Abstract
Some, but not all, types of learning and memory can influence neurogenesis in the adult hippocampus. Trace eyeblink conditioning has been shown to enhance the survival of new neurons, whereas delay eyeblink conditioning has no such effect. The key difference between the two training procedures is that the conditioning stimuli are separated in time during trace but not delay conditioning. These findings raise the question of whether temporal discontiguity is necessary for enhancing the survival of new neurons. Here we used two approaches to test this hypothesis. First, we examined the influence of a delay conditioning task in which the duration of the conditioned stimulus (CS) was increased nearly twofold, a procedure that critically engages the hippocampus. Although the CS and unconditioned stimulus are contiguous, this very long delay conditioning procedure increased the number of new neurons that survived. Second, we examined the influence of learning the trace conditioned response (CR) after having acquired the CR during delay conditioning, a procedure that renders trace conditioning hippocampal-independent. In this case, trace conditioning did not enhance the survival of new neurons. Together, these results demonstrate that associative learning increases the survival of new neurons in the adult hippocampus, regardless of temporal contiguity.
Keywords: classical conditioning, plasticity, dentate gyrus, hippocampus, learning and memory, BrdU
Introduction
The role of the hippocampus in learning and memory has long been recognized. However, this brain region has been associated with a range of learning tasks, such as trace classical conditioning, contextual fear conditioning, spatial navigation learning, and delayed nonmatch to sample, which do not readily fall into a single category. One attempt at a unifying theory suggests that the hippocampus is necessary for learning associations between stimuli separated in time or space (Wallenstein et al., 1998; Bangasser et al., 2006). As such, conditioning procedures that involve an interval of time, or a “trace” between the presentation of the conditioned stimulus (CS) and unconditioned stimulus (US), require the hippocampus for acquisition, whereas those that present both stimuli in an overlapping manner (“delay” conditioning) typically do not (Solomon et al., 1986; Moyer et al., 1990; McEchron et al., 1998; Beylin et al., 2001; Bangasser et al., 2006).
Numerous studies have suggested an association between learning and structural plasticity in the hippocampus (for review, see Lamprecht and LeDoux, 2004; Shors, 2004). Adult neurogenesis is a fundamental form of plasticity because it leads to the growth of new dendrites, axons, and synapses (Hastings and Gould, 1999; van Praag et al., 2002; Zhao et al., 2006). In the dentate gyrus of the adult rat, neurogenesis has been related to various types of learning and memory (Kempermann et al., 2004; Becker, 2005; Leuner et al., 2006), and learning certain tasks can alter the number of new neurons (Gould et al., 1999; Ambrogini et al., 2000; Dobrossy et al., 2003; Leuner et al., 2004; Hairston et al., 2005; Olariu et al., 2005). However, not all types of learning affect adult neurogenesis. For example, trace eyeblink conditioning enhances the survival of new neurons, whereas delay eyeblink conditioning does not (Gould et al., 1999). These results raise the issue of whether learning-induced effects on neurogenesis require the association of stimuli that do not occur together in time.
Two alternate versions of trace and delay eyeblink conditioning can be used to determine whether a temporal gap between stimuli is essential for enhancing the survival of new neurons in the hippocampus. In one version, referred to here as very long delay, the conditioning stimuli are contiguous in time but learning the task depends on the hippocampus (Beylin et al., 2001). In the other version, trace eyeblink conditioning is rendered hippocampal independent by first training rats with a delay arrangement (Beylin et al., 2001). Using these procedures, we found that training on the very long delay task was as effective as the trace task at increasing the number of new neurons. Furthermore, previous training on delay eyeblink conditioning rendered trace conditioning ineffective at increasing the number of newly born cells. Together, these data suggest that temporal discontiguity is not an essential feature for enhancing adult neurogenesis.
Materials and Methods
Adult male Sprague Dawley rats (60–80 d of age) were individually housed, provided with access to food and water ad libitum, and maintained on a 12 h light/dark cycle. All experiments conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1: delay conditioning with a very long delay.
Rats were injected intraperitoneally with bromodeoxyuridine (BrdU; 200 mg/kg), a marker of dividing cells. One week later, rats underwent 800 trials of eyeblink conditioning (200 trials/d for 4 d) using a trace (n = 8), delay (n = 7), or very long delay (n = 11) procedure (Fig. 1). An additional group of naive rats (n = 10) was injected with BrdU but did not receive stimulus exposure. All rats were perfused 24 h after the last block of training trials (i.e., 13 d after BrdU injection). The timing of BrdU injection, training, and perfusion was similar to those of our previous studies, which reported increases in numbers of BrdU-labeled cells after training with trace eyeblink conditioning (Gould et al., 1999; Leuner et al., 2004).
