Abstract
Pelvic organ prolapse is a vaginal protrusion of female pelvic organs. It has high prevalence worldwide and represents a great burden to the economy. The pathophysiology of pelvic organ prolapse is multifactorial and includes genetic predisposition, aberrant connective tissue, obesity, advancing age, vaginal delivery and other risk factors. Owing to the long course prior to patients becoming symptomatic and ethical questions surrounding human studies, animal models are necessary and useful. These models can mimic different human characteristics – histological, anatomical or hormonal, but none present all of the characteristics at the same time. Major animal models include knockout mice, rats, sheep, rabbits and nonhuman primates. In this article we discuss different animal models and their utility for investigating the natural progression of pelvic organ prolapse pathophysiology and novel treatment approaches.
Keywords: aberrant connective tissue, animal model, female, mesh repair, pelvic organ prolapse, POP, vaginal delivery
Pelvic organ prolapse (POP) consists of descent of the anterior, posterior or apical vaginal compartments. While not life-threatening, POP often results in a significant reduction in quality of life, including shame, embarrassment and sexual dysfunction. POP is also a significant economic burden to millions of women and the healthcare system since it is one of the major indications for benign gynecological surgery, accounting for over 225,000 inpatient procedures and costing more than US$1 billion per year in the USA alone [1].
The pathophysiology of POP is still not well understood, but is known to involve genetic predisposition, aberrant connective tissue (CT) metabolism, pregnancy and hormonal effects, vaginal delivery, and other risk factors such as previous hysterectomy, obesity, advancing age and constipation [1]. POP often develops decades after the greatest risk factor, vaginal delivery, suggesting an additional impact of aging. Given the long time course and the complex and multifaceted nature of this disorder, animal models are a potentially useful way to improve our understanding of POP.
Animal models are particularly appropriate for studying the natural progression of pathologies and investigating novel treatment approaches. However, the development of applicable animal models for POP is challenging since humans, as the only strict bipeds, have particularly difficult childbirth delivery process and a unique pelvic orientation with regard to gravity. Several animal species, including mice, rabbits, nonhuman primates (NHP), sheep, cows, pigs, dogs, domestic cats, deer, bongos, horses, buffalos, donkeys and cheetahs have been documented to spontaneously develop forms of POP [2–17]. Although most of these species are not conducive for use in laboratory research, over the past few decades several of these species have been extensively studied and may serve as valuable animal models. In this review we discuss these models and their utility for investigating pathophysiology and potential new treatment approaches.
Relevant human anatomy
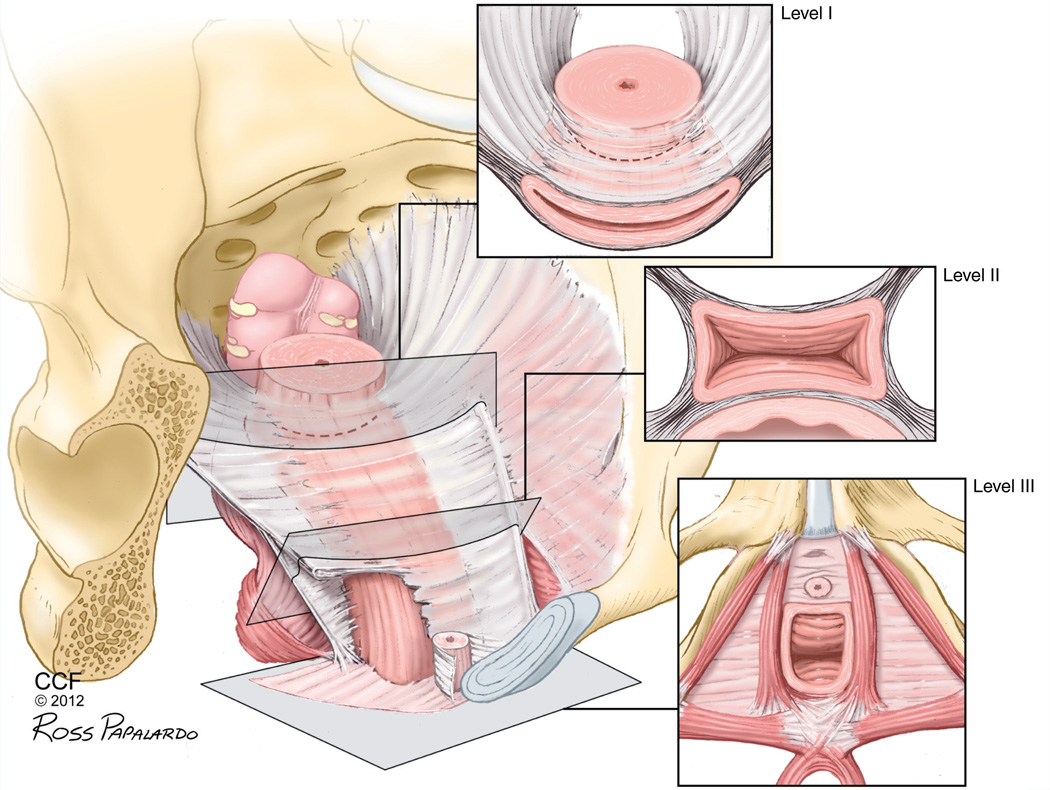
The female pelvis is a dynamic system that must provide sufficient pelvic organ support while allowing physiological motility for storage and passage of urine and feces, copulation, pregnancy and parturition [18]. Critical structural support is provided by connective and muscular tissues within the pelvis. Traditionally, this structural support has been categorized in three distinct levels highlighting support of the upper third of the vagina by the cardinal and uterosacral ligaments (level I), paravaginal attachments of the middle half of the vagina to the arcus tendineus fascia (level II), and the fusion of the lower third of the vagina to the perineal membrane and perineal body (level III; Figure 1). Endopelvic fascia includes these ligaments and a looser areolar tissue that provides resilience and distensibility [19]. Combined, these support structures oppose the forces against the pelvic floor, including gravity, increased intra-abdominal pressure and the maternal trauma of vaginal delivery. Some authors speculate that POP can be caused by an injury to the levator ani during vaginal childbirth [20], although most of the time this injury is undetected. DeLancey et al. found that primiparous women presented abnormalities in the levator ani compared with nulliparous women [21]. Similarly, using a simulated vaginal birth model, Lien et al. showed that the muscles of the levator ani are at significant risk for stretch related injury in childbirth [22].
