Abstract
Most effector T cells are generated in the periphery following an encounter with a foreign antigen and exposure to soluble and membrane-bound mediators. There are, however, some T cell subsets, such as γδ T cells and natural killer T cells, that acquire their effector potential in the thymus before their emigration to the periphery. This developmental preprogramming enables these cells to differentiate rapidly into cytokine-producing effectors during the host immune response. This review focuses on murine interleukin (IL)-17–producing γδ T (γδ-17) cells, which have been shown, through their early production of IL-17, to have a critical role in multiple infectious and autoimmune diseases. Specifically, we discuss what is currently known about the genetic requirements for their generation and compare it with what is known about that of the more extensively studied IL-17–producing helper T (Th17) cells. Based on this comparison, we propose a model for murine γδ-17 development and differentiation.
Keywords: thymus, T cell development, T cell differentiation, IL-17, signal transduction, fate specification
I. INTRODUCTION
Since the discovery of an interleukin (IL)-17-producing subset of murine γδ T (γδ-17) cells just 7 years ago,1 immunologists have made considerable progress in defining the role of these cells in the immune response. First, it has been shown that this functional subset plays a protective role in host defense to microbes, such as Mycobacterium tuberculosis,2 Mycobacterium bovis,3,4 Listeria monocytogenes,5–8 Escherichia coli,9 and Candida albicans.10 In these infection models, γδ-17 cells produce IL-17 at an early stage in the immune response, and this early IL-17 production is required for the recruitment of neutrophils to the site of infection and subsequent clearance of the pathogen. Significantly, in the M. bovis and L. monocytogenes models, γδ-17 cells also are required for granuloma formation and optimal αβ T cell effector responses.3–5,8 Second, γδ-17 cells have a pathogenic role in multiple autoimmune diseases. The early production of IL-17 by γδ T cells contributes to pathogenesis in mouse models of collagen-induced arthritis,11 colitis,12 and experimental autoimmune encephalitis (EAE).13,14 In the colitis model, their mode of action is to promote IL-17 production by colitogenic Th17 cells,12 whereas in the EAE model, γδ-17 cells not only promote IL-17 production by interleukin-17–producing helper T (Th17) cells, but also inhibit the development of regulatory T cells.13,14 Last, γδ-17 cells have opposing roles in tumor immunity, depending on the tumor model employed. Chemotherapeutic agents that induce tumor cell death elicit a tumor-specific cytotoxic T lymphocyte response that is capable of resolving the tumor.15,16 Notably, the emergence of these cytotoxic T lymphocytes after chemotherapy is dependent upon the influx, at an earlier time point, of γδ-17 effectors into the tumor bed.16 However, in multiple transplantable tumor models, γδ-17 cells exert protumor activities by inducing vascularization and subsequent growth of the tumor.17 Together, these data demonstrate that by rapidly producing IL-17 during an immune response, γδ-17 cells have the ability to control the actions of immune and nonimmune cells alike.
It is interesting that cytokine-producing γδ-17 and Th17 cells are elicited in many of the same inflammatory diseases, but functional γδ-17 cells appear days to weeks before functional Th17 cells.2,12,18,19 The difference in the kinetics of their appearance may be explained by the fact that peripheral γδ-17 cells are preprogrammed in the thymus to produce IL-17,18,20 whereas naïve CD4+ T cells are programmed to become Th17 cells in the periphery in the context of an immune response.21–23 In this review, we compare the current knowledge regarding the genetic requirements for the generation of γδ-17 and Th17 effectors, with the goal of determining whether the respective priming microenvironments dictate the requirements for specific effector fates. This comparison not only identifies signaling pathways that are shared by, and unique to, each IL-17–producing effector but also serves as the basis for a model of γδ-17 development and differentiation.
II. TH17 DIFFERENTIATION PATHWAY
The development of an in vitro culture system in which naïve CD4+ T cells can be induced to differentiate into various types of effectors has aided greatly in the identification of molecules that are sufficient for the generation of Th17 cells. Using such an in vitro culture system, it was shown that transforming growth factor (TGF)-β and IL-6, together with T cell receptor (TCR) engagement, induce the differentiation of naïve CD4+ T cells into Th17 cells.24–26 More in-depth analyses have revealed that TCR, TGF-β, and IL-6 signaling pathways synergize to induce expression of the transcription factor RORγt,27 the chemokine receptor CCR6,28 the cytokine IL-21,29 and the cytokine receptors IL-1R and IL-23R.29,30 These phenotypic changes enable Th17 cells to migrate to inflammatory sites28 and to secrete IL-17 in response to IL-23 and IL-1.29,30
Subsequent in vivo studies using genetically modified mice demonstrate that Th17 cell differentiation is more complex than originally thought. First, factors other than the ones previously mentioned also positively regulate Th17 differentiation. These include Notch,31,32 prostaglandin E2,33 sphingosine-1 phosphate,34,35 B-cell activating factor,36,37 and the transcription factors c-Rel,38–40 BATF,41 interferon regulatory factor-4,42,43 Runx1,44 aryl hydrocarbon receptor,45,46 and Iκbς.47 Second, it was shown recently that there is a differential requirement for IL-6 in Th17 differentiation, depending on whether naïve T cells are primed in mucosal tissues (IL-6 dependent) or the spleen (IL-6 independent).48 Notably, this differential requirement for IL-6 in priming Th17 cells in mucosal and secondary lymphoid tissues is because of differences in the properties of their resident dendritic cells (DCs). DC populations from the lamina propria contain a mixture of CD103+ and CD103− cells, whereas those in the spleen are primarily CD103−. Interestingly, the CD103+ DCs, but not the CD103− DCs, produce high levels of TGF-β and retinoic acid, which inhibit Th17 differentiation and promote regulatory T cell development in the absence of IL-6.48 Thus, these data demonstrate that the cytokine requirements for Th17 differentiation differ when naïve CD4+ T cells are primed in mucosal tissues versus secondary lymphoid tissues.48 As we discuss in Section IV, the idea that the priming microenvironment is able to dictate the cytokine requirements for effector T cell differentiation will prove to be important in our understanding of γδ-17 development in the thymus.