Figure 1.
Schematic diagram of delay, trace, and very long delay conditioning procedures. During delay conditioning, an 850 ms CS overlapped and coterminated with a 100 ms US. During trace conditioning, a 250 ms CS was separated from a 100 ms US by a 500 ms stimulus-free trace interval. The ISI for both tasks was 750 ms. During very long delay conditioning, the ISI was extended to 1400 ms such that a 1500 ms CS overlapped and coterminated with a 100 ms US.
Experiment 2: trace conditioning after delay conditioning.
Rats were injected intraperitoneally with BrdU (200 mg/kg) and, 4 d later, underwent 400 trials of delay eyeblink conditioning (200 trials/d for 2 d) followed by 800 trials of trace conditioning (200 trials/d for 4 d) (n = 6). An additional group of naive rats (n = 6) was injected with BrdU but did not receive stimulus exposure. As before, rats were perfused 24 h after the last block of training trials (i.e., 13 d after BrdU injection). Importantly, the trace conditioning procedure began 1 week after the BrdU injection, the time when training with trace conditioning alone increases cell survival (Gould et al., 1999; Leuner et al., 2004).
Classical conditioning.
Rats were anesthetized with sodium pentobarbital supplemented by isoflurane inhalant. A headstage with four electrodes was secured to the skull. Electrodes consisted of stainless steel wire implanted subcutaneously to emerge through and around the eyelid. Two electrodes recorded electromyograph (EMG) activity for determination of the eyeblink, and two delivered the periorbital stimulation to elicit the eyeblink reflex.
For eyeblink conditioning, headstages were connected to a cable that allowed free movement within the conditioning chamber. Twenty-four hours before conditioning, rats were acclimated to the conditioning apparatus for 1 h. For experiment 1, rats underwent trace, delay, or very long delay conditioning (Fig. 1). All conditioning procedures utilized an 83 dB burst of white noise CS and a 0.7 mA periorbital shock US. During trace conditioning, a 250 ms CS was separated from a 100 ms US by a 500 ms stimulus-free interval. To equate the interstimulus interval (ISI) (750 ms) between the CS and US in the delay versus trace paradigms, an 850 ms CS that overlapped and coterminated with a 100 ms US was used during delay conditioning. Thus, this version of delay conditioning differs from the standard delay procedure, which typically incorporates a shorter CS and ISI (Solomon and Groccia-Ellison, 1996; Gould et al., 1999; Ivkovich and Stanton, 2001). During very long delay conditioning, the ISI was extended to 1400 ms such that a CS of 1500 ms overlapped and coterminated with a 100 ms US. For experiment 2, rats were first trained with the delay procedure (850 ms CS) for 2 d. Twenty-four hours later, rats began trace eyeblink conditioning, as described above, with the same ISI (750 ms) as delay conditioning. Each block of conditioning consisted of 100 trials with every 10 trial sequence composed of one CS-alone presentation, four paired presentations of the CS and US, one US-alone presentation, and four paired presentations of the CS and US. The intertrial interval was 25 ± 5 s. To detect the occurrence of an eyeblink, the maximum EMG response occurring during a 250 ms prestimulus baseline recording period was added to four times its SD. Responses that exceeded that value and were longer than 3 ms were recorded as eyeblinks. Eyeblinks were considered conditioned responses (CRs) if they began 500 ms before US onset. Eyeblink performance was calculated as the percentage of trials during which a CR was produced in response to a CS.
Immunohistochemistry.
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 m phosphate buffer. Brains were dissected from the skulls and postfixed for at least 2 d. Coronal sections (40 μm) throughout the entire rostrocaudal extent of the dentate gyrus were cut with a vibratome from one hemisphere into a bath of 0.1 m PBS, pH 7.5. For BrdU peroxidase staining, a 1:12 series of sections were mounted onto glass slides, dried, and pretreated by heating in 0.1 m citric acid, pH 6.0. Slides were then rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 m HCl:PBS for 30 min, rinsed, and incubated with mouse antibodies to BrdU (diluted 1:250 with 0.5% Tween 20; Vector Laboratories, Burlingame, CA). The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200; Vector Laboratories) for 60 min, rinsed, incubated with avidin–biotin complex, rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2. Slides were counterstained with cresyl violet, dehydrated, cleared, and coverslipped under Permount (Fisher Scientific, Fair Lawn, NJ).