Figure 1. Vaginal levels of support.
The three levels are anatomically connected and continuous, providing support to the vagina and uterus by a complex interaction of connective tissue, ligaments and muscle.
Reprinted with permission from the Cleveland Clinic Center for Medical Art & Photography © 2012. All Rights Reserved.
POP can be measured and assessed on physical exam using the standardized POP quantification system (POP-Q). In this system, prolapse can be divided in five different stages (stage 0 to stage IV). Stage 0 indicates normal anatomy with no prolapse and stage IV indicates complete eversion of the total length of the vagina [23].
Human female anatomy has evolved to accommodate an upright posture, enabling greater efficiency of walking and the use of both hands while ambulating; however, it has brought great complexity and complications to parturition [24]. The resultant differences between human and animal anatomy are potentially the most significant challenge to any nonhuman model of POP. Often thought of as the ideal animal model for POP, NHP are not strictly bipedal, spend a portion of their time in a quadruped orientation and have a different pelvic anatomy from humans [25,26]. In rodents and other quadrupeds, the musculature of the pelvic floor is situated dorsally to control the tail [27]. This partially shifts the axis of support of the pelvic viscera from the pelvic floor to the abdominal wall. However, some quadrupeds, including sheep, still rely on the pelvic viscera for support due to topography of the land and/or intrinsically elevated abdominal pressures [28]. Animal models, therefore, must be utilized in studies designed to minimize their limitations to reduce bias in the study of POP. In this review, we present several animal models and discuss the similarities and differences to humans, as well as the advantages and limitations of each model.
Animal models of POP
Mice & rats
Rodents are the most widely used animal models for investigation of the development of spontaneous POP in women, as well as to test novel treatments and improve existing therapies. There are several advantages to rodent models of POP, including ease of handling, short lifespan and relatively low cost. Rodents also have predictable and short estrous cycles and lengths of gestation that make POP development less time intensive to study [29]. Large numbers of subjects can be easily acquired and studied, enabling investigations with population-based epidemiological outcomes.
However, there are several anatomical differences between rodents and humans that must be considered when interpreting results from rodent studies. For example, unlike humans, the pelvic floor is oriented horizontally in rodents and they have a much smaller fetal head-to-birth canal ratio than humans. Despite this, genetically manipulated mouse models display a POP phenotype similar to the clinical condition [30,31]. In addition, standard laboratory rodents have been utilized to study POP pathogenesis and evaluate surgical implants for prolapse repair [32–35].
Mice with aberrant elastin metabolism
Lysyl oxidase like-1 (LOXL1)-deficient mice closely resemble the clinical phenotype of POP. LOXL1 is the enzyme responsible for crosslinking tropoelastin, the monomeric form of elastin [36]. Therefore, LOXL1 is critical to elastic fiber homeostasis and repair. LOXL1-deficient mice primarily prolapse after delivery, similar to humans. Lee et al. determined that parity significantly increases the rate of prolapse in LOXL1-deficient mice, and that prolapse significantly decreases leak point pressure, a measure of stress urinary incontinence, a pelvic floor disorder closely associated with POP [30]. Elastin von Gieson staining of the external urethral sphincter in prolapsed mice showed increased elastin clusters, which the authors suggest is associated with abnormal elastin repair, compared with both nonprolapsed LOXL1-knockout (KO) mice and wild-type mice. On MRI, mouse POP was found to include multiple organs, paralleling the heterogeneity of human prolapse [37]. Liu et al. also studied bladder and urethral sphincter function in LOXL1-KO mice compared with wild-type controls and found that prolapsed mice had lower mean bladder capacity and lower voiding pressure compared with controls [38]. Gustilo-Ashby et al. determined that cesarean section delays, but does not fully eliminate prolapse development, similar to what is seen in the human condition [6,39].
To further elucidate the role of elastin metabolism in prolapse development, Drewes et al. characterized mice deficient in fibulin 5 (FBLN5), which colocalizes with both LOXL1 enzyme and elastin [40]. FBLN5-KO mice develop prolapse as early as 12 weeks of age, even if nulliparous. Parous mice prolapse earlier, likely because of a deficiency of the enzymes required for repair after parturition. Isolating the mechanical injury of childbirth, Rahn et al. showed that vaginal distension increased the rate of prolapse, presumably due to an increase in demand of elastin repair enzymes in response to excessive stretch [41,42]. This theory is supported by a separate study demonstrating that the vagina is weaker after POP in these mice [41].
Budatha et al. recently determined that an evolutionarily conserved motif on the FBLN5 gene regulates the expression of matrix metalloproteinases (MMPs), enzymes that degrade elastin and collagen, particularly MMP9 [43]. Mice in which this motif is disrupted have increased MMP9 expression and activity, but normal elastic fibers and do not develop POP. Furthermore, FBLN5-KO mice with reduced MMP9 levels were partially rescued from the POP phenotype, supporting the theory that FBLN5 regulates the CT of the pelvic floor by two separate mechanisms, elastic fiber organization and MMP inhibition [43].
POP in LOXL1- and FBLN5-deficient mice can be quantified by a grading system, the mouse organ prolapse quantification scale (MOP-Q), which was verified and validated on FBLN5 mice and subsequently used on LOXL1-KO mice [6,30,44]. The differences in staging POP in mice and humans highlight the differences in anatomy that limit the models. Mouse prolapse involves an external perineal bulge, whereas human prolapse is a herniation through one of the vaginal compartments (anterior, apex or posterior) and is externalized only in severe cases.
The relevance of mouse models deficient in LOXL1 and FBLN5 are supported by clinical studies that show decreased expression of these enzymes in women with POP [45,46]. However, some studies have failed to find significant decreases in LOXL1 expression in women with POP and have even found increased levels [47]. This discrepancy in the literature may be explained by cause and effect when examining levels of enzymes in a dynamic system, such as the pelvic floor. Although studies attempt to control for hormone levels and other variables it is unknown how abnormal CT, as in prolapse, affects expression of these enzymes. Prospective longitudinal human studies examining tissue biopsies from healthy women without prolapse then re-examining tissue from those who eventually prolapse would be ideal, although extremely difficult in practice and would often have uncontrolled confounding variables in clinical studies. Animal models that predictably prolapse, therefore, have the potential to be used to study cause and effect between POP and molecular events such as enzyme expression, given the relative ease with which animal tissue can be acquired for analysis.