III. PHENOTYPE OF γδ-17 CELLS
The phenotypes of Th17 and γδ-17 cells are, for the most part, similar (see Table 1 for phenotype of γδ-17 cells). Both express CCR6, IL-23R, CD44, TLR2, and RORγt.13,27,49–53,56 Interestingly, γδ-17 cells also have been reported to express CD25 (IL-2Rα),51,52 the C-type lectin receptor dectin-1,50 the scavenger receptor SCART2,54 and the Src family kinase, kinase, B lymphoid kinase (Blk).55 Although Th17 cells express neither CD25 nor dectin-1,21–23 it is not known whether they express SCART2 or Blk because their expression has not been examined in Th17 cells.
TABLE 1.
Phenotype of γδ-17 cells
| Markers | References |
|---|---|
| Surface antigens | |
| CCR6 | Haas et al.;49 Martin et al.50 |
| CD44 | Shibata et al.;51 Haas et al.;49 Do et al.;52 Martin et al.50 |
| CD25 | Shibata et al.;51 Do et al.52 |
| IL-23R | Awasthi et al.;53 Sutton et al.13 |
| SCART2 | Kisielow et al.55 |
| TLR1/2 | Martin et al.50 |
| Dectin | Martin et al.50 |
| Signaling molecules | |
| Blk | Laird et al.56 |
| Transcription factors | |
| Rorγt | Ivanov et al.;27 Lochner et al.54 |
Notably, the vast majority of γδ-17 cells express γδTCRs that use either the Vγ6 or Vγ4 gene segment.5,11,51 Vγ6+ γδ T cells are only generated early in ontogeny, during the second wave of γδ T cell development in the fetal thymus.57 Because of the lack of terminal deoxynucleotidyl transferase expression in these cells, the rearranged TCRγ and –δ chains lack nontemplated nucleotide additions and, as a result, lack diversity.58–62 Around the time of birth, γδ T cells expressing this invariant γδTCR exit the thymus and colonize the lung, tongue, nasal lymphoid tissue, peritoneal cavity, and female reproductive tract.9,63–66 Vγ4+ γδ T cells, on the other hand, are generated in both the fetal and adult thymus,57,67 and those generated postnatally express a diverse TCR repertoire.60,62 Vγ4+ γδ T cells are found not only in secondary lymphoid tissues but also in the lung.65,68 Because both Vγ6+ and Vγ4+ γδ T cell subsets reside in the lung, it is important to note that their colonization and subsequent residency occur in a sequential fashion, with Vγ6+ γδ T cells being the major γδ T cell population from birth until about 8 to 10 weeks of age; Vγ4+ γδ T cells predominate from that age on.65
Despite the extensive number of markers that can be used to identify γδ-17 cells, the vast majority of studies examining the role of specific genes in γδ-17 development, function, or both have enumerated γδ-17 cells based on their ability to produce IL-17 after treatment with phorbol 12-myristate 13-acetate (PMA) and ionomycin (which together simulate antigen receptor signaling by activating protein kinase C and raising intracellular Ca2+ levels). However, the use of this functional assay comes with the assumption that protein kinase C– and calcium-regulated signaling pathways mimic all signaling pathways that elicit IL-17 production from γδ-17 cells. As shown in the following example, this assumption is flawed, as it is indeed possible for γδ-17 cells from a mutant mouse to produce wild-type levels of IL-17 after activation with PMA and ionomycin yet produce reduced levels of IL-17 after activation with physiological stimuli. Homozygous knock-in IL-23R green fluorescent protein (GFP) reporter mice, which lack a functional IL-23R, generate γδ-17 cells in numbers equivalent to those in heterozygous IL-23R GFP reporter mice, indicating that this cytokine receptor is not required for γδ-17 development.7 To assess the functional ability of these cells, γδ-17 cells were activated in vitro with PMA and ionomycin or activated in vivo by infection with L. monocytogenes. Remarkably, while in vitro IL-17 production was found to be comparable between wild type and IL-23R–deficient γδ-17 cells7; in vivo IL-17 production by γδ-17 cells was markedly reduced in Listeria-infected IL-23R−/− mice compared with Listeria-infected wild-type mice (Fig. 1C, D).7 These data demonstrate not only that IL-23 is required for the expansion of γδ-17 effectors, but also that the use of PMA and ionomycin to assess γδ-17 cell effector function leads to inconclusive results if not performed in combination with an in vivo functional assay. For this reason, we only review the studies that show unequivocal evidence that a specific gene has a role in γδ-17 development, differentiation, or both.
FIGURE 1.
