Abstract
Synthesis of eukaryotic messenger RNA by RNA polymerase II is governed by the concerted action of a set of general transcription factors that control the activity of polymerase during both the initiation and elongation stages of transcription. To date, five general elongation factors [P-TEFb, SII, TFIIF, Elongin (SIII) and ELL] have been defined biochemically. Here, we discuss these transcription factors and their roles in controlling the activity of the RNA polymerase II elongation complex.
In recent years the pace of research on the elongation stage of eukaryotic mRNA synthesis has accelerated. Current studies suggest that eukaryotes possess two classes of elongation factors: general elongation factors, whose functions are to expedite efficient transcription of all eukaryotic protein-coding genes1–4; and regulatory elongation factors5–8, whose functions are to control expression of specific genes or gene families by regulating transcription of particular protein-coding genes. Because no cellular proteins known to function as gene-specific regulatory elongation factors have yet been isolated, we will confine our review to a discussion of those biochemically defined cellular proteins whose activities suggest they could function as general elongation factors (Table I).
Table I.
RNA polymerase II general elongation factors
| Factor | Structure | Polypeptides (kDa) | Activities |
|---|---|---|---|
| P-TEFb | Heterodimer | 124, 43 | Prevents arrest, DRBa sensitive |
| SII (TFIIS) | Monomer | 38 | Prevents arrest, promotes nascent transcript cleavage |
| TFIIF | Heterodimer | 30, 70 | Suppresses pausing |
| Elongin (SIII) | Heterotrimer of Elongin A, B and C | Suppresses pausing | |
| Elongin A | 110 | Transcriptionally active subunit | |
| Elongin B | 18 | Regulatory subunit | |
| Elongin C | 15 | Regulatory subunit | |
| ELL | 80 | Suppresses pausing |
5,6-dichloro-1-β-d-ribofuranosylbenzimidazole.
A role for general elongation factors
The existence of a set of general elongation factors is predicted by several notable features of the mechanism of elongation by RNA polymerase II (Pol II). First, studies of elongation by RNA Pol II in cultured cells have revealed that the nucleotide analog 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) is a potent inhibitor of total hnRNA synthesis and acts by dramatically increasing the frequency at which transcribing polymerase suffers arrest at poorly defined sites within a few hundred nucleotides of the promoter9. Because DRB does not affect elongation by purified RNA Pol II in promoter-independent assays or in highly purified, reconstituted transcription systems, these findings suggest the existence of a DRB-sensitive general elongation factor(s) that regulates the activity of RNA Pol II at an early stage in the elongation of mRNA.
Second, biochemical studies have revealed that purified RNA Pol II cannot extend RNA chains in vitro at rates sufficient to account for the observed rates of mRNA synthesis in vivo. Whereas mRNA synthesis is carried out efficiently in eukaryotic cells at rates of 1200–2000 nucleotides min−1, elongation by purified RNA Pol II is an inherently discontinuous process that proceeds optimally in vitro at rates of only 100–300 nucleotides min−1 and is interrupted by frequent pausing and, in some cases, arrest. As a consequence, general elongation factors that suppress pausing or prevent arrest of transcribing RNA Pol II might be predicted to be vital for expression of many eukaryotic protein-coding genes, including extremely large genes such as dystrophin, which spans more than 2 mb of chromosomal DNA and requires nearly 17 hours to be transcribed, and genes such as Drosophila antennapedia (100 kb) and ultrabithorax (70 kb), whose expression must be precisely timed during development.
General elongation factors that prevent arrest
Two structurally unrelated cellular proteins that prevent arrest, P-TEFb and SII, have been defined biochemically. P-TEFb was originally identified in Drosophila extracts and purified to homogeneity by Price and co-workers10 by its ability to protect elongating RNA Pol II from arrest in a partially purified Drosophila transcription system. P-TEFb is inhibited by DRB and is therefore an excellent candidate to regulate the DRB-sensitive step in elongation by RNA Pol II.
How P-TEFb protects polymerase from arrest is not yet clear. P-TEFb is a heterodimer composed of ~124 kDa and ~43 kDa polypeptides and has been shown to possess a protein kinase activity that can phosphorylate the RNA Pol II carboxy-terminal domain (CTD) (N. Marshall and D. Price, pers. commun.). It is therefore possible that P-TEFb promotes elongation by a mechanism involving phosphorylation of RNA Pol II or other proteins involved in DRB-sensitive transcription.
