that the extent of DNA bending is accentuated at lower temperature (Marini et al., 1984; Griffith et al., 1986) and supports the idea that a bend in this template is causally involved in arrest (Kerppola and Kane, 1990; Reines, 1992).
Elongation complexes at most template positions cannot be forced into an SII-dependent form since pol II held at a variety of template positions by nucleotide starvation or lac repressor remains elongation competent even after dwell times of >1 h (Reines and Mote, 1993). The arrest pathway is apparently a site-specific option for pol II, i.e. there is a thermodynamic “well” found at intrinsic arrest sites. If so, the absence of arrest at most template locations is not necessarily due to kinetic escape via rapid insertion of the next base which outcompetes a pathway to arrest. Instead, there may not be an accessible arrest conformation for pol II under these conditions. Similar conclusions have been drawn for termination by E. coli RNA polymerase (von Hippel and Yager, 1992). Termination by E. coli RNA polymerase and RNA palymerase I has been described as a two-step process, a slowed rate of next-nucleotide insertion and a template location where elongation capacity can be lost (Yager and von Hippel, 1987; McDowell et al., 1994; Lang et al., 1994). The efficiency of pol II arrest seems to involve similar steps. The features that determine these requirements are not known but may be related to the number of template A residues that encode the U-rich 3′ termini characteristic of many arrested RNAs (Izban and Luse, 1993b) and a bend in or near the arrest site (Kerppola and Kane, 1990). A U-rich RNA terminus and/or the unusual structure of the DNA helix at this site or in the transcription bubble could serve to bath reduce polymerization step time and potentiate the rapid decay in elongation potential identified here. Additional signals or factors may be needed for pol II to terminate transcription perhaps in conjunction with processing events such as polyadenylation (Logan et al., 1987; Connelly and Manley, 1988).
In the absence of NTPs, factor-dependent and factor-independent complexes carry out SII-activated nascent RNA cleavage. Dinucleotides or 7–14 base oligonucleotides are liberated from SII-independent and SII-dependent complexes, respectively (Izban and Luse, 1993a, 1993b). Luse and co-workers have suggested that this difference in cleavage increment is indicative of a structural distinction between the two states. The difference between SII-dependent and independent elongation complexes may be the loss of proximity of the polymerization catalytic site relative to the 3′-end of the RNA (Izban and Luse, 1993b; Borukhov et al, 1993; Chamberlin, 1994). The decay of elongation competence measured here may reflect in part the rate of this presumptive conformational change. A covalent modification of one or more components of the elongation complex cannot be ruled out, although any such modification is not expected to involve the carboxyl-terminal domain of pol II which does not affect arrest or SII-activated readthrough in vitro (Christie et al., 1994). Exonuclease III footprinting reveals a subtle, 1 base pair downstream movement of the arrested complex after nascent RNA cleavage (Gu et al., 1993). There is a large difference between SII-dependent and independent complexes with respect to the position of the RNA 3′-end relative to the downstream boundary (Gu et al., 1993). Arrested complexes have 3′-ends which lie closer to the leading edge than do factor-independent complexes. Similar structural differences have been observed between elongation-competent and arrested E. coli RNA polymerases (Krummel and Chamberlin, 1992b; Borukhov et al., 1993; Nudler et al., 1994). The defining features of arrest sites in general have not been thoroughly elucidated. The locations and frequencies of occurrence of such sites in bacterial or eukaryotic genomes are not known.
It has been suggested that SII and TFIIF act by different means to influence elongation by pol II. TFIIF only affects readthrough if present before RNA polymerase encounters an arrest site, whereas SII is effective even after the encounter (Bengal et al., 1991; Izban and Luse, 1992a). SII works via arrested RNA cleavage which resolves the fate of those enzymes that do succumb to arrest and can only take place after arrest (Reines, 1994). Bath TFIIF and NH4Cl enhance the ability of pol II to use a limiting level of NTPs (Figs. 4 and 6) as has recently been described for a new elongation factor, SIII (Bradsher et al., 1993b). The kinetic step accelerated by these agents is unknown. The ability of TFIIF (and NH4+ ions) to support high elongation rates and to reduce dwell time, is a possible explanation for how it reduces the probability that pol II will acquire factor dependence at an arrest site and why it is required before pol II arrives at an arrest site. We cannot exclude the possibility that these agents alter the configuration of the elongation complex such that it is less prone to arrest in a manner that is independent of dwell time. Two RNA polymerase-binding proteins, greA and greB, have been described that enable E. coli RNA polymerase to readthrough arrest sites (Borukhov et al., 1993; Nudler et al., 1994; Chamberlin, 1994). Like TFIIF, greA must be present prior to arrest to enable readthrough while greB, like SII, can reactivate arrested complexes via RNA cleavage (Borukhov et al., 1993). Unlike greA, however, TFIIF has not yet been reported to activate nascent RNA cleavage. These and perhaps other factors (Bengal et al., 1991; Bradsher et al., 1993a, 1993b) likely collaborate to minimize the possibility that RNA polymerases will became trapped an transcription units in the absence of physiological transcript release signals.