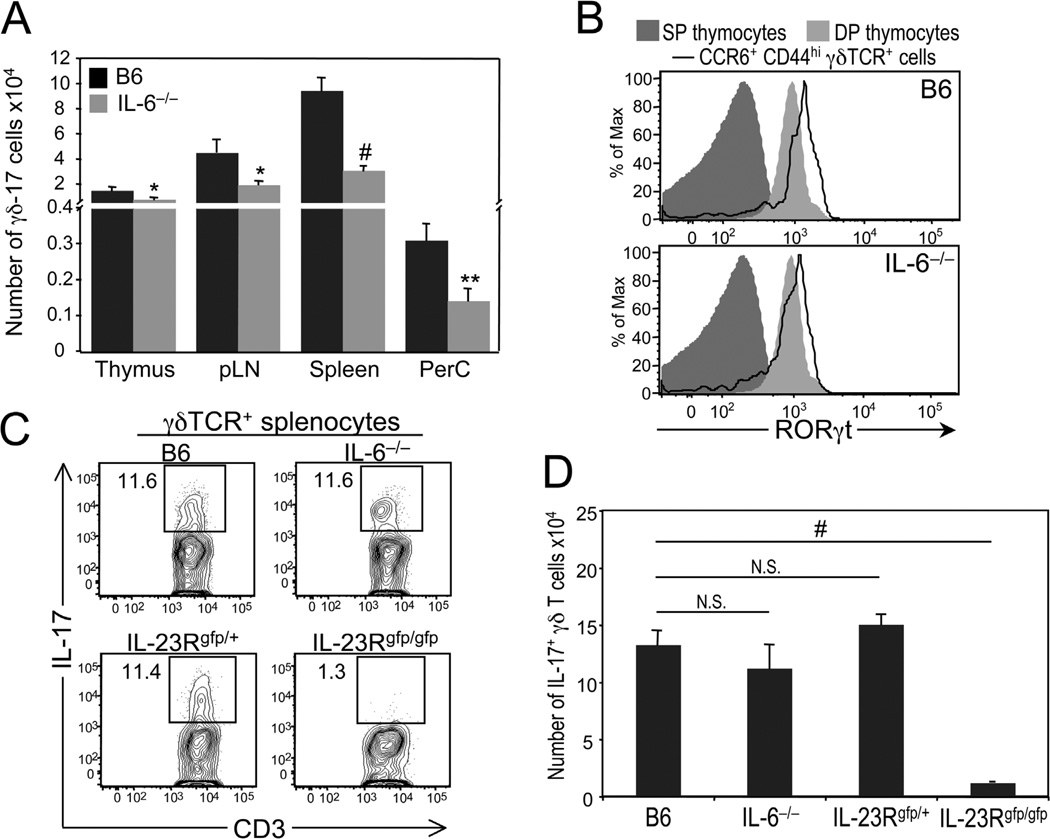
Phenotypic and functional analysis of γδ-17 cells in interleukin (IL)-6–deficient mice. (A) Comparison of the numbers of γδ-17 cells, defined as CCR6+ and CD44hi, in the thymus, peripheral lymph nodes (pLN), spleen, and peritoneal cavity (PerC) of 5- to 6-week-old C57BL/6 (B6) and IL-6−/− mice. *P ≤ 0.05; **P ≤ 0.01; #P ≤ 0.001. (B) Histograms showing representative staining of RORγt in wild-type and IL-6–deficient γδ-17 cells from pLN. RORγt expression levels on CD4+ and CD8+ single-positive (SP) thymocytes are shown as a negative control, whereas those on CD4+CD8+ double-positive (DP) thymocytes are shown as a positive control. (C) Six-week-old B6, IL-6−/−, IL-23Rgfp/+ (IL-23R+/−), and IL-23Rgfp/gfp (IL-23R−/−) were infected intraperitoneally with a 0.3 LD50 dose of wild-type Listeria (3 × 105 pfu). Five days after infection, splenocytes were harvested and restimulated in vitro with IL-1, IL-23, and Pam3Cys (a TLR2/1 agonist) for 4 hours in the presence of brefeldin A. Representative dot plots show IL-17 versus CD3 staining in gated γδ T cells. Numbers represent percentage of IL-17+ γδ T cells. (D) Summary of data presented in C. # P ≤ 0.001. N.S., not significant.
IV. GENES REQUIRED FOR THE DEVELOPMENT OF γδ-17 CELLS IN THE THYMUS
To date, no in vitro culture system has been developed to identify the factors that play a role in γδ-17 development and differentiation, most likely because a functionally uncommitted γδ thymocyte (which would be comparable to the naïve CD4+ T cell in the in vitro culture system discussed earlier) has yet to be identified. Consequently, we must rely on the use of genetically modified mice to determine whether a specific gene is required for the generation of γδ-17 effectors. In this section and the next, we discuss the genes required for γδ-17 development and differentiation by their associated signaling pathways (see Table 2 for a list of required genes).
TABLE 2.
Genes required for the development and differentiation of γδ–17 cells.