For double-labeling immunofluorescence for BrdU and the neuronal markers class III β-tubulin (TuJ1) or neuronal nuclei (NeuN), free-floating sections were denatured in 2 m HCl:TBS for 30 min, rinsed in TBS, and incubated with rat anti-BrdU (1:200 with 0.5% Tween 20; Accurate Chemical, Westbury, NY) plus mouse anti-TuJ1 (1:500; Covance, Princeton, NJ) or mouse anti-NeuN (1:500; Chemicon, Temecula, CA) for 2 d. Then sections were rinsed, incubated with biotinylated anti-rat (1:250; Chemicon) for 90 min, rinsed, and incubated for 30 min in the dark with streptavidin-conjugated Alexa 568 (1:1000; Invitrogen, Carlsbad, CA) to visualize BrdU and with goat anti-mouse Alexa 488 (1:500; Invitrogen) to visualize TuJ1 or NeuN. Finally, sections were rinsed, mounted onto coated slides, and coverslipped using glycerol in TBS (3:1).
Microscopic data analysis.
Quantitative analysis was conducted blind to behavioral condition. For BrdU peroxidase, estimates of total numbers of BrdU-labeled cells were determined using a modified stereology protocol previously reported to successfully quantify BrdU labeling (Gould et al., 1999). BrdU-labeled cells in the subgranular zone (SGZ) and granule cell layer (GCL) on every 12th unilateral section throughout the entire rostrocaudal extent of the dentate gyrus were counted at 1000× (100× objective with a 10× ocular) on an Olympus (Tokyo, Japan) BX-50 light microscope, avoiding cells in the outermost focal plane. Counts were multiplied by 24 to obtain estimates of BrdU-labled cells per brain. For immunofluorescence, the percentage of BrdU-labeled cells in the SGZ and GCL that expressed NeuN or TuJ1 was determined using a Zeiss (Oberkochen, Germany) Axiovert confocal laser scanning microscope (510 LSM; lasers, argon 458/488 and helium–neon 543; Zeiss). For each brain, 25 randomly selected BrdU-labeled cells per marker were analyzed. Optical stacks of 1-μm-thick sections were obtained through putatively double-labeled cells. To verify double labeling throughout their extent, cells were examined in orthogonal planes.
Statistical analysis.
For experiment 1, the percentage of CRs during the first 100 trials in 20 trial blocks were analyzed using repeated-measures ANOVA because it has been shown that trace, delay, and very long delay conditioning differ with regard to their rates of acquisition during early training (Beylin et al., 2001). The percentage of CRs over the total 800 trials in 100 trial blocks was analyzed separately using repeated-measures ANOVA to evaluate overall performance throughout the training period. The effects of training condition on the numbers of BrdU-labeled cells and numbers of double-labeled cells were analyzed using one-way ANOVA followed by Newman–Keuls post hoc analysis. For experiment 2, separate repeated-measures ANOVAs were used to analyze the percentage of CRs during the 400 trials of delay conditioning and the 800 trials of trace conditioning. To evaluate the effects of pretraining on the delay task on subsequent trace conditioning, the percentage of CRs during the last 100 trials of delay conditioning and the first 20 trials of trace conditioning were compared using ANOVA. The effect of training condition on the number of BrdU-labeled cells was analyzed using a two-tailed t test.