A similar, yet more severe, POP phenotype is observed in mice deficient in fibulin 3 (FBLN3), a related scaffold protein with less clearly understood function. McLaughlin et al. created a targeted KO of FBLN3 that develops with reduced fertility and decreased lifespan, decreased body mass, lordokyphosis, and fat, muscle and organ atrophy [31]. POP in these mice has similar risk factors and biochemical expression as FBLN5-deficient mice [12]. Interestingly, POP and other generalized hernia phenotypes developed in FBLN3-deficient mice on a C57B/6 genetic background, but not on a BALB/c background, suggesting that genetic modifiers on the BALB/c background compensate for the loss of FBLN3. This compensation, which has yet to be investigated in LOXL1-or FBLN5-deficient mice, demonstrates the complexity of elastin homeostasis and repair. The presence of severe defects, in addition to POP, highlights the implications of using organism-wide-KO mice to study pelvic floor disorders. Refinement of each mouse model with aberrant elastin metabolism may lie in creating site specific KOs, although it is unknown whether enzyme dysregulation is site specific in humans with POP [48].
Hoxa11 knockdown mouse model
Working specifically with Level I support, Connell et al. investigated the role of homeobox gene Hoxa11 in the development of the uterosacral ligaments (USL) [49]. They found that mice deficient in Hoxa11 lack a USL and have dysregulated expression of MMPs. Using USL tissue from prolapsed and nonprolapsed women undergoing hysterectomy they found that women with POP have decreased Hoxa11 mRNA expression compared with controls. Hoxa11 also increases proliferation of fibroblasts and human USL cells in vitro and may do so via repression of p53, a known cell-cycle suppressor [50]. Knockdown of Hoxa11 decreases collagen and increases MMP expression in mice mimicking, at least in part, the enzymatic derangements seen in women with POP [51–53].
Hoxa11-deficient mice elucidate a possible pathway that combines molecular derangements and anatomic failure to explain the pathogenesis of POP. This concept is supported by human studies implicating a role for Hoxa11 in women with POP [49]. However, Hoxa11-deficient mice do not spontaneously prolapse. Although it is likely that the unique rodent pelvic anatomy reduces the importance of the USL in preventing prolapse, it may be difficult to design studies to test novel therapeutics to prevent or attenuate POP without phenotypic outcomes that do not require sacrifice and harvest of pelvic tissues.
UPII-SV40T mice
The serendipitous discovery of spontaneous prolapse in a strain of UPII-SV40T mice engineered to develop bladder cancer has become another animal model of POP [54]. These mice reliably develop urothelial cell carcinoma due to expression of SV40T specifically in urothelium, leading to cancer development. POP was first observed in a male mouse and has since been characterized with gross dissection and MRI evaluation in both males and females. POP has been shown to be independent of SV40T or UPII expression using a pedigree analysis in these mice. McNanley et al. therefore suggest that this is a novel mutation leading to prolapse development in this strain [54]. The group plans to screen candidate genes to identify the defect. The two primary limitations to this model are the observation of prolapse in males, who do not develop prolapse in the human condition, and the development of prolapse in nulliparous females. While some women will develop POP even without having delivered children, these cases are far less common than POP associated with vaginal delivery.
Rats to study prolapse pathogenesis
Rat models have also been used to study the biomechanics of the female reproductive tract and the effects of pregnancy, labor and delivery, providing critical insight into the pathogenesis of POP. Distensibility of rat vaginal tissue changes dramatically during pregnancy to accommodate pending parturition [33] and does not return to a prepregnant state up to 4 weeks after delivery [35]. The authors speculate that impaired return to a prepregnant state leaves the vaginal tissue susceptible to further injury, weakening and eventual prolapse. Future studies, however, are necessary to assess recovery beyond 4 weeks. Studies such as these in normal rodent models may enable development of more accurate animal models, as well as a better understanding of human prolapse pathophysiology.
Rabbits
A case series of eight spontaneous vaginal prolapses over a span of 5 years has been documented in a colony of specific pathogen-free IIIVO/JU rabbits [15]. Six of the eight prolapsed female rabbits (does) were parous while two were nulliparous. All does with prolapse were in a period of increased sexual activity, but parous does were at least 2 months post-parturition. Litter size of does that prolapsed were smaller than average, implicating possible generalized abnormalities of the reproductive tract and/or genetic abnormalities. The authors concluded that this was likely a spontaneous mutation given the isolated observation in the colony and the variability in reproductive history between cases [15].
The vast majority of rabbits, however, do not spontaneously prolapse. Furthermore, while it is appealing to study POP in a small mammal model, the anatomy of the rabbit vagina differs significantly from that of humans. The vagina of the rabbit is extensive, consisting of both an internal and external portion. The internal portion is more similar to the small intestine in gross and histological structure, and a large portion of the anterior wall of the external vagina includes the clitoris [27]. Owing to these differences in anatomy, the best use of rabbit models may be to study biocompatibility of surgical implants.
Large animals
Several large animal species develop spontaneous POP. However, the scientific data mostly exists for sheep, cows and pigs, which we review here [5,10,17,28,55,56].
Sheep
The female domestic sheep (ewe) has been well documented to acquire spontaneous POP [10,17,27,28,57]. The ewe is the only domesticated animal that frequently suffers antepartum POP [58]. Although few epidemiological studies have been performed, the prevalence of POP in sheep may be as high as 15% [59]. Similar to humans, sheep of all ages can develop POP but the incidence is highest in mature, multiparous sheep and the risk increases with each successive pregnancy [28]. However, as is the case in humans, parturition is not required for the development of POP. In general, POP in ewes occurs months after delivery, but virgin animals may also develop POP [28,60]. Numerous studies document the pathological findings and potential etiology of prolapse in ewes [28,55,58,61–68]. Additionally, studies have been conducted that use the sheep as a model to study biomechanics of the vagina [69] and as a model for investigating surgical treatments for POP [70–74].
The most commonly used staging system for prolapse in the ewe was proposed by Bosse et al. [10,75]. Although this system is not as detailed as POP-Q, it simulates this classification system as well as the Baden–Walker halfway system [23,76]. Additionally, radiological methods have been developed to track the displacements of the various components of the ewe genital tract, enabling quantification of descent of the cervix throughout progression of POP [77].