Acknowledgments
We thank John Mate, Jr., for expert technical assistance and Drs. M. Chamberlin, L. Coluccio, R. Conaway, J. Conaway, C. Moran, S. Warren, and K. Wilkinson for helpful discussions and/or a critical reading of the manuscript.
Footnotes
This work was supported by grants from the National Institutes of Health and the American Cancer Society.
The abbreviation used is: pol II, RNA polymerase II.
W. Gu, unpublished results.
REFERENCES
- Arndt KM, Chamberlin MJ. J. Mol. Biol. 1990;213:79–108. doi: 10.1016/S0022-2836(05)80123-8. [DOI] [PubMed] [Google Scholar]
- Bengal E, Flores O, Krauskopf A, Reinberg D, Aloni Y. Mol. Cell Biol. 1991;11:1195–1206. doi: 10.1128/mcb.11.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S, Sagitov V, Goldfarb A. Cell. 1993;72:459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- Bradsher JN, Jackson KW, Conaway RC, Conaway JW. J. Biol Chem. 1993a;268:25587–25593. [PubMed] [Google Scholar]
- Bradsher JN, Tan S, McLaury H-J, Conaway JW, Conaway RC. J. Biol. Chem. 1993b;268:25594–25603. [PubMed] [Google Scholar]
- Carpousis AJ, Gralla JD. J. Mol Biol. 1985;183:165–177. doi: 10.1016/0022-2836(85)90210-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin MJ. Harvey Lect. 1994;88:1–21. [PubMed] [Google Scholar]
- Chan CL, Landick R. In: Transcription Mechanisms and Regulation. Conaway RC, Conaway JW, editors. New York: Raven Press; 1994. pp. 297–321. [Google Scholar]
- Chen HC, England L, Kane CM. Gene (Amst.) 1992;116:253–258. doi: 10.1016/0378-1119(92)90522-q. [DOI] [PubMed] [Google Scholar]
- Christie KR, Awrey DE, Edwards AM, Kane CM. J. Biol Chem. 1994;269:936–943. [PubMed] [Google Scholar]
- Connelly S, Manley JL. Genes & Dev. 1988;2:440–452. doi: 10.1101/gad.2.4.440. [DOI] [PubMed] [Google Scholar]
- Eilat D, Hechberg M, Fischel R, Laskov R. Proc. Natl. Acad. Sci. U. S. A. 1982;79:3818–3822. doi: 10.1073/pnas.79.12.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie DA, Yager TD, von Hippel PH. Ann. Rev. Biophys. Biomol. Struct. 1992;21:379–415. doi: 10.1146/annurev.bb.21.060192.002115. [DOI] [PubMed] [Google Scholar]
- Erie DA, Hajiseyedjavadi O, Young MC, von Hippel PH. Science. 1993;262:867–873. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- Flores O, Maldonado E, Reinberg D. J. Biol. Chem. 1989;264:8913–8921. [PubMed] [Google Scholar]
- Griffith J, Bleyman M, Rauch C, Kitchin P, Englund P. Cell. 1986;46:717–724. doi: 10.1016/0092-8674(86)90347-8. [DOI] [PubMed] [Google Scholar]
- Gu W, Powell W, Mete J, Jr, Reines D. J. Biol. Chem. 1993;268:25604–25616. [PMC free article] [PubMed] [Google Scholar]
- Guo H, Price DH. J. Biol. Chem. 1993;268:18762–18770. [PubMed] [Google Scholar]
- Hagler J, Shuman S. Science. 1992;255:983–986. doi: 10.1126/science.1546295. [DOI] [PubMed] [Google Scholar]
- Hirashima S, Hirai H, Nakanishi Y, Natori S. J. Biol. Chem. 1988;263:3858–3863. [PubMed] [Google Scholar]
- Izban MG, Luse DS. Genes & Dev. 1991;5:683–696. doi: 10.1101/gad.5.4.683. [DOI] [PubMed] [Google Scholar]
- Izban MG, Luse DS. J. Biol. Chem. 1992a;267:13647–13655. [PubMed] [Google Scholar]
- Izban MG, Luse DS. Genes & Dev. 1992b;6:1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- Izban MG, Luse DS. J. Biol. Chem. 1993a;268:12864–12873. [PubMed] [Google Scholar]
- Izban MG, Luse DS. J. Biol. Chem. 1993b;268:12874–12885. [PubMed] [Google Scholar]
- Jin DJ, Burgess RR, Richardson JP, Gross CA. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1453–1457. doi: 10.1073/pnas.89.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Kuzuhara T, Sekimizu K, Natori S. J. Biochem. (Tokyo) 1991;109:674–677. doi: 10.1093/oxfordjournals.jbchem.a123439. [DOI] [PubMed] [Google Scholar]
- Kane CM. In: Transcription Mechanisms and Regulation. Conaway RC, Conaway JW, editors. New York: Raven Press; 1994. pp. 279–296. [Google Scholar]
- Kerppola TK, Kane CM. Biochemistry. 1990;29:269–278. doi: 10.1021/bi00453a037. [DOI] [PubMed] [Google Scholar]
- Krummel B, Chamberlin MJ. Biochemistry. 1989;28:7829–7842. doi: 10.1021/bi00445a045. [DOI] [PubMed] [Google Scholar]