SII is an ~38 kDa protein, which was originally purified to homogeneity from Ehrlich ascites tumor cells by Natori and co-workers11. SII promotes passage of elongating RNA Pol II through a wide variety of transcriptional impediments, including some nucleoprotein complexes as well as specific DNA sequences referred to as intrinsic arrest sites, which are found scattered throughout the transcribed regions of eukaryotic protein-coding genes1,12. Unlike P-TEFb, SH does not protect RNA Pol II from DRB-sensitive arrest. As discussed in more detail below, SII promotes the passage of RNA Pol II through impediments by an unusual mechanism involving re-iterative endonucleolytic cleavage and re-extension of nascent transcripts held in the polymerase active site.
SII and nascent transcript cleavage
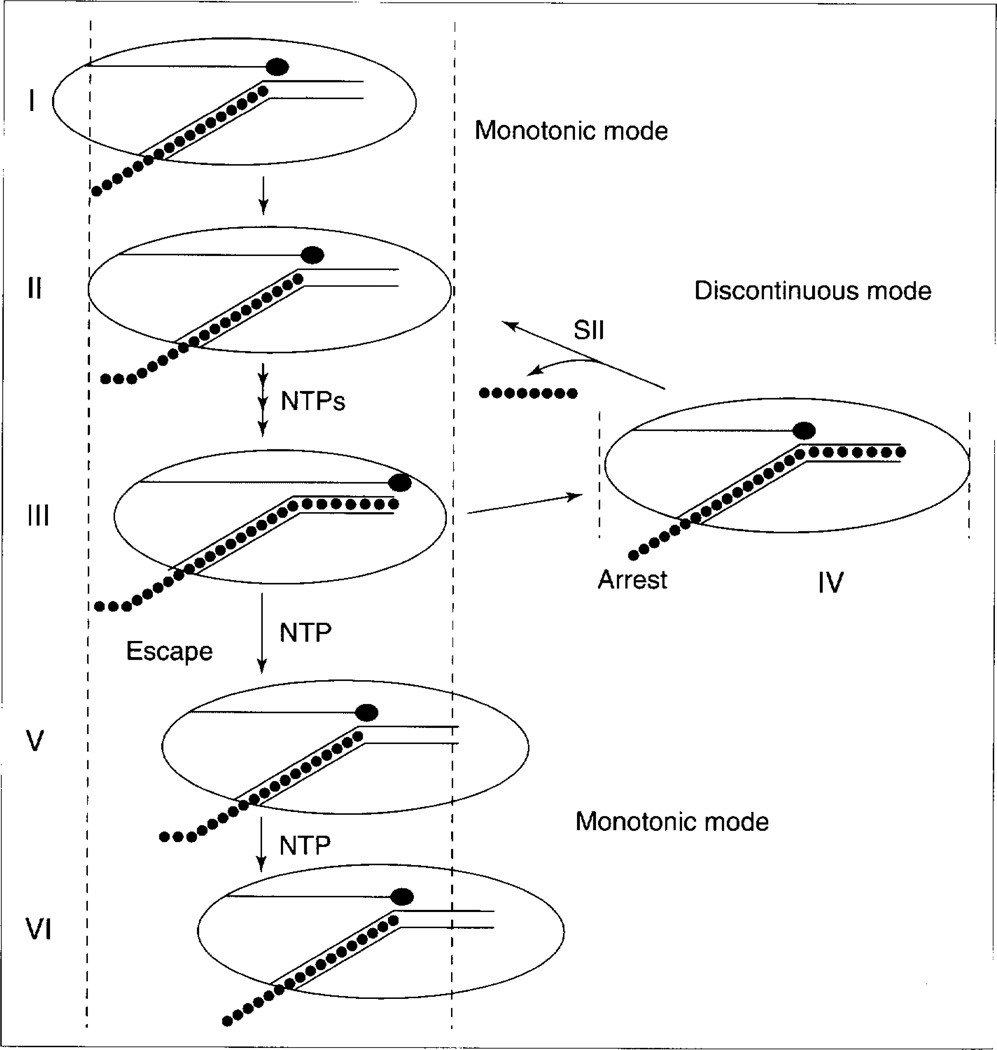
Efforts to understand how SII prevents arrest have been greatly aided by biochemical studies of the mechanics of elongation by RNA Pol II. A variety of evidence suggests that elongation by RNA Pol II proceeds by one of two mechanisms referred to as monotonic elongation and discontinuous elongation13,14 (Fig. 1). During monotonic elongation, RNA Pol II translocates each time a ribonucleotide is added to the growing transcript. When RNA Pol II approaches an intrinsic arrest site or other impediment, the enzyme begins discontinuous elongation and ceases to translocate even though the transcript continues to grow. Current models propose that, under these conditions, polymerase is forced into a strained conformation that can be resolved in one of two ways: the leading edge of the enzyme can move forward to escape arrest and resume monotonic elongation, or else the catalytic site of the enzyme can slip backwards, out of contact with the 3′-hydroxyl terminus of the nascent transcript, to enter the arrested state.