| Gene | References |
|---|---|
| Thymus | |
| Tgfb1 | Do et al.52 |
| Smad3 | Do et al.52 |
| Il6 | Petermann et al.14 |
| Relb | Powolny-Budnicka et al.76 |
| Ltbr | Powolny-Budnicka et al.76 |
| Nfkb2 | Powolny-Budnicka et al.76 |
| Rorc | Ivanov et al.;27 Lochner et al.;54 Shibata et al.74 |
| Hes1 | Shibata et al.74 |
| Ptgir | Jaffar et al.80 |
| Blk | Laird et al.56 |
| Periphery | |
| Il23 | Meeks et al.6 |
| Il23r | Riol-Blanco et al.;7 |
| Il1r1 | Sutton et al.13 |
| Il2 | Shibata et al.51 |
| Il2ra | Shibata et al.51 |
| Hes1 | Shibata et al.74 |
A. γδTCR Signaling
Signaling through the αβ TCR is a mandatory step in T helper cell differentiation; however, it is still unclear whether γδTCR signaling, through recognition of self-ligands expressed in the thymus, is required for functionally uncommitted γδ thymocytes to acquire the IL-17 effector fate. This uncertainty stems from the fact that there are lines of evidence both for and against a requirement for γδTCR signaling in the IL-17 effector fate specification. The first line of evidence in favor of a role for γδTCR signaling is that γδ-17 thymocytes, defined by their expression of CCR6, express high levels of CD44,49–52 an activation marker that is induced on antigen-experienced T cells.72,73 The second line of evidence is that CCR6+ γδ thymocytes express the zinc finger transcription factor ThPOK at levels significantly higher than those in thymocytes that have recently chosen the γδ lineage.74 Because ThPOK levels are positively regulated by TCR signaling, these data suggest that elevated levels of ThPOK in γδ-17 thymocytes are the result of γδTCR engagement.74 The last line of evidence is that γδ-17 thymocytes, but not γδ thymocytes committed to another effector fate, express the Src family kinase Blk,54 which we recently have shown to be a negative regulator of both TCR and B cell receptor signal transduction.54,75 In the absence of Blk, γδ-17 development is severely impaired, as evidenced by the significant loss of Vγ4+ γδ-17 cells and the almost complete absence of Vγ6+ γδ-17 cells in the thymus and periphery of Blk−/− mice.54 Given its role as a negative regulator of antigen receptor signaling, it is conceivable that the expression of Blk in γδ-17 thymocytes serves as a mechanism by which a high γδTCR signaling threshold is set in γδ thymocytes with an affinity for self-ligands. Of course, it cannot be ruled out that Blk, being an Src family kinase, functions in signaling pathways that are coupled to receptors other than the TCR.
The evidence against a role for γδTCR signaling in γδ-17 development is from a study in which the development of T10-/T22-specific γδ T cells was assessed in mice that have extremely low expression levels of T10/T22 (i.e., β2m−/− mice).18 Interestingly, although it was found that the number of T10-/T22-specific γδ T cells, defined by tetramer staining, is comparable between wild-type and β2m−/− mice, the phenotype of these cells is strikingly different. Specifically, in wild-type mice, the T10-/T22-specific γδ T cell population contained a combination of CD122+ (IL-2Rβ) and CD122− cells, whereas this population in β2m−/− mice was primarily CD122−.18 Subsequent functional analysis of wild-type CD122+ and CD122− T10-/T22-specific γδ T cells revealed that, after TCR stimulation, CD122+ γδ T cells produced interferon (IFN) γ and CD122− γδ T cells produced IL-17.18 On the basis of these findings, it was concluded that CD122− γδ thymocytes, which include γδ-17 thymocytes, did not require ligand for their generation.18 However, because this study was performed before the identification of γδ-17–specific markers, it is still not known whether the development, function, or both of CCR6+ tetramer-positive γδ thymocytes are indeed normal in β2m−/− mice.
B. TGFβ and IL-6 Signaling
TGFβ signaling is required for thymic development of γδ-17 cells because in the absence of TGFβ1 or Smad3, a component of the TGFβ signaling pathway, the number of IL-17+ γδ thymocytes is reduced drastically relative to that of wild-type mice.52 Likewise, IL-6 signaling is important because there is a significant decrease in the number of γδ-17 cells, defined by the expression of CCR6, IL-23R and/or CD44 in the thymus, peripheral lymph nodes, spleen, and peritoneal cavity of IL-6−/− mice compared to wild-type mice (Fig. 1A).14 Interestingly, despite being present in reduced numbers, the γδ-17 cells in IL-6−/− mice are comparable to those in wild-type mice in their expression of RORγt (Fig. 1B)56 and in their ability to expand and differentiate into IL-17 producers after Listeria infection (Fig. 1C, D). Together, these data suggest that IL-6 does not act directly on functionally uncommitted γδ thymocytes to promote the acquisition of the γδ-17 effector fate, but instead it acts indirectly by regulating the size of the thymic microenvironment that supports γδ-17 development. Consistent with this idea are the findings that (1) IL-6 positively regulates the expression of Delta-like 4, a Notch ligand, on thymic epithelial cell lines76 and (2) Notch signaling is required for γδ-17 development (see below).70
C. Lymphotoxin-β receptor Signaling
Lymphotoxin-β receptor (LTβR) is a member of the tumor necrosis factor receptor superfamily, and signaling through this receptor is dependent on activation of the alternative nuclear factor (NF)-κB pathway.77 It was shown recently that the generation of IL-17+ γδ thymocytes is severely impaired in mice deficient in components of the LTβR signaling pathway (i.e., LTβR−/−, Nfkb2−/−, T cell–specific Relb−/−).69 The LTβR ligands that regulate this developmental process are produced by CD4+CD8+ thymocytes, not epithelial cells.69,78 The mechanism by which the LTβR signaling pathway controls γδ-17 development is by regulating Rorc expression in γδ thymocytes committing to the γδ-17 lineage.69 Rorc encodes the transcription factor RORγt, which acts as a master regulator of IL-17–producing cells.27 Accordingly, RORγt is also required for the development of γδ-17 cells because IL17+ γδ-17 cells are virtually absent from the spleen, lamina propria, and peritoneal cavity of Rorc−/− mice.27,56,70
The generation of Th17 cells is not dependent on RelB.69 Instead, Th17 cell differentiation requires c-Rel, an NF-κB family member belonging to the classical pathway.39,40 c-Rel is activated by TCR signaling, and it is responsible for inducing Rorc expression.39,40 Therefore, both γδ-17 and Th17 cells utilize NF-κB, albeit through the activation of different receptors, to regulate RORγt expression and drive commitment to the IL-17 effector fate.