The advantages of the ewe as a model of prolapse lie in the similarities between human and sheep reproductive anatomy. The dimensions of the ewe and human vagina are similar in both length and diameter [73,78]. Additionally, the ewe’s pelvic architecture relies on three levels of support, similar to those detailed by DeLancey in women [19,27]. It is possible that the introduction of assisted birthing in domesticated ewes has selected for larger lamb birth weight while maintaining the size of the pelvic inlet. Among other risk factors, prolapse in the ewe has been attributed to a high incidence of dystocia, which is correlated to smaller pelvic inlet area and large lamb size [79].
It has also been suggested that the ruminant abdominal anatomy generates increased pressures [72]. Ewes grazing on hills rest with their head uphill, and studies have shown that flocks that graze hills tend to have higher incidence of prolapse [28,63]. A survey of approximately 120,000 ewes in New Zealand found that the incidence of vaginal prolapse was significantly higher on hilly land than flat land [63]. McLean et al. studied the effect of topographical elevation on the intra-abdominal pressure (IAP) of ewes [63]. They found that sheep lying on a slope of 16 degrees have significantly higher IAP at the vaginal level than sheep lying on a horizontal platform [63,80]. These data suggest that increased IAP predisposes ewes to prolapse, similar to the increased risk in women with obesity [1].
Similar to humans, the bladder is often the organ contained within the prolapse. Scott et al. found that the bladder alone was present in the 41.7% cases of ewe vaginal prolapse, the uterine horn(s) in 16.7%, and both bladder and uterus in 25% [65]. Urethral constriction or kinking was observed in vaginal prolapse involving the bladder, as is often seen in women with POP and occult stress urinary incontinence [65,81]. These results suggest a high similarity between the presentation of POP in humans and ewes [82].
The pathophysiology of POP in sheep also appears to be similar to that of humans. Ennen et al. found that ewes with prolapse had decreased expression of the α-2 chain of collagen I, increased MMP1 and decreased TIMP1 compared with late-term pregnant ewes [55]. Additionally, although they found significantly decreased levels of estrogen receptor-α in prolapsed ewes, the authors were unable to determine the role of estrogens in the development of prolapse [55]. Given that the studies of CT metabolism and hormonal effects on POP in women and rodent models has gained significant traction [6,36,40,42,82–85], the finding that ewes with prolapse display significant alterations in the metabolism of vaginal collagen during the antepartum period has profound implications for the use of sheep as a model of POP.
Phytoestrogens contained in the grazing pastures of some sheep flocks affect the hypothalamic–pituitary–ovarian axis and lead to increased risk of prolapse, among other reproductive abnormalities [28,61,86–88]. Thus, estrogenic diets can produce pronounced effects on the support of the genital tract of ewes [28,61,86,88–91]. Non-hormonal-induced prolapse using specific diets that result in excess fat deposition, impacted rumen contents and greater rumen size may also be useful in developing models of prolapse in sheep [68]. Genetics may also play a significant role in the development of prolapse in the ewe since certain ram pedigrees generate ewes that prolapse at unusually higher rates [28,92].
While the ewe as a model of prolapse has the advantages of similar normal anatomy, anatomical derangement in prolapse and similar risk factors, several logistic disadvantages must be considered when using sheep, particularly the space required to board sheep. The ewe may be best used as an animal model of female pelvic anatomy and support with which to study vaginal repair techniques.
Cows
Like sheep, cows often develop prolapse and the predisposing factors seem to be similar, including inherited factors, estrogenic diets and increases in IAP [10]. Beef breeds are particularly affected, with prevalence as high as 1.1% in Hereford cattle. Unlike sheep, most cases of vaginal prolapse in cows are observed during the last 2 months of gestation. The prolapse often begins with the mucosa of the anterior vagina just cranial to the urethral meatus protruding towards the introitus. As the prolapse progresses, the entire anterior wall and cervix may extend through the introitus. Similar to humans, sheep and other animal models, the disease is progressive and may lead to ulceration, necrosis and infections [10].
Pigs
Prolapse is well documented to occur in pigs at estrus and following diets high in estrogens [10,56]. Interesting, prolapse is common in gilts following diets of moldy corn with up to 40% of animals demonstrating POP [3]. Marked degrees of prolapse are seen in gilts as young as 4 months old and signs of hyperestrogenism are apparent in gilts as young as 1 week old [56]. This model may be particularly useful for studying the effects of diets and hormones on POP development given the short interval between the start of diet and the signs of POP [3].
Few studies have been performed to directly evaluate the use of the pig as a model of POP in humans. However, Gruber et al. studied the anatomy and histology of the pig vagina and pelvic support structures to determine its efficacy as a model of human pelvic floor disorders [93]. They found that the anatomy of the uterosacral and cardinal ligaments had similar origins and insertions as humans. Elastin content is higher in the utersosacral ligament than the cardinal ligament and the pig vagina is thinner and contains less elastin than human vaginas [93]. This latter finding may be due to the fact that the pigs they studied were young and nulliparous, whereas studies of human vaginal tissues are typically of older parous women. The authors concluded that the pig model represents a potentially valuable model for studying pelvic floor disorders [93].
Nonhuman primates
NHP are often thought to be the best animal model to study POP, given the similarity to humans in gross anatomy and histological structure [25,94,95]. The reproductive cycle, process of gestation/parturition, large head relative to the pelvic outlet [96] and hormone effects on the pelvic organs resemble those seen in humans [27]. Vaginal delivery increases the risk of POP in NHP, similar to humans [25]. NHP also more accurately simulate human posture. They are intermittently bipedal, which places downward forces on the pelvic floor, as in humans. Perhaps owing to these similarities, NHP have been known to present with spontaneous POP [4,94,97]. Disadvantages of this model include lengthy pregnancy and time to develop spontaneous POP, as well as cost of maintenance and boarding. Currently studied species of NHP include the rhesus macaque, squirrel monkeys and baboons.