Figure 1.
Monotonic and discontinuous modes of elongation (based on models proposed by Chamberlin13, Nudler et al.14 and W. Gu, PhD Thesis, Emory University, 1996). During monotonic elongation (states I, II), nucleotides (●) are incorporated into RNA, polymerase translocates along the DNA and the extended transcript is extruded into solution. Discontinuous elongation (states II, III) occurs when polymerase continues to incorporate nucleotides into RNA, but fails to translocate downstream. Under these conditions, nascent RNA (●) occupies an extended product groove in polymerase (referred to as site I in Ref. 13). The conformation of polymerase might be ‘strained’ in state III as its catalytic site moves relative to the rest of the enzyme, but might decay into an arrested conformation (IV) in which the catalytic site (red ball on stick) loses contact with the RNA 3′-end. SII releases polymerase from arrest by inducing RNA cleavage, which results in the release of an oligonucleotide (●). A correctly positioned RNA 3′-end can now be productively elongated (II). An escape transition from III to V avoids arrest owing to polymerase translocation and threading of RNA out of the enzyme. The relative frequency at which state III goes to IV rather than to V is arrest efficiency. Another round of monotonic (V, VI) or discontinuous elongation, depending upon DNA sequence, follows.
Evidence indicates that discontinuous elongation does not always result in arrest. A fraction of RNA Pol II molecules encountering intrinsic arrest sites can escape without assistance from SII. In addition, RNA can be extended very close to the site of a template-bound protein, suggesting that the polymerase active site has continued to move forward even as translocation is blocked. This is a reversible state, as RNA synthesis resumes without SII or RNA cleavage when the protein is stripped from the DNA15.
Evidence suggests that SII releases RNA Pol II from arrest by activating a polymerase-associated endoribonuclease, which cleaves the nascent transcript upstream of its 3′-hydroxyl terminus, resulting in the creation of a new 3′-hydroxyl terminus, which is correctly positioned with respect to the polymerase catalytic site and can be extended12. SII does not affect the fraction of individual RNA Pol II molecules that pass through potential arrest sites during any given encounter. Instead, SII-activated transcript cleavage and re-extension appears to provide additional opportunities for arrested polymerases to escape arrest. Accordingly, transcriptional impediments such as template-strand thymidine dimers, which block elongation completely, cannot be overcome solely by SII-activated transcript cleavage and re-extension16.
The active site responsible for nascent transcript cleavage resides in RNA Pol II, because transcript cleavage requires a physical interaction between polymerase and the cleaved transcript and is inhibited by low concentrations of α-amanitin12. In addition, whereas purified SII has no detectable transcript cleavage activity in the absence of polymerase, highly purified RNA Pol II elongation complexes carry out low, but detectable levels of transcript cleavage. Furthermore, Luse and co-workers observed that SII-activated transcript cleavage and pyrophosphorolysis result in cleavage of the same internal phosphodiester bonds of nascent transcripts, suggesting that both RNA chain polymerization and transcript cleavage are catalysed by the same active site in polymerase17. Because the specific active-site residues responsible for polymerization and transcript cleavage have not yet been identified, it is not clear if they are identical.