Results
Temporal discontiguity is not essential for increasing neurogenesis
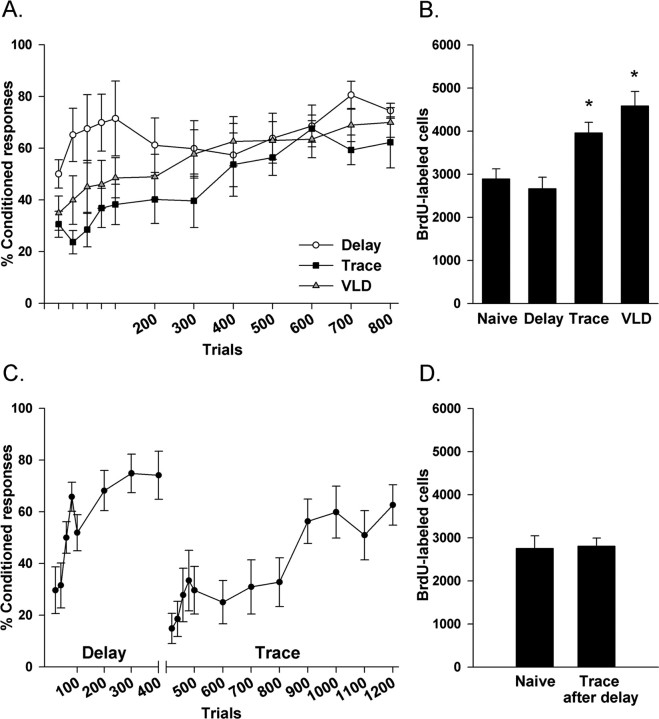
Training condition had a significant effect on the number of BrdU-labeled cells in the dentate gyrus (F(3,32) = 11.3; p < 0.00005) (Figs. 2B, 3A). As shown previously, trace conditioning increased the number of BrdU-labeled cells relative to naive rats (p < 0.01) or rats trained with the delay procedure (p < 0.01). Similarly, a greater number of BrdU-labeled cells were observed in the very long delay group compared with naive rats (p < 0.005) and those that underwent delay conditioning (p < 0.0005). The number of BrdU-labeled cells in rats that underwent trace conditioning did not differ from the number in rats trained with very long delay (p = 0.14). Also, the number of BrdU-labeled cells in naive rats did not differ from the number in rats trained with the delay procedure (p = 0.81).
Figure 2.
Temporal discontiguity is neither necessary nor sufficient for enhancing the survival of new neurons in the dentate gyrus. A, Data are represented as a mean ± SEM percentage of CRs with the first 100 trials divided into 20 trial blocks and the remaining 700 trials divided into blocks of 100. During the first 100 trials, rats trained on the delay conditioning task emitted more CRs than those trained on trace or very long delay (VLD). B, Bars represent mean ± SEM number of BrdU-labeled cells in the dentate gyrus. Both trace and VLD conditioning increased the number of BrdU-labeled cells when compared with naive rats or rats trained on delay conditioning. C, Data are represented as a mean ± SEM percentage of CRs. For both delay and trace, the first 100 trials are divided into blocks of 20 trials, and the remaining trials are divided into blocks of 100. Rats quickly acquired the CR during delay conditioning. Although conditioned responding decreased when trace conditioning began, responding during the trace phase reached a criterion of 60% trials with a CR. D, Bars represent mean ± SEM number of BrdU-labeled cells in the dentate gyrus. The number of BrdU-labeled cells in rats trained on trace after delay conditioning did not differ from naive controls. *p < 0.01.
Figure 3.
Trace and very long delay conditioning enhance adult neurogenesis. A, More BrdU-labeled cells (arrows) were observed in the dentate gyrus after both trace and very long delay (VLD) conditioning relative to naive rats and those that were trained on the delay task. B, C, Confocal laser-scanning images of BrdU-labeled cells (red) colabeled (arrow) with TuJ1 (B, green), a marker of immature and mature neurons, or the mature neuronal marker NeuN (C, green). The majority of BrdU-labeled cells expressed one of the neuronal markers, and the number of colabeled cells did not vary across groups. gcl, Granule cell layer.
Most BrdU-labeled cells expressed the markers of immature or mature neurons: ∼80% expressed TuJ1, whereas ∼70% colabeled with NeuN (Fig. 3B,C). There were no differences in the percentage of BrdU-labeled cells that expressed either neuronal marker among any of the training conditions (p values >0.05), suggesting that the increase in BrdU-labeled cell number associated with trace and very long delay conditioning represents enhanced neurogenesis.
The differential effects of training condition on the number of BrdU-labeled cells were accompanied by differences in performance during acquisition of the CR. During early training (first 100 trials), rats in the delay conditioning group emitted a greater percentage of CRs than those trained on trace or very long delay (F(2,23) = 3.8; p < 0.05) (Fig. 2A). Analysis of eyeblink conditioning performance across the 800 conditioning trials revealed that rats in each behavioral condition expressed more CRs as training progressed (F(7,161) = 6.89; p < 0.0000001). The percentage of CRs emitted across all 800 trials did not differ among groups exposed to the delay, trace, and very long delay tasks (F(14,161) = 1.02; p = 0.43).