Rhesus macaque
Rhesus macaques have been studied as a model for human POP owing to their similarities in anatomy, and since they have infants with relative large heads, requiring a fivefold vaginal diameter dilation during vaginal delivery [94]. Feola et al. showed that vaginal parity is correlated with loss of collagen alignment and that modification of vaginal biomechanics can lead to POP [25]. Otto et al. studied the effects of estradiol and progesterone on rhesus paravaginal attachment and demonstrated that it is ligamentous and hormone sensitive [94]. They also showed that the rhesus macaque vagina has the same components as human women and respond with thickening to estrogen treatment. These suggest the presence of estrogen receptors in fibroblasts like in humans [94].
Squirrel monkey
Squirrel monkeys have a long gestation, approximately 153 days, and have disproportionately sized neonates for the maternal pelvic outlet, since the newborns are close to 17% as large as their mothers [98]. Labor duration in squirrel monkeys is long, approximately 12 h. Hormonal cycles of squirrel monkeys are different from humans, but they demonstrate similar variations in circulating hormone levels (estradiol and progesterone) throughout the breeding period [99].
Coates et al. observed pelvic relaxation in 50% of a group of 28 squirrel monkeys and, as in humans, they presented loss of support in different areas of the vagina (anterior and posterior compartments and cervix) [99]. In a larger study with 164 squirrel monkeys they showed that, with increasing parity, the percentage of females with normal pelvic support decreases significantly. The age of these animals varied from 3 to 17 years, indicating that this model may be impractical owing to the high variability and long time for phenotype presentation [4].
Pierce et al. showed that female squirrel monkeys have similar intrapelvic skeletal muscle anatomy to humans and that the levator ani nerve originates from the S2 spinal root with no innervation from the pudendal nerve [95]. Similarly, Barber et al. showed that the innervation of the levator ani in humans originates from the S3–S5 level of the spinal cord and the pudendal nerve is not related to levator ani innervation [100]. These authors suggest further that studies on animal models are essential due to the impossibility of performing definitive studies, such as nerve transection in humans [100].
In a later study Pierce et al. also demonstrated that myogenic changes in the levator ani muscle increase with age as in women, and that gross disruption or atrophy of the levator ani does not lead to pelvic prolapse [101]. In contrast, studies in humans suggest that levator ani disruption can lead to POP [20,21]. Despite the similarities in histology and anatomy of CT and muscle between squirrel monkeys and humans, this is a difficult animal model to work with and not many investigations are ongoing.
Baboon
Mattson et al. found that baboons had similar anatomy to women and squirrel monkeys [26]. However, they found no evidence of spontaneous POP in baboons. Unlike humans, baboon neonates are relatively small compared with the maternal pelvic outlet, and the pelvic floor stresses due to erect posture are different. Nonetheless, baboons could be useful to assess surgical procedures, such as meshes and their complications due to their similar anatomy to humans [26].
All the NHP present histological, hormonal and anatomical similarity with humans. However, they are often impractical to work with because of expense, long lifespan and study timelines, and the expertise needed to handle them.
Investigations of surgical implants for POP
Surgical implants, such as vaginal meshes, can correct a defect such as POP, by taking the load during increased IAP. As vaginal meshes for prolapse repair have become more commonly used worldwide and increasingly controversial in the USA, investigators have turned to animal models to study novel synthetic mesh materials. Standard laboratory rats and rabbits subjected to vaginal or abdominal wall defects have been utilized extensively as injury and biocompatibility models to compare synthetic implants [32,102–105]. Pierce et al. compared polypropylene to porcine subdermis meshes in rabbits and found that lightweight polypropylene was less compromised after long duration implantation [32]. Ho et al. used small intestine submucosa seeded with stem cells for vaginal reconstruction compared with small intestine submucosa alone, and found that the stem cells enhanced tissue repair [106]. More recently, Letouzey et al. aimed to prevent infection of implanted synthetics by coating meshes with antibiotics in a rat model and found that this method provided full protection against infection [34]. While the lack of comparable anatomy limits the potential to use rodents in pure efficacy studies of novel synthetic implants, investigation of biocompatibility has proven feasible and useful in these animal models. A potentially novel avenue of investigation involves testing newly available biomaterials in animal models with incomplete or inadequate tissue repair capabilities and/or spontaneous POP development, potentially mimicking the clinical situation of women with failed meshes.
Similar to rodents, sheep have been employed as a model of the female genital tract for studying surgical implants, including materials used in female pelvic reconstructive surgery [70,71,73,74]. De Tayrac et al., found that vaginal mesh extrusion was more common with noncoated polypropylene meshes than with coated meshes in the ewe vagina [71]. However, there was no difference with regard to mesh shrinkage, tissue in-growth, inflammatory response and position of the mesh within the vaginal wall [71]. Krause et al. studied both the rabbit and sheep model to identify a practical animal model for vaginal implantation of surgical mesh [73]. They found that implantation into the sheep genital tract was technically easier given the size of nulliparous and primiparous sheep vaginas. Extrusion rates in sheep and rabbit vaginas were high [73] suggesting that these models may be useful for studying pelvic mesh extrusion in women, a significant complication of POP repair [107].
The sheep model has been utilized to study a new tension-free vaginal tape-like mesh intended for use in the treatment of female stress urinary incontinence [74]. The authors chose sheep in part because they believed it offered the most comparable attributes to human female anatomy regarding both tissue properties and anatomical size [74]. They found that the initial pullout force of the new tension-free vaginal tape-like mesh increased from 1 to 12 weeks suggesting engraftment within the pelvic tissues. They also reported no histological abnormalities and good histological tissue integration. Alcalay et al. utilized sheep to determine the pullout force of polypropylene mesh deployed by the EndoFast Reliant™ system (Endogun Medical Systems, Israel) and compared three different methods of deployment of mesh straps (EndoFast, pocket, or tunneling technique) [70]. The feasibility and successful outcomes of these studies support the use of sheep as a model for studying surgical implants for female pelvic surgery.
Although pigs have been utilized to study several types of meshes these studies have involved abdominal and peritoneal implantation as opposed to implantation in the vagina [108]. While investigations in pigs may not provide specific advantages over those in sheep or other animal models, given the current controversy over pelvic meshes, it is important to take any opportunity to study these surgical implants.
Animal models can be used to test the local reaction to surgical implants, such as meshes. They also can be useful for testing biomechanics and other material properties after implantation in a biological system. However, no animal model replicates the IAPs in humans due to the different postural orientation of different animals; humans are the only ones with a strictly bipedal posture.