More detailed information concerning the molecular mechanism of SII action is likely to emerge from ongoing studies of its structure and function. The gene encoding SII is ubiquitously expressed and highly conserved in species as divergent as yeast, archaebacteria and humans. The least conserved region of SII is its amino terminus, which shares sequence similarity with the amino terminus of Elongin A. The highly conserved carboxy-terminal half of SII is sufficient for all its known functions, including binding to RNA Pol II, preventing arrest, and activating nascent transcript cleavage. Interestingly, although intact SII does not detectably bind to nucleic acids at physiological ionic strength, SII mutants lacking ~70 amino acids from the central region of the protein bind without sequence specificity to both double- and single-stranded nucleic acids, leading to the suggestion that the SII carboxyl terminus contains a cryptic nucleic acid-binding activity that is revealed by the interaction of SII with the RNA Pol II elongation complex18.
The carboxy-terminal 50 amino acids of SII form an unusual structure containing three anti-parallel β-sheets with four zinc-liganding cysteine residues19. Based on sequence similarity and molecular modelling, this ‘zinc ribbon’ structure might resemble a region of the ninth subunit of RNA Pol II. The role of the zinc ribbon in SII function is unknown; it contains neither nucleic acid nor RNA Pol II-binding activities. The central portion of Saccharomyces cerevisiae SII between residues 131 and 240 can bind independently to RNA Pol II (D. Awrey, pers. commun.). Based on its solution nuclear magnetic resonance (NMR) structure, this SII region forms a highly α-helical domain20. It is notable that, although SII and the bacterial protein greA are functional homologs, neither their primary sequences nor their three-dimensional structures appear related.
Thus far, information concerning the role of SII in transcription in vivo has come from investigations of S. cerevisiae SII. Yeast strains carrying inactivating mutations in the SII gene are sensitive to 6-azauracil, which is known to lower intracellular pools of ribonucleoside triphosphates and thus might be expected to have a dramatic effect on elongation by RNA Pol II. The sensitivity of yeast cells lacking functional SII to 6-azauracil can be overcome by expression of genes encoding transcriptionally active SII derivatives in these cells21. Notably, the sensitivity of yeast cells carrying RNA Pol II large-subunit mutations to 6-azauracil can also be suppressed by overproduction of SII in these cells22. In addition, some RNA Pol II mutations that result in 6-azauracil sensitivity in vivo also reduce the affinity of polymerase for SII or influence the elongation properties of the enzyme in vitro23,24.
Elongation factors that suppress pausing
Three structurally unrelated cellular proteins, TFIIF, Elongin (SIII) and ELL, which all increase the rate of elongation by RNA Pol II by suppressing transient pausing, have been defined biochemically. As with SII, these proteins can bind to RNA Pol II, but unlike SII, neither TFIIF, Elongin (SIII) nor ELL can release RNA Pol II from arrest or promote nascent transcript cleavage. In view of their ability to stimulate the rate of elongation by RNA Pol II on oligo(dC)-tailed templates in the absence of auxiliary proteins, TFIIF, Elongin (SIII) and ELL appear to function through a direct interaction with one or more components of the ternary elongation complex.
Exactly how these proteins suppress pausing is not known. A possible clue to their mechanism of action was provided by the observation that both Elongin (SIII)25 and TFIIF (Y. Takagi et al., unpublished) promote a dramatic increase in the ability of RNA Pol II to carry out template-directed extension of the 3′-hydroxyl termini of duplex DNA fragments. It has been suggested that DNA template-directed addition of ribonucleotides to the 3′-hydroxyl termini of DNA by RNA Pol II occurs in a reaction that mimics transcription by the ternary elongation complex. According to this model, RNA Pol II binds the 3′-hydroxyl terminus of DNA in its active site, just as the enzyme binds the 3′-end of an elongating RNA molecule, and the polymerase catalytic site for ribonucleotide addition uses the DNA 3′-hydroxyl terminus as if it were the 3′-hydroxyl terminus of the nascent RNA transcript. Thus, the finding that Elongin (SIII) and TFIIF promote extension by RNA Pol II of the 3′-hydroxyl termini of DNA primers is consistent with the idea that Elongin (SIII) and TFIIF might facilitate proper positioning of the 3′-hydroxyl termini of nascent transcripts with respect to the polymerase catalytic site. If transient pausing occurs when the polymerase catalytic site is only slightly displaced from its position at the 3′-end of growing transcripts (whereas arrest occurs when the catalytic site loses contact with the 3′-end of the transcript), it is possible that Elongin (SIII) and TFIIF suppress pausing by holding the catalytic site and 3′-hydroxyl terminus of the nascent transcript in register.