Temporal discontiguity is not sufficient for increasing neurogenesis
The number of BrdU-labeled cells in the dentate gyrus of rats trained with trace after delay conditioning did not differ from naive controls (t(10) = 0.15; p = 0.88) (Fig. 2D). Rats increased responding over training trials during both delay (F(3,15) = 6.44; p < 0.005) and trace (F(7,35) = 7.51; p < 0.0005) conditioning phases. Although conditioned responding decreased from the last 100 trials of delay to the first 20 trials of trace conditioning (F(1,5) = 34.01; p < 0.005), responding during the trace phase reached a criterion of 60% trials with a CR (Fig. 2C).
Discussion
In the present experiments, we used two different training procedures to determine whether the effect of classical eyeblink conditioning on adult neurogenesis was limited to tasks that involve a temporal separation between stimuli. First, we compared the number of new cells in animals that were trained on trace conditioning with the number of cells in animals that were trained on two types of delay conditioning tasks, one that depends on the hippocampus (very long delay) and one that does not (delay) (Beylin et al., 2001). Both delay tasks, however, are characterized by temporal contiguity. We observed increases in the number of new neurons after training with either the trace or very long delay procedures but not after training with the delay procedure. Second, we examined the influence of trace conditioning after training with the delay procedure, a manipulation that renders trace conditioning hippocampal independent. In this case, trace conditioning did not enhance the survival of new neurons.
The only difference between very long delay and delay conditioning was the length of the CS, and yet one procedure increased cell survival and the other did not. However, there were differences in the way that the animals responded during these tasks. Animals trained with the very long delay procedure exhibited a slower rate of acquisition during early training than did the animals trained with the delay procedure, suggesting that the task becomes more difficult to learn as the temporal distance between the CS and US is extended. This observation is consistent with a previous study in which lengthening the interval between the CS and US increased the number of trials required to acquire the CR (Beylin et al., 2001). Given such a long ISI, it is more difficult for the animal to predict when the US will occur. Notably, all groups did eventually display comparable levels of conditioning, and therefore performance at the end of training does not contribute to the group differences in cell survival. Instead, it appears that new neurons are sensitive to the formation of the CS–US association and possibly the task demands of the initial association.
Animals with hippocampal lesions cannot acquire the CR during trace conditioning, even if they are exposed to more than 1000 trials (Moyer et al., 1990; Beylin et al., 2001). However, if they are first trained with delay, they can learn and express the trace response (Beylin et al., 2001). Thus, the hippocampus is necessary for the initial association if a trace interval is present but becomes unnecessary if the association has already been formed using stimuli that overlap in time. Here we show that this training procedure does not increase the survival of new cells, even though a trace interval is present between the CS and US. Together, these data suggest that a factor other than temporal discontiguity determines whether or not learning increases neurogenesis. The data to date suggest that the most effective tasks are dependent on the hippocampus (Gould et al., 1999; Leuner et al., 2004), although it remains unclear whether the effect is limited to hippocampus-dependent learning.
A minimum degree of learning and/or cognitive engagement may be necessary to alter the number of new neurons in the dentate gyrus. Animals trained with only 200 trials of trace conditioning do not as a group retain more cells than animals exposed to training with unpaired stimuli, although a significant correlation between learning and the number of cells that survive has been observed (Leuner et al., 2004). In the case of the delay followed by trace paradigm, the lack of an effect on cell survival might arise because animals first trained on delay conditioning are better prepared to acquire the trace task and therefore do so more readily. That is, if learning to associate the two stimuli during delay conditioning subsequently enhances the ability to associate the stimuli across time, the later learning experience might be insufficient to increase cell survival. This would be a likely explanation if learning the delay task reduced the number of trials necessary to reach learning criterion during trace conditioning, but it did not. Instead, when switched to the trace paradigm, animals previously trained on delay showed a decrement in responding and performed similarly to animals that were trace conditioned for the first time. Acquisition of the CR took several hundred trials in these animals, suggesting that learning to express the trace CR remained difficult. Nevertheless, the animals had already learned that the CS and US were associated by the time that they underwent trace conditioning. This is most likely why trace conditioning does not require the hippocampus when it follows delay conditioning (Beylin et al., 2001) and why this procedure was not sufficient to rescue new cells from death.