It is important to note that LTβR signaling also is required for the generation of IFNγ-producing γδ T (γδ-IFNγ) cells in the thymus.78 The finding that both γδ-17 and γδ-IFNγ effector lineages are dependent on LTβR signaling suggests that other signaling pathways coordinate with LTβR signaling to direct functionally uncommitted γδ thymocytes to either the IL-17 or IFNγ effector fate. Importantly, for this coordinated signaling to occur, there would have to be physically separate thymic niches that promote and support each effector fate.
D. Notch Signaling
Notch signaling is involved in many cell fate decisions, including those of T helper cell effectors.79It has been shown recently that Notch signaling is required for γδ-17 and Th17 development.31,32,70 In fetal mice that lack Hes-1, a target of the Notch signaling pathway, IL-17+ γδ thymocytes fail to develop.70 From the Th17 studies, we know that mechanism of action of Notch is to regulate transcriptional activity at the Rorc promoter.31,32
E. Prostaglandin Signaling
Prostaglandins are lipid mediators that can inhibit or stimulate immune cells.80 In regard to the generation of IL-17–producing effectors, prostaglandins have been shown to promote both γδ-17 and Th17 development.33,71 Prostaglandin I2 regulates γδ-17 development, as evidence by the marked reduction in IL-17+ γδ T cells in the thymus and lung of prostaglandin I2 receptor-deficient mice compared with wild-type mice.71 Another prostaglandin, namely prostaglandin E2, has been shown to play a role in Th17 differentiation. Prostaglandin E2 promotes Th17 cell function through the upregulation of both IL-23R and IL-1R expression.33 It is tempting to speculate that prostaglandin I2 may function in a similar manner in developing γδ-17 thymocytes.
V. GENES REQUIRED FOR THE DIFFERENTIATION OF γδ-17 CELLS IN THE PERIPHERY
A. IL-23R and IL-1R Signaling
IL-23 and IL-1 are produced by innate immune cells, such as DCs, as a consequence of signaling through pattern recognition receptors.81 Both of these cytokines are crucial for IL-17 production by γδ T cells, as evidenced by the significant reduction in IL-17+ γδ T cells after L. monocytogenes infection in IL-23−/− and IL-23R−/− mice (Fig. 2)6,7 or in IL-1R1−/− mice with EAE.13 Notably, other IL-17–producing cells, including Th17 cells and natural killer T cells, share the requirement for IL-23 and IL-1 to differentiate into cytokine-producing effectors.30,82,83
FIGURE 2.
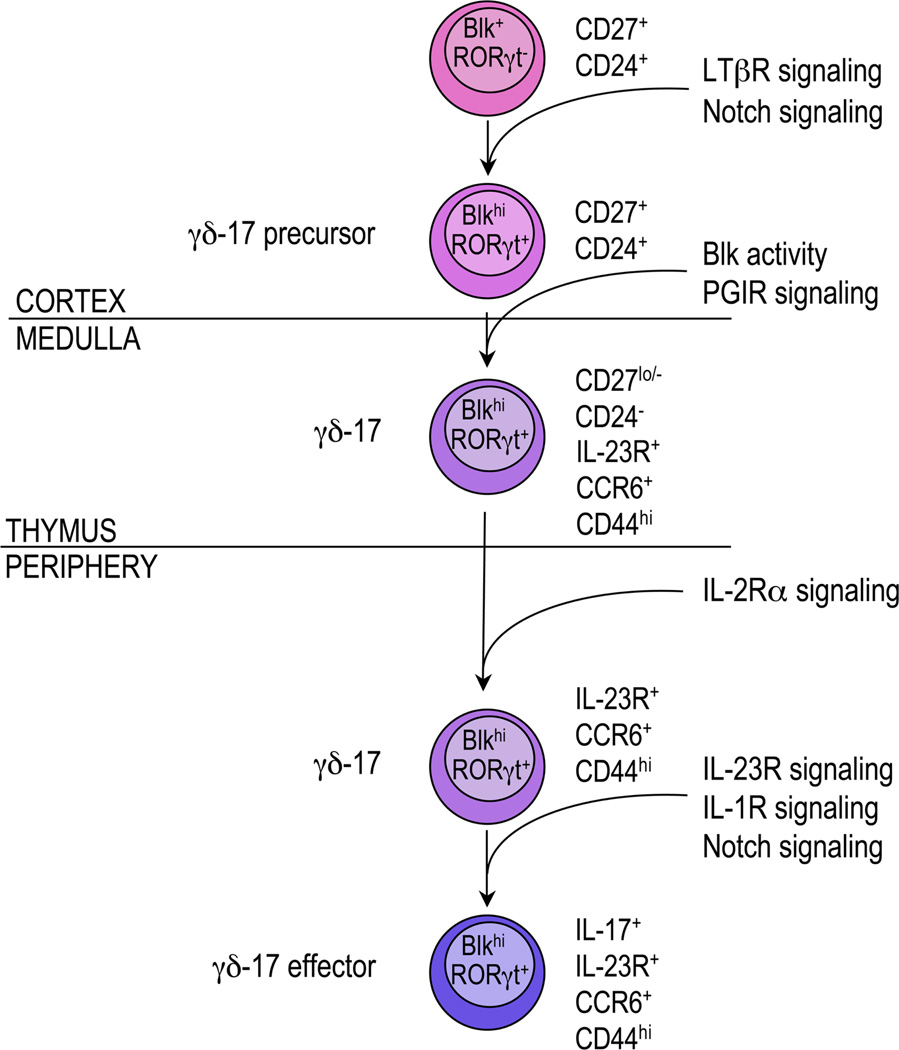
A proposed 3-stage model for the development and differentiation of γδ-17 cells. In the first stage, functionally uncommitted γδ thymocytes commit to the γδ-17 lineage and begin to express RORγt. These RORγT+ γδ thymocytes are referred to as γδ-17 precursors. In the next stage, these γδ-17 precursors mature by acquiring expression of CCR6 and interleukin (IL)-23R and then emigrate to the periphery. At the last stage, peripheral γδ-17 cells differentiate into cytokine-producing effectors. See text for more details. Blk, B lymphoid kinase; LTβR, lymphotoxin-β receptor; PGIR, Prostaglandin I2 receptor.