TFIIF
TFIIF is unique among the general transcription factors by virtue of its ability to control the activity of RNA Pol II at both the initiation and elongation stages of transcription. A role for TFIIF in elongation was first brought to light by Greenleaf and co-workers26. Although TFIIF cannot release RNA Pol II from arrest at SII-sensitive sites, it has been shown that interaction of TFIIF with transcribing polymerase before an encounter with an arrest site decreases the likelihood that it will suffer arrest27. Thus, TFIIF and SII appear to regulate elongation by RNA Pol II in different, yet complementary ways: whereas TFIIF decreases the duration of pausing by RNA Pol II and protects the elongation complex from arrest, SII re-activates elongation complexes that have arrested. Whether Elongin (SIII) and ELL also protect the RNA Pol II elongation complex from arrest is not known.
Although it is not clear how TFIIF promotes elongation, structure–function studies of its RAP30 and RAP74 subunits have revealed that TFIIF initiation and elongation activities are carried out by different functional domains. Analysis of RAP74 mutants suggests that most of RAP74 is required for initiation, whereas only the RAP74 amino terminus is required for elongation28. Analysis of RAP30 mutants suggests that a RAP30 carboxy-terminal region required for initiation and that appears to bind DNA in the preinitiation complex is dispensable for elongation, whereas a RAP30 region in the central portion of the protein is required for elongation29. Interestingly, this RAP30 region resembles the Pol-binding domains of σ70 from Escherichia coli and σ43 from Bacillus subtilis, and McCracken and Greenblatt have obtained evidence that TFIIF indeed interacts with RNA Pol II through this region30.
The Elongin (SIII) complex
Elongin (SIII) was initially purified from rat liver nuclei as a multimeric complex composed of A, B and C subunits of ~110, 18 and 15 kDa31. Biochemical studies32 have revealed that Elongin A is the transcriptionally active subunit of the Elongin (SIII) complex, and that Elongin B and C are positive regulatory subunits capable of forming a stable binary complex that can strongly induce Elongin A transcriptional activity (Fig. 2). Elongin B and C regulate Elongin A activity by different mechanisms. Elongin C can bind directly to Elongin A in the absence of Elongin B to form an AC complex with increased specific activity in transcription, suggesting that Elongin C functions as a bona fide inducer of Elongin A activity. By contrast, Elongin B, a member of the ubiquitin homology (UbH) gene family, does not bind stably to Elongin A in the absence of Elongin C. Instead, Elongin B has a chaperone-like role in assembly of the Elongin (SIII) complex by binding to Elongin C and promoting its interaction with Elongin A.
Figure 2.
Model for regulation of Elongin (SIII) transcriptional activity by the von Hippel–Lindau (VHL) tumor suppressor protein. (a) The Elongin BC complex binds to Elongin A and induces its transcriptional activity. The fully assembled Elongin ABC complex strongly stimulates the overall rate of elongation by RNA polymerase II. (b) The VHL tumor suppressor protein can bind to the Elongin BC complex. Binding of the VHL protein and Elongin A to the Elongin BC complex is mutually exclusive. In addition, binding of the VHL protein to the Elongin BC complex prevents Elongin BC from interacting with Elongin A. One role of the VHL tumor suppressor protein, therefore, might be to regulate the activity of the RNA polymerase II (Pol II) elongation complex. Other abbreviations used: A, Elongin A; B, Elongin B; C, Elongin C; V, VHL.
Evidence implicating Elongin (SIII) in human genetic disease was obtained recently from experiments revealing that Elongin (SIII) is a potential target for regulation by the product of the von Hippel–Lindau (VHL) tumor suppressor gene33,34. The VHL gene is mutated in families with VHL disease, a rare genetic disorder (incidence ~1 in 36 000), which predisposes affected individuals to a variety of cancers, including clear-cell renal carcinoma, central nervous system hemangioblastomas, retinal hemangiomas, multiple endocrine neoplasias and pheochromocytomas. Of greater general clinical importance, functional loss of both VHL alleles is observed in the majority of patients with sporadic clear-cell renal carcinoma.