Another issue to consider relates to timing of the CR. Some have suggested that the hippocampus is involved in accurate timing of the CR, especially during eyeblink conditioning in which the response is timed in milliseconds (Solomon et al., 1986; Moyer et al., 1990). In a previous study, animals with hippocampal lesions could adapt the CR to a new ISI, provided that they had been trained previously with contiguous stimuli during delay conditioning (Beylin et al., 2001). We did not vary the ISI in the present study, which was 750 ms for both delay and trace conditioning. Nonetheless, because the animals first trained on the delay task did show evidence of reacquisition during subsequent trace conditioning, it would appear that learning to time the CR is not a critical feature for enhancing cell survival.
The learning-induced increase in adult neurogenesis is not limited to associative eyeblink conditioning but also occurs with training on spatial navigation tasks requiring the hippocampus (Gould et al., 1999; Hairston et al., 2005; Epp et al., 2006). The effect depends on how well the animals learn (Leuner et al., 2004; Sisti and Shors, 2006) and on the maturity of cells at the time of training (Ambrogini et al., 2000; Epp et al., 2006). However, the biological mechanisms promoting the survival of newly generated neurons after learning are presently unknown. Because enhanced neuronal excitability accompanies both hippocampal-dependent and -independent tasks (Weisz et al., 1984; Moyer et al., 1996), some other factor must be responsible for the maintenance of new cells after hippocampal-dependent learning. For example, manipulations of central cholinergic systems have been shown to affect trace but not delay conditioning (Kaneko and Thompson, 1997) and to modulate neurogenesis (Kaneko et al., 2006), raising the possibility that these processes are related. Another putative mechanism may involve growth hormone (GH) and its downstream effector insulin-like growth factor-I (IGF-I). GH expression is increased in response to trace conditioning (Donahue et al., 2002), and, thus, GH may serve as a neurogenic signal during hippocampal-dependent associative learning. Indeed, GH/IGF-1 has been shown to influence neurogenesis in the dentate gyrus by supporting cell survival (Lichtenwalner et al., 2006).
Decreases in adult neurogenesis have been associated with deficits in trace but not delay conditioning (Shors et al., 2001, 2002). In contrast, depletion of new neurons does not affect spatial navigation learning (Shors et al., 2001; Madsen et al., 2003; Raber et al., 2004; Snyder et al., 2005), although there are effects on retention (Snyder et al., 2005). There are mixed reports about context conditioning, with some studies finding deficits and others not (Shors et al., 2002; Saxe et al., 2006; Winocur et al., 2006). The discrepancies across studies may be explained by differences in factors such as task difficulty, rate of acquisition during training, strength of the memory, and the temporal parameters of the task. Thus, although the present findings have shown that bridging a temporal gap between stimuli is neither necessary nor sufficient for enhancing the survival of new neurons in the hippocampus, they do not exclude the possibility that newly generated cells are somehow involved in learning to associate events across time, especially events that are difficult to predict.
Footnotes
This work was supported by National Institutes of Health Grants MH59970 and MH59740 and National Science Foundation Grant IOB-0444364. We thank M. E. McBreen for her assistance.
References
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler D, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;5:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Donahue CP, Jensen RV, Ochiishi T, Eisenstein I, Zhao M, Shors T, Kosik KS. Transcriptional profiling reveals regulated genes in the hippocampus during memory formation. Hippocampus. 2002;12:821–833. doi: 10.1002/hipo.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new cells in the dentate gyrus during the axon-extension period of cell maturation. Soc Neurosci Abstr. 2006;32:266–8. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus- dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hastings N, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiol Learn Mem. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Thompson RF. Disruption of trace conditioning of the nictitating membrane response in rabbits by central cholinergic blockade. Psychopharmacology. 1997;131:161–166. doi: 10.1007/s002130050279. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83:199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases Ca1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Shore LE, Brewer MD, Cameron HA. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–762. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: Neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Shors TJ. Learning predicts how many new neurons survive. Soc Neurosci Abstr. 2006;32 266.2. [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Groccia-Ellison ME. Classic conditioning in aged rabbits: delay, trace, and long-delay conditioning. Behav Neurosci. 1996;110:427–435. doi: 10.1037//0735-7044.110.3.427. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Weisz D, Clark GA, Thompson RF. Increased responsivity of dentate granule cells during nictitating membrane response conditioning in rabbit. Behav Brain Res. 1984;12:145–154. doi: 10.1016/0166-4328(84)90037-8. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]