B. Notch Signaling
Recent evidence suggests that Notch signaling in γδ-17 cells also is required in the periphery to maintain their optimal function. When Hes-1 is conditionally deleted in peripheral γδ-17 cells, there are significantly fewer IL-17+ γδ T cells in these mice than in wild-type mice.73 This reduction in IL-17–producing γδ T cells may be explained by the ability of Notch signaling to regulate positively both Rorc and IL-17 transcription.31,32
C. IL-2/IL-2Rα Signaling
γδ-17 cells express CD25,51 a surface phenotype shared by regulatory T cells but not Th17 cells.21–23 The loss of either IL-2 or CD25 has no effect on γδ-17 development because the number of IL-17–producing γδ thymocytes is comparable among CD25−/−, IL-2−/−, and wild-type mice.51 Remarkably, their loss does have an effect on γδ-17 cell maintenance because IL-17+ γδ T cells are virtually undetectable in the peritoneal cavity of CD25−/− mice and severely reduced in the peritoneal cavity of IL-2−/− mice.51 It is not known how IL-2 signaling regulates the maintenance of γδ-17 cells; the cellular source(s) of IL-2, and the mechanism by which CD25 signals in γδ-17 cells, remain unresolved.51
VI. A THREE-STAGE MODEL FOR THE DEVELOPMENT AND DIFFERENTIATION OF γδ-17 CELLS
By comparing the genetic requirements for the generation of γδ-17 and Th17 cells, we have found that many of the signaling pathways that regulate their development and function are, in fact, shared. In this section, we build on this comparison to define the stages of γδ-17 development and differentiation and, on the basis of the quantity of supporting data, identify the genes that act at each stage (see Fig. 2).
A. Stage One: Commitment to the γδ-17 Lineage
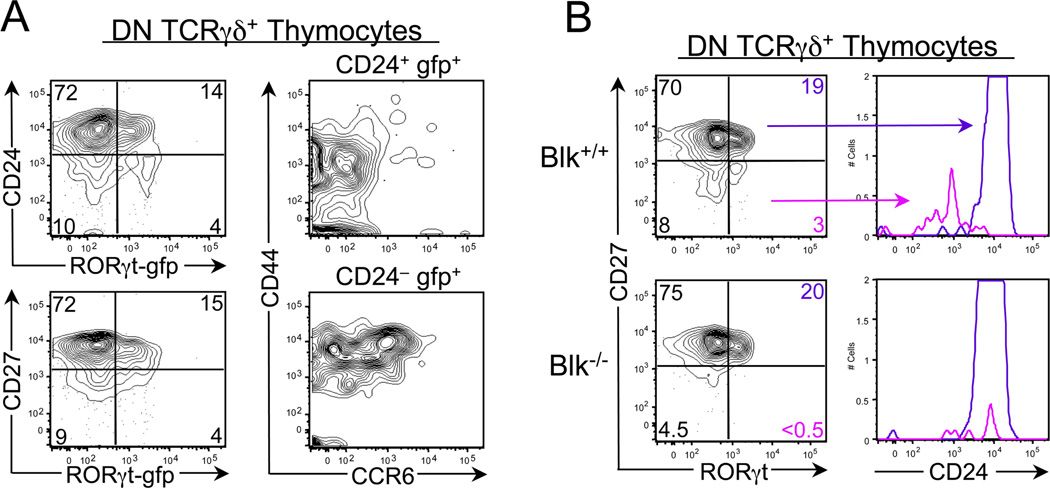
Both γδ-IFNγ and γδ-17 thymocytes arise from a CD27+CD24+ γδ thymocyte,20 which is not committed to any effector lineage.49 During the first stage of γδ-17 development, these functionally uncommitted cells encounter, in the thymic cortex, LTβR ligands produced by CD4+CD8+ thymocytes69,78 and delta-like 4, a Notch1 ligand expressed by cortical thymic epithelial cells.84 Signaling through both LTβR and Notch leads to the induction of Rorc transcription.31,32,69 In fact, there may be synergy between the NF-κb and Notch signaling pathways in inducing RORγt expression because synergy has been noted between these 2 pathways in other developmental processes.85,86 These RORγT+ γδ thymocytes, which we have termed γδ-17 precursors, express no or low levels of CCR6, IL-23R, and CD44 and high levels of CD27 and CD24. Importantly, these γδ-17 precursors can be detected in the thymus of RORγtgfp/+ mice and of wild-type mice using an anti-RORγt antibody (Fig. 3).It is notable that mice that lack expression of Blk possess wild-type numbers of γδ-17 precursors (Fig. 3), suggesting that this kinase is required at a later stage of γδ-17 development in the thymus.
FIGURE 3.