The VHL protein binds tightly and specifically to the Elongin BC complex both in vitro and in cells. A subset of naturally occurring VHL mutants from VHL tumors and clear-cell renal carcinomas exhibit substantially reduced binding to the Elongin BC complex, arguing that the VHL–Elongin BC interaction is likely to be important for the tumor suppressor activity of the VHL protein. Binding of VHL and Elongin A to the Elongin BC complex in vitro is mutually exclusive and depends on a short sequence conserved in VHL and Elongin A. Furthermore, binding of the VHL protein to the Elongin BC complex inhibits its ability to interact with Elongin A, leading to the suggestion that tumor suppression by the VHL protein could involve regulation of Elongin A transcriptional activity.
ELL
ELL is an ~80 kDa elongation factor that was originally purified to homogeneity from rat liver nuclei35. The human ELL (eleven-nineteen lysine-rich leukemia) gene on chromosome 19p13.1 was initially isolated as a gene that undergoes frequent translocations with the mixed lineage leukemia (MLL) gene on chromosome 11q23 in acute myeloid leukemia36,37. The human ELL gene encodes a 621 amino acid protein that contains no obvious structural motifs characteristic of transcription factors. The MLL gene encodes a protein of 3968 amino acids that contains amino-terminal AT-hook DNA-binding and methyltransferase-like domains and several carboxy-terminal regions resembling those encoded by the Drosophila trithorax gene, including multiple contiguous zinc finger motifs and a transcriptional activation domain. In addition, MLL appears to regulate the expression of homeotic genes, also like the Drosophila trithorax protein.
It is not clear what role, if any, the ELL protein and its activity in elongation play in the development of acute myeloid leukemia. Characterization of chromosomal abnormalities in a large number of human cancers has revealed that the MLL gene is a recurring target for translocations in a variety of clinically distinct leukemias. Genes encoding six MLL translocation partners, in addition to ELL, have been cloned. A remarkable feature of these translocations is that their breakpoints result in the creation of a putative oncogene. This ‘oncogene’ encodes nearly the entire translocation partner fused to the amino-terminal ~1400 amino acids of the MLL protein, including its AT-hook and methyltransferase-like domains, but lacking its carboxy-terminal zinc fingers and transcriptional activation domain. The finding that these translocations all occur within the same region of the MLL gene, but are associated with clinically distinct leukemias, has led to the suggestion that the MLL translocation partner might play an important role in establishing the leukemic phenotype. The discovery of a second RNA Pol II elongation factor implicated in oncogenesis provides further support for a close connection between the regulation of transcription elongation and cell growth.
Acknowledgements
Work in the authors’ laboratories is supported by National Institutes of Health Grants GM46331 (D. R.) and GM41628 (J. W. C. and R. C. C.) and by funds provided to the Oklahoma Medical Research Foundation by the H. A. and Mary K. Chapman Charitable Trust (J. W. C. and R. C. C).
Contributor Information
Daniel Reines, Department of Biochemistry, Emory University School of Medicine, Atlanta, GA 30322, USA.
Joan Weliky Conaway, Program in Molecular and Cell Biology, Oklahoma Medical Research Foundation, 825 N. E. 13th Street, Oklahoma City, OK 73104, USA.