- Krummel B, Chamberlin MJ. J. Mol Biol. 1992a;225:221–237. doi: 10.1016/0022-2836(92)90917-9. [DOI] [PubMed] [Google Scholar]
- Krummel B, Chamberlin MJ. J. Mol. Biol. 1992b;225:239–250. doi: 10.1016/0022-2836(92)90918-a. [DOI] [PubMed] [Google Scholar]
- Lang WH, Morrow BE, Ju Q, Warner JR, Reeder RH. Cell. 1994;79:527–534. doi: 10.1016/0092-8674(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Levin JR, Krummel B, Chamberlin MJ. J. Mol. Biol. 1987;196:85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- Logan J, Falck-Pederson E, Darnell JE, Shenk T. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8306–8310. doi: 10.1073/pnas.84.23.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J, Effron P, Goodman T, Singleton C, Wells R, Wartell R, Englund P. J. Biol. Chem. 1984;259:8974–8979. [PubMed] [Google Scholar]
- Marshall TK, Guo H, Price DH. Nucleic Acids Res. 1990;18:6293–6298. doi: 10.1093/nar/18.21.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JC, Roberts JW, Jin DJ, Gross C. Science. 1994;266:822–825. doi: 10.1126/science.7526463. [DOI] [PubMed] [Google Scholar]
- Metzger W, Schickor P, Heumann H. EMBO J. 1989;8:2745–2754. doi: 10.1002/j.1460-2075.1989.tb08416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J, Ghanouni P, Reines D. J. Mol. Biol. 1994;236:725–737. doi: 10.1006/jmbi.1994.1185. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Nakano A, Nomura K, Sekimizu K, Natori S. J. Biol. Chem. 1992;267:13200–13204. [PubMed] [Google Scholar]
- Nudler E, Goldfarb A, Kashlev M. Science. 1994;265:793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- Price DH, Sluder A, Greenleaf AL. Mol. Cell Biol. 1989;9:1465–1475. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D. J. Biol. Chem. 1991;266:10510–10517. [PMC free article] [PubMed] [Google Scholar]
- Reines D. J. Biol. Chem. 1992;267:3795–3800. [PMC free article] [PubMed] [Google Scholar]
- Reines D. In: Transcription. Conaway R, Conaway J, editors. New York: Raven Press; 1994. pp. 263–278. [Google Scholar]
- Reines D, Mote J., Jr Proc. Natl. Acad. Sci. U. S. A. 1993;90:1917–1921. doi: 10.1073/pnas.90.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D, Chamberlin MJ, Kane CM. J. Biol. Chem. 1989;264:10799–10809. [PubMed] [Google Scholar]
- Reines D, Ghanouni P, Li Q-q, Mote J. J. Biol. Chem. 1992;267:15516–15522. [PMC free article] [PubMed] [Google Scholar]
- Roberts J. In: Transcriptional Regulation. McKnight SL, Yamamoto KR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 389–406. [Google Scholar]
- Sluder AE, Price DH, Greenleaf AL. J. Biol. Chem. 1988;263:9917–9925. [PubMed] [Google Scholar]
- Tan S, Conaway RC, Conaway JW. BioTechniques. 1994;16:824–828. [PubMed] [Google Scholar]
- Thummel CS. Science. 1992;255:39–40. doi: 10.1126/science.1553530. [DOI] [PubMed] [Google Scholar]
- Tsuboi A, Conger K, Garrett KP, Conaway RC, Conaway JW, Arai N. Nucleic Acids Res. 1992;20:3250. doi: 10.1093/nar/20.12.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PH, Yager TD. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2307–2311. doi: 10.1073/pnas.88.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PH, Yager TD. Science. 1992;255:809–812. doi: 10.1126/science.1536005. [DOI] [PubMed] [Google Scholar]
- Wang D, Hawley DK. Proc. Natl. Acad. Sci. U. S. A. 1993;90:843–847. doi: 10.1073/pnas.90.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DK, Hawley DK. Mol. Cell. Biol. 1990;10:5782–5792. doi: 10.1128/mcb.10.11.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DK, Wang D, Hawley DK. J. Biol. Chem. 1992;267:7733–7744. [PubMed] [Google Scholar]
- Xu Q, Nakanishi T, Sekimizu K, Natori S. J. Biol. Chem. 1994;269:3100–3103. [PubMed] [Google Scholar]
- Yager TD, von Hippel PH. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhart F, editor. Washington, D. C.: American Society for Microbiology; 1987. pp. 1241–1270. [Google Scholar]
- Yager TD, von Hippel PH. Biochemistry. 1991;30:1097–1115. doi: 10.1021/bi00218a032. [DOI] [PubMed] [Google Scholar]
- Yoo OJ, Yoon HS, Baek KH, Jeon CJ, Miyamoto K, Uene A, Agarwal K. Nucleic Acids Res. 1991;19:1073–1079. doi: 10.1093/nar/19.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]