Identification of γδ-17 precursors in the thymus. (A) dot plots show representative CD24 (top left) and CD27 (bottom left) staining versus GFP (representing RORgt expression) on gated DN TCR γδ+ thymocytes from 3.5-week-old RORγtgfp/+ mice. Numbers represent percentages of cells in each quadrant. Dot plots show representative CD44 versus CCR6 staining on gated CD24+gfp+ DN TCRγδ+ thymocytes (top right) and on gated CD24logfp+ DN TCRγδ+ thymocytes (bottom right). (B) Dot plots show representative CD27 versus RORγt staining on gated DN TCRγδ+ thymocytes from 5-week-old Blk+/+ and Blk−/− mice. Numbers represent percentages of cells in each quadrant. Adjacent histograms show CD24 staining on gated CD27+ROR γt+ subset (purple histogram) and on gated CD27-RORγt+ subset (magenta histogram). Blk, B lymphoid kinase; DN, double negative; GFP, green fluorescent protein; TCR, T cell receptor.
B. Stage Two: Maturation of γδ-17 Precursors into γδ-17 Thymocytes
Within a wild-type thymus, there is a small population of CCR6+CD44hi γδ thymocytes that produce IL-17 when stimulated in vitro.49,52 We propose that these mature cells are derived from the pool of γδ-17 precursors in response to factors whose signaling pathways require Blk activity. It also is conceivable that prostaglandin I2 acts at this stage, if it induces IL-23R and IL-1R expression as prostaglandin E2 does.33 Moreover, because these cells are mature, they are probably located in the medulla, where they are positioned to leave the thymus.
C. Stage Three: Differentiation of γδ-17 Cells into IL-17–Producing Effectors
Once γδ-17 cells exit the thymus, they migrate to mucosal and lymphoid tissues, where IL-2/IL-2Rα signaling is required for their homeostasis.51 During an immune response, IL-23 and IL-1 are produced81 and delta-like 4 levels are increased,87,88 all of which act to induce IL-17 production from γδ-17 cells.6,7,13
VII. CONCLUDING REMARKS
Through their early production of IL-17, γδ-17 cells play a critical role in controlling the immune response, be it protective, as in the case of microbial infection, or pathogenic, as in the case of autoimmune disease. By reviewing what currently is known about the genetic requirements for γδ-17 cell generation, we have gained a better understanding of the molecules and associated signaling pathways that regulate their development and activation. There is also the realization that more tools are required to study these cells, such as an in vitro culture system, not only to identify factors that promote and support their commitment and maturation but also to determine their mechanism of action. Moreover, when investigating the role of a specific gene in γδ-17 development and function, it would be beneficial to use reporter mice that express GFP under the control of the Rorc or Il23r promoter to track maturation, differentiation, and migration of wild-type and mutant γδ-17 cells under steady-state conditions and during disease states. The knowledge gained will enable the development of vaccines and strategies that promote or suppress γδ-17 cell function.
ACKNOWLEDGEMENTS
This work was supported by the Hendricks Fund for Medical Research and the National Institutes of Health grant no. AI081068. We thank Drs. Michael Princiotta and Paul Love for critical review of the manuscript.
ABBREVIATIONS
- γδ-17 cells
interleukin-17–producing γδ T cells
- Blk
B lymphoid kinase
- DCs
dendritic cells
- EAE
experimental autoimmune encephalitis
- GFP
green fluorescent protein
- IFN
interferon
- IL
interleukin
- LTβR
lymphotoxin-β receptor
- NF
nuclear factor
- PMA
phorbol 12-myristate 13-acetate
- TCR
T cell receptor
- TGF
transforming growth factor
- Th17 cells
interleukin-17–producing helper T cells
REFERENCES
- 1.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 3.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 5.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O’Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 7.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulated unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. IL-17A-producing γδ T cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J Immunol. 2010;185:5879–5887. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 9.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 10.Dejima T, Shibata K, Yamada H, Hara H, Iwakura Y, Naito S, Yoshikai Y. Protective role of naturally occurring interleukin-17A-producing γδ T cells in the lung at the early stage of systemic candidiasis in mice. Infect Immun. 2011;79:4503–4510. doi: 10.1128/IAI.05799-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do JS, Visperas A, Dong C, Baldwin WM, 3rd, Min B. Cutting edge: generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J Immunol. 2011;186:4546–4550. doi: 10.4049/jimmunol.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VJ, Oukka M, Korn T. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an Interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Génin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, André F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesnière A, Ibrahim N, Déchanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L. Contribution of IL-17-producing γδ T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor via promoting angiogenesis. Eur J Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 18.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong C. Th17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 23.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 26.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 30.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, Anguita J, Juncadella I, Nickoloff BJ, Le Poole IC, Miele L, Osborne BA. Notch signaling regulates mouse and human Th17 differentiation. J Immunol. 2011;187:692–701. doi: 10.4049/jimmunol.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 35.Huang MC, Watson SR, Liao JJ, Goetzl EJ. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 36.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Xia Z, Lan Q, Wang J, Su W, Han YP, Fan H, Liu Z, Stohl W, Zheng SG. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e23629. doi: 10.1371/journal.pone.0023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Hardy K, Bunting K, Daley S, Ma L, Shannon MF. Regulation of the IL-21 gene by the NF-κB transcription factor c-Rel. J Immunol. 2010;185:2350–2359. doi: 10.4049/jimmunol.1000317. [DOI] [PubMed] [Google Scholar]