Ronald C. Conaway, Program in Molecular and Cell Biology, Oklahoma Medical Research Foundation, 825 N. E. 13th Street, Oklahoma City, OK 73104, USA
References
- 1.Kerppola TK, Kane CM. FASEB. J. 1991;5:2833–2841. doi: 10.1096/fasebj.5.13.1916107. [DOI] [PubMed] [Google Scholar]
- 2.Kane CM. In: Transcription: Mechanisms and Regulation. Conaway RC, Conaway JW, editors. Raven Press; 1994. pp. 279–296. [Google Scholar]
- 3.Aso T, Shilatifard A, Conaway JW, Conaway RC. J. Clin. Invest. 1996;97:1561–1569. doi: 10.1172/JCI118580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aso T, Conaway JW, Conaway RC. FASEB. J. 1995;9:1419–1428. doi: 10.1096/fasebj.9.14.7589983. [DOI] [PubMed] [Google Scholar]
- 5.Yankulov K, et al. Cell. 1994;77:749–759. doi: 10.1016/0092-8674(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 6.Krumm A, Hickey LB, Groudine M. Genes Dev. 1995;9:559–572. doi: 10.1101/gad.9.5.559. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Lis JT. In: Transcription: Mechanisms and Regulation. Conaway RC, Conaway JW, editors. Raven Press; 1995. pp. 459–475. [Google Scholar]
- 8.Jones KA, Peterlin BM. Annu. Rev. Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 9.Fraser NW, Sehgal PB, Darnell JE. Nature. 1978;272:590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- 10.Marshall NF, Price DH. J. Biol. Chem. 1995;270:12335–12338. doi: 10.1074/jbc.270.21.12335. [DOI] [PubMed] [Google Scholar]
- 11.Sekimizu K, Kobayashi N, Mizuno D, Natori S. Biochemistry. 1976;15:5064–5070. doi: 10.1021/bi00668a018. [DOI] [PubMed] [Google Scholar]
- 12.Reines D. In: Transcription: Mechanisms and Regulation. Conaway RC, Conaway JW, editors. Raven Press; 1994. pp. 263–278. [Google Scholar]
- 13.Chamberlin MJ. Harvey Lectures. 1995;88:1–21. [PubMed] [Google Scholar]
- 14.Nudler E, Goldfarb A, Kashlev M. Science. 1994;265:793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- 15.Reines D, Mote J. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1917–1921. doi: 10.1073/pnas.90.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue BA, et al. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd MD, Izban MG, Luse DS. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8057–8061. doi: 10.1073/pnas.91.17.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal K, et al. Biochemistry. 1991;30:7842–7851. doi: 10.1021/bi00245a026. [DOI] [PubMed] [Google Scholar]
- 19.Quian X, et al. Nature. 1993;365:277–279. doi: 10.1038/365277a0. [DOI] [PubMed] [Google Scholar]
- 20.Morin P, Awrey D, Edwards AM, Arrowsmith C. Proc. Natl. Acad. Sci. U. S. A. doi: 10.1073/pnas.93.20.10604. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi T, Shimoaraiso M, Kubo T, Natori S. J. Biol. Chem. 1995;270:8991–8995. doi: 10.1074/jbc.270.15.8991. [DOI] [PubMed] [Google Scholar]
- 22.Archambault J, Lacroute F, Ruet A, Friesen JD. Mol. Cell. Biol. 1992;12:4142–4152. doi: 10.1128/mcb.12.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell W, Reines D. J. Biol. Chem. 1996;271:6866–6873. doi: 10.1074/jbc.271.12.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, et al. Proc. Natl. Acad. Sci. U. S. A. (in press) [Google Scholar]
- 25.Takagi Y, Conaway JW, Conaway RC. J. Biol. Chem. 1995;270:24300–24305. doi: 10.1074/jbc.270.41.24300. [DOI] [PubMed] [Google Scholar]
- 26.Price DH, Sluder AE, Greenleaf AL. Mol. Cell. Biol. 1989;9:1465–1475. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Reines D. J. Biol. Chem. 1995;270:11238–11244. doi: 10.1074/jbc.270.19.11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kephart DD, Wang BQ, Burton ZF, Price DH. J. Biol. Chem. 1994;269:13536–13543. [PubMed] [Google Scholar]
- 29.Tan S, Conaway RC, Conaway JW. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6042–6046. doi: 10.1073/pnas.92.13.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken S, Greenblatt J. Science. 1991;253:900–902. doi: 10.1126/science.1652156. [DOI] [PubMed] [Google Scholar]
- 31.Bradsher JN, Jackson KW, Conaway RC, Conaway JW. J. Biol. Chem. 1993;268:25587–25593. [PubMed] [Google Scholar]
- 32.Aso T, Lane WS, Conaway JW, Conaway RC. Science. 1995;269:1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- 33.Duan DR, et al. Science. 1995;269:1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 34.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 35.Shilatifard A, et al. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 36.Thirman MJ, et al. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12110–12114. doi: 10.1073/pnas.91.25.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitani K, et al. Blood. 1995;85:2017–2024. [PubMed] [Google Scholar]