Expert commentary
The multifactorial etiology of POP includes genetic predisposition, evolutionarily disadvantaged pelvic structure, vaginal delivery and common comorbidities that further increase risk. Each animal model should be exploited strategically for its unique attributes relating to POP pathogenesis. Despite the fact that there is no ideal animal model recapitulating the human situation with all human characteristics present simultaneously, animal models are still our best option for investigations into which controlling confounding variables are important.
Rodent models provide the most critical insight into the CT components of POP pathophysiology. Accordingly, CT enzyme deficiencies, backed by clinical data, have shed significant light on the molecular pathophysiology that predisposes and contributes to POP. Adding risk factors beyond genetic predisposition, such as vaginal delivery and vaginal distension, improves the utility of these models.
Rodents also form a significant fraction of animal models used to study surgical implants. Rodents, similar to any in vivo mammalian system, are often used to test biocompatibility of the material. However, NHP or sheep may be more anatomically relevant models. Furthermore, surgical implants destined for vaginal placement should be tested in the particular environment of the vagina. This transvaginal approach is easier in larger animals.
Large animals serve as particularly useful models of POP given the anatomical similarities of the pelvic soft tissues and similarities in predisposing risk factors. The pathophysiology and risk factors of POP in sheep and NHP seem to follow a similar multifactorial pathway as in women. Although many of the studies examining POP in sheep were performed in the 1950s and 1960s, more recent studies illustrate the marked similarities in pathophysiology including CT content, MMP-mediated tissue turnover and hormonal changes. This model may serve as an ideal starting point for studying the effects of hormones on prolapse. Perhaps the most exciting data regarding prolapse in sheep is the potential for developing breeds of sheep that predictably prolapse with increasing age and parity. This would enable progress in the field given the limited number of animals that reliably and spontaneously prolapse (Table 1). Despite anatomical and hormonal similarity to humans, NHP have not been used as a model for surgical implants due the high cost of maintenance and long duration of the studies.
Table 1.
Characteristics of animal models of pelvic organ prolapse.
| Animal model |
Lifespan (years) |
Spontaneous POP |
Age spontaneous prolapse appears |
Increased POP with parity |
Difficult labor |
Duration of hormonal cycle (days) |
Feasibility for pelvic repair surgery |
Similar pelvic anatomy to women |
Genetically modified |
Cost |
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | ||||||||||
| LOXL1 KO | 1–2 | Yes | ~12 weeks+ | Yes | No | 4–5 | Difficult | Poor | Yes | $ |
| FBLN5 KO | 1–2 | Yes | ~12 weeks+ | Yes | No | 4–5 | Difficult | Poor | Yes | $ |
| FBLN3 KO | 1–2 | Yes | ~12 weeks+ | No | No | 4–5 | Difficult | Poor | Yes | $ |
| Hoxa11 KO | 1–2 | No | NA | NA | INA | 4–5 | Difficult | Poor | Yes | $ |
| UPII/SV40T | 1–2 | Yes | ~16 weeks+ | INA | INA | 4–5 | Difficult | Poor | Yes | $ |
| Rats | ||||||||||
| 2.5–4 | No | NA | No | No | 4–5 | Intermediate | Poor | No | $ | |
| Rabbits | ||||||||||
| 5–6 | No† | NA | No | No | NA | Intermediate | Poor | No | $$ | |
| Sheep | ||||||||||
| 6–11 | Yes | 2 years+ | Yes | Yes | 13–19 | Good | Good | No | $$$ | |
| Cows | ||||||||||
| 20–25 | Yes | ~3 years+ | INA | Yes | 18–24 | Good, but logistically difficult | Good | No | $$$ | |
| Pigs | ||||||||||
| ~15 | Yes | 4 months+ | INA | Yes | 18–22 | Good | Fair | No | $$$ | |
| NHP | ||||||||||
| Rhesus macaque | ~25 | Yes | ~13 years‡ | INA | Yes | 26–29 | Good | Excellent | No | $$$$$ |
| Squirrel monkey | ~20 | Yes | 3 years+ | Yes | Yes | 8–12 | Good | Excellent | No | $$$$$ |
| Baboon | 30–40 | No | NA | No | No | 30–40 | Good | Excellent | No | $$$$$ |
Although there have been reports of spontaneous prolapse in rabbits, this has only been observed in one colony [15].
Rhesus monkey age of spontaneous prolapse obtained from Clark et al. [111].
$–$$$$$: Relative cost of working with each animal model; INA: Information not available; KO: Knockout; NA: Not applicable; NHP: Nonhuman primates; POP: Pelvic organ prolapse.
In light of the recent US FDA warning and the controversies surrounding the use of synthetic mesh for vaginal prolapse repair and its complications, NHP and sheep may also be invaluable for studying extrusion/intrusion, one of the most relevant complications of surgery with mesh. The fact that sheep develop mesh extrusion at unusually high rates makes them an excellent model for investigating vaginal surgical mesh.
Five-year view
In their current state, animal models have focused on characterization and elucidation of the anatomy, biomechanics, histology and molecular pathophysiology of POP. However, animal models should also provide a basis to develop novel therapeutics and improve upon existing treatments. The most efficacious current treatments are surgical, with or without pelvic meshes. Treatments on the horizon include cell-based therapies and tissue regeneration techniques [106,109,110]. These therapies have great potential but will take time and ample funding to investigate thoroughly.
As they are currently used, animal models to study surgical meshes largely focus on biocompatibility. Future work in this area should focus on biocompatibility and efficacy of meshes in inherently diseased animals. Given that women with prolapse probably have abnormal CT homeostasis, surgical meshes should be tested in animal models with similarly abnormal CT homeostasis. Furthermore, novel materials for vaginal implantation should continually be sought out. The standard polypropylene mesh, despite modification and improvement, still presents a high rate of rejection and complications [107]. Given the environment of the vagina, biomedical engineers and researchers are challenged with the task of developing appropriate materials to be tested in animal models.
Most treatments for POP, however, are instituted once gross anatomical deformity and symptoms have occurred. Ideal treatment should involve early intervention and prophylaxis in appropriately screened populations. Animal models can also be used to test novel techniques that can target prevention or early detection, such as cell-based therapies. They could potentially be combined with mesh to reduce complications and improve efficacy. These techniques have great potential but take many years and research funding to be translated to clinical practice.