- 39.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORγ-RORγT transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S, Daley S, Shannon MF. The NF-κB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 41.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls TH17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brustle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 43.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 46.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IκBζ regulates TH17 development by cooperating with ROR nuclear receptors. Nature. 2010;464:1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 48.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2011;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas JD, González FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 50.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 52.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing γδ T cells in the thymus occurs via a TGF-β1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult γδ T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 55.Laird RM, Laky K, Hayes SM. Unexpected role for the B cell-specific Src family kinase B lymphoid kinase in the development of IL-17-producing γδ T cells. J Immunol. 2010;185:6518–6527. doi: 10.4049/jimmunol.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito K, Bonneville M, Takagachi Y, Nakanishi N, Kanagawa O, Krecko EG, Tonegawa S. Different γδ T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989;86:631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothenberg E, Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983;130:1627–1633. [PubMed] [Google Scholar]
- 59.Gregoire KE, Goldschneider I, Barton RW, Bollum FJ. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues to rat and mouse. J Immunol. 1979;123:1347–1352. [PubMed] [Google Scholar]
- 60.Bogue M, Gilfillan, Benoist C, Mathis D. Regulation of N-region diversity in antigen receptors of rat and mouse. Proc Natl Acad Sci U S A. 1992;89:11011–11015. doi: 10.1073/pnas.89.22.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elliot JF, Rock EP, Patten PA, Davis MM, Chien Y-H. The adult T-cell receptor δ-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988;311:627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- 62.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor gd genes: implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;58:859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 63.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a γδ thymocyte subset with homogenous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 64.Nandi D, Allison JP. Phenotypic analysis and γδ T-cell receptor repertoire of murine T-cells associated with vaginal epithelium. J Immunol. 1991;147:1773–1778. [PubMed] [Google Scholar]
- 65.Sim GK, Rajaserkar R, Dessing M, Augustin A. Homing and in situ differentiation of resident pulmonary lymphocytes. Int Immunol. 1994;6:1287–1295. doi: 10.1093/intimm/6.9.1287. [DOI] [PubMed] [Google Scholar]
- 66.Kim CH, Witherden DA, Havran WL. Characterization and TCR variable region use of mouse resident nasal γδ T lymphocytes. J Leukoc Biol. 2008;84:1259–1263. doi: 10.1189/jlb.0108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperling AI, Cron RQ, Decker DC, Stern DA, Bluestone JA. Peripheral T cell receptor γδ variable gene repertoire maps to the T cell receptor loci and is influenced by positive selection. J Immunol. 1992;149:3200–3207. [PubMed] [Google Scholar]
- 69.Powolny-Budnicka I, Riemann M, Yanzer S, Schmid RM, Hehlgans T, Weih F. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent Interleukin-17 production by γδ T cells. Immunity. 2011;34:364–374. doi: 10.1016/j.immuni.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, Kawamoto H, Toh H, Yoshikai Y. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood. 2011;118:586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 71.Jaffar Z, Ferrini ME, Shaw PK, FitzGerald GA, Roberts K. Prostaglandin I2 promotes the development of IL-17-producing γδ T cells that associate with the epithelium during allergic lung inflammation. J Immunol. 2011;187:5380–5391. doi: 10.4049/jimmunol.1101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 73.Butterfield K, Fathman CG, Budd RC. A subset of memory CD4+ helper T lymphocytes identified by expression of Pgp-1. J Exp Med. 1989;169:1461–1466. doi: 10.1084/jem.169.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park K, He X, Lee HO, Hua X, Li Y, Wiest D, Kappes DJ. TCR-mediated ThPOK induction promotes development of mature (CD24−) γδ thymocytes. EMBO J. 2010;29:2329–2341. doi: 10.1038/emboj.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samuelson EM, Laird RM, Maue AC, Rochford R, Hayes SM. Blk haploinsufficiency impairs the development, but enhances the functional responses, of MZ B cells. Immunol Cell Biol. 2011 Sep 6; doi: 10.1038/icb.2011.76. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki M, Yamamoto M, Sugimoto A, Nakamura S, Motoda R, Orita K. Delta-4 expression on a stromal cell line is augmented by interleukin-6 via STAT3 activation. Exp Hematol. 2006;34:1143–1150. doi: 10.1016/j.exphem.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 77.Muller KR, Siebenlist U. Lymphotoxin β receptor induces sequential activation of distinct NF-κB factors via separate signaling pathways. J Biol Chem. 2003;278:12006–12012. doi: 10.1074/jbc.M210768200. [DOI] [PubMed] [Google Scholar]
- 78.Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of γδ cell differentiation of αβ T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- 79.Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 80.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med (Berl) 2009;87:1015–1022. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 81.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doisne JM, Soulard V, Bécourt C, Amniai L, Henrot P, Havenar-Daughton C, Blanchet C, Zitvogel L, Ryffel B, Cavaillon JM, Marie JC, Couillin I, Benlagha K. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1− invariant NKT cells to bacteria. J Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 84.Fiorini E, Ferrero I, Merck E, Favre S, Pierres M, Luther SA, MacDonald HR. Cutting edge: thymic crosstalk regulates delta-like 4 expression on cortical epithelial cells. J Immunol. 2008;181:8199–8203. doi: 10.4049/jimmunol.181.12.8199. [DOI] [PubMed] [Google Scholar]
- 85.Ang H-L, Tergaonkar V. Notch and NFκB signaling pathways: do they collaborate in normal vertebrate brain development and function? Bioessays. 2007;29:1039–1047. doi: 10.1002/bies.20647. [DOI] [PubMed] [Google Scholar]
- 86.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 87.Schaller MA, Neupane R, Rudd BD, Kunkel SL, Kallal LE, Lincoln P, Lowe JB, Man Y, Lukacs NW. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med. 2007;204:2925–2934. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reynolds ND, Lukacs NW, Long N, Karpus WJ. Delta-like ligand 4 regulates central nervous system T cell accumulation during experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2803–2813. doi: 10.4049/jimmunol.1100160. [DOI] [PMC free article] [PubMed] [Google Scholar]