Key issues.
The prolonged natural history, difficulty in obtaining tissue samples and multifactorial etiology of pelvic organ prolapse (POP) in humans necessitates the development of appropriate and accurate animal models.
Animal models can mimic different human characteristics including histological, anatomical or hormonal characteristics, but none present all characteristics at the same time.
Mice deficient in critical enzymes involved in elastin homeostasis develop spontaneous POP that simulates the human condition.
Developmental abnormalities in level I support may contribute to POP development as elucidated by a unique rodent model and supported by human biopsy studies.
Standard laboratory rats have been used to elucidate the pathophysiology of POP and improve current surgical implants.
Large animal models, particularly sheep, may serve as excellent models for studying the influence of hormones and biomechanics on the pathophysiology of POP.
Sheep can also serve as models for investigations into the mechanisms of pelvic mesh extrusion.
Nonhuman primates are similar to humans in anatomy, histology, hormones and pregnancy/parturition, making them excellent models for surgical implants and surgical complications. However, they are impractical for many investigators.
Animal models of POP are in their infancy, but, as in other fields, we expect them to provide useful clinically relevant pathophysiological information as the field matures.
Future studies should utilize these animal models to discover new mechanisms in POP pathophysiology, novel treatments, improve upon existing surgical implants and translate novel therapeutics to clinical practice.
Acknowledgements
The authors would like to thank Ross Papalardo (Staff Illustrator, Cleveland Clinic) for the artwork.
This work was supported in part by NIH R01 HD059859 and the Rehabilitation Research and Development Service of the Department of Veterans Affairs
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1. Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. •• Thorough review addressing updated and important issues on prevalence, pathophysiology and treatment of human pelvic organ prolapse.
- 2.Alan M, Cetin Y, Sendag S, Eski F. True vaginal prolapse in a bitch. Anim. Reprod. Sci. 2007;100(3–4):411–414. doi: 10.1016/j.anireprosci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Bristol FM, Djurickovic S. Hyperestrogenism in female swine as the result of feeding mouldy corn. Can. Vet. J. 1971;12(6):132–135. [PMC free article] [PubMed] [Google Scholar]
- 4.Coates KW, Gibson S, Williams LE, et al. The squirrel monkey as an animal model of pelvic relaxation: an evaluation of a large breeding colony. Am. J. Obstet. Gynecol. 1995;173(6):1664–1669. doi: 10.1016/0002-9378(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon KS, Singh BB, Kumar H, Bal MS, Singh J. Treatment of vaginal prolapse in cows and buffaloes. Vet. Rec. 2006;158(9):312. doi: 10.1136/vr.158.9.312-b. [DOI] [PubMed] [Google Scholar]
- 6.Gustilo-Ashby AM, Lee U, Vurbic D, et al. The impact of cesarean delivery on pelvic floor dysfunction in lysyl oxidase like-1 knockout mice. Fem. Pelv. Med. Recon. Surg. 2010;16(1):21–30. doi: 10.1097/SPV.0b013e3181d00035. [DOI] [PubMed] [Google Scholar]
- 7.Gyimesi ZS, Linhart RD, Burns RB, Anderson DE, Munson L. Management of chronic vaginal prolapse in an eastern bongo (Tragelaphus eurycerus isaaci) J. Zoo. Wildl. Med. 2008;39(4):614–621. doi: 10.1638/2007-0012.1. [DOI] [PubMed] [Google Scholar]
- 8.Mason BJ. Uterine prolapse in a donkey. Vet. Rec. 1970;86(22):675. doi: 10.1136/vr.86.22.675. [DOI] [PubMed] [Google Scholar]
- 9.Miesner MD, Anderson DE. Management of uterine and vaginal prolapse in the bovine. Vet. Clin. North Am. Food Anim. Pract. 2008;24(2):409–419. doi: 10.1016/j.cvfa.2008.02.008. ix. [DOI] [PubMed] [Google Scholar]
- 10.Noakes D, Parkinson TJ, England G. Arthur’s Veterinary Reproduction and Obstetrics (8th Edition) PA, USA: WB Saunders; 2001. pp. 145–153. [Google Scholar]
- 11.Nothling JO, Knesl O, Irons P, Lane E. Uterine prolapse with an interesting vascular anomaly in a cheetah: a case report. Theriogenology. 2002;58(9):1705–1712. doi: 10.1016/s0093-691x(02)01081-6. [DOI] [PubMed] [Google Scholar]
- 12.Rahn DD, Acevedo JF, Roshanravan S, et al. Failure of pelvic organ support in mice deficient in fibulin-3. Am. J. Pathol. 2009;174(1):206–215. doi: 10.2353/ajpath.2009.080212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riera FL, Hinrichs K, Hunt PR, Kenney RM. Cervical hyperplasia with prolapse in a mare. J. Am. Vet. Med. Assoc. 1989;195(10):1393–1394. [PubMed] [Google Scholar]
- 14.Twomey DF, Boon JD, Sayers G, Schock A. Arcanobacterium pyogenes septicemia in a southern pudu (Pudu puda) following uterine prolapse. J. Zoo. Wildl. Med. 2010;41(1):158–160. doi: 10.1638/2009-0008.1. [DOI] [PubMed] [Google Scholar]
- 15.Van HH, Hesp AP, Versluis A, Zwart P, Van Zutphen LF. Prolapsus vaginae in the IIIVO/JU rabbit. Lab. Anim. 1989;23(4):333–336. doi: 10.1258/002367789780746097. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan L, McGuckin S. Uterine prolapse in a cat. Vet. Rec. 1993;132(22):568. doi: 10.1136/vr.132.22.568. [DOI] [PubMed] [Google Scholar]
- 17.Zacharin RF. Genital prolapse in ruminants. Aust. N. Z. J. Obstet. Gynaecol. 1969;9(4):236–239. doi: 10.1111/j.1479-828x.1969.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 18.Stepp KJ, Walters MD. In: Anatomy of the Lower Urinary Tract, Rectum, and Pelvic Floor. Walters MD, Karram MM, editors. MO, USA: Mosby Elsevier; 2007. p. 26. [Google Scholar]
- 19.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 1992;166(6 Pt 1):1717–1724. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 20.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Munoz A. Pelvic floor disorders 5–10 years after caginal or cesarean childbirth. Obstet. Gynecol. 2011;118(4):777–784. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet. Gynecol. 2003;101(1):46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet. Gynecol. 2004;103(1):31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am. J. Obstet. Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 24.Wittman AB, Wall LL. The evolutionary origins of obstructed labor: bipedalism, encephalization, and the human obstetric dilemma. Obstet. Gynecol. Surv. 2007;62(11):739–748. doi: 10.1097/01.ogx.0000286584.04310.5c. [DOI] [PubMed] [Google Scholar]
- 25.Feola A, Abramowitch S, Jones K, Stein S, Moalli P. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am. J. Obstet. Gynecol. 2010;203(6):595–598. doi: 10.1016/j.ajog.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson JA, Kuehl TJ, Yandell PM, Pierce LM, Coates KW. Evaluation of the aged female baboon as a model of pelvic organ prolapse and pelvic reconstructive surgery. Am. J. Obstet. Gynecol. 2005;192(5):1395–1398. doi: 10.1016/j.ajog.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 27. Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144(Suppl. 1):S146–S158. doi: 10.1016/j.ejogrb.2009.02.022. • Compares the biomechanics and feasibility of different animal models for pelvic organ prolapse.
- 28. McLean JW. Vaginal prolapse in sheep. NZ Vet. J. 1956;4(2):38–55. •• Landmark paper regarding pelvic organ prolapse in sheep, discussing symptoms, presentation, epidemiology, etiology and biomechanics.
- 29.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol. Reprod. 1982;27(2):327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 30.Lee UJ, Gustilo-Ashby AM, Daneshgari F, et al. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am. J. Physiol. Renal Physiol. 2008;295(2):F545–F555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin PJ, Bakall B, Choi J, et al. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 2007;16(24):3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- 32.Pierce LM, Grunlan MA, Hou Y, Baumann SS, Kuehl TJ, Muir TW. Biomechanical properties of synthetic and biologic graft materials following long-term implantation in the rabbit abdomen and vagina. Am. J. Obstet. Gynecol. 2009;200(5):549–548. doi: 10.1016/j.ajog.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet. Gynecol. 2007;109(1):136–143. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 34.Letouzey V, Lavigne JP, Garric X, Coudane J, de Tayrac R, Callaghan DO. Is degradable antibiotic coating for synthetic meshes provide protection against experimental animal infection after fascia repair? J. Biomed. Mater. Res. B. Appl. Biomater. 2011 doi: 10.1002/jbm.b.31973. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 35.Alperin M, Feola A, Duerr R, Moalli P, Abramowitch S. Pregnancy- and delivery-induced biomechanical changes in rat vagina persist postpartum. Int. Urogynecol. J. 2010;21(9):1169–1174. doi: 10.1007/s00192-010-1149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. •• First identified LOXL1-deficient mice as a model of post-partum pelvic organ prolapse and characterizes the molecular role of LOXL1 in elastin repair.
- 37.Kenton K, Shott S, Brubaker L. Vaginal topography does not correlate well with visceral position in women with pelvic organ prolapse. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1997;8(6):336–339. doi: 10.1007/BF02765592. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Daneshgari F, Li M, et al. Bladder and urethral function in pelvic organ prolapsed lysyl oxidase like-1 knockout mice. BJU Int. 2007;100(2):414–418. doi: 10.1111/j.1464-410X.2007.06929.x. [DOI] [PubMed] [Google Scholar]
- 39.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet. Gynecol. 2006;107(6):1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 40.Drewes PG, Yanagisawa H, Starcher B, et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am. J. Pathol. 2007;170(2):578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am. J. Obstet. Gynecol. 2008;198(5):590–596. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahn DD, Acevedo JF, Word RA. Effect of vaginal distention on elastic fiber synthesis and matrix degradation in the vaginal wall: potential role in the pathogenesis of pelvic organ prolapse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(4):R1351–R1358. doi: 10.1152/ajpregu.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Budatha M, Roshanravan S, Zheng Q, et al. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J. Clin. Invest. 2011;121(5):2048–2059. doi: 10.1172/JCI45636. •• Demonstrates a dual role for FBLN5 in the pathogenesis of pelvic organ prolapse and identifies similar molecular changes in women with the disorder.
- 44.Wieslander CK, Rahn DD, McIntire DD, et al. Quantification of pelvic organ prolapse in mice: vaginal protease activity precedes increased MOPQ scores in fibulin 5 knockout mice. Biol. Reprod. 2009;80(3):407–414. doi: 10.1095/biolreprod.108.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klutke J, Ji Q, Campeau J, et al. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet. Gynecol. Scand. 2008;87(1):111–115. doi: 10.1080/00016340701819247. [DOI] [PubMed] [Google Scholar]
- 46.Takacs P, Nassiri M, Viciana A, Candiotti K, Fornoni A, Medina CA. Fibulin-5 expression is decreased in women with anterior vaginal wall prolapse. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009;20(2):207–211. doi: 10.1007/s00192-008-0757-x. [DOI] [PubMed] [Google Scholar]
- 47.Jung HJ, Jeon MJ, Yim GW, Kim SK, Choi JR, Bai SW. Changes in expression of fibulin-5 and lysyl oxidase-like 1 associated with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;145(1):117–122. doi: 10.1016/j.ejogrb.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Man WC, Ho JY, Wen Y, Sokol ER, Polan ML, Chen B. Is lysyl oxidase-like protein-1, alpha-1 antitrypsin, and neutrophil elastase site specific in pelvic organ prolapse? Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009;20(12):1423–1429. doi: 10.1007/s00192-009-0905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Connell KA, Guess MK, Chen H, Andikyan V, Bercik R, Taylor HS. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J. Clin. Invest. 2008;118(3):1050–1055. doi: 10.1172/JCI34193. • Elucidates a possible pathway that combines molecular derangements and anatomic failure to explain the pathogenesis of pelvic organ prolapse.
- 50.Connell KA, Guess MK, Chen HW, Lynch T, Bercik R, Taylor HS. HOXA11 promotes fibroblast proliferation and regulates p53 in uterosacral ligaments. Reprod. Sci. 2009;16(7):694–700. doi: 10.1177/1933719109334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabriel B, Watermann D, Hancke K, et al. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006;17(5):478–482. doi: 10.1007/s00192-005-0045-y. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y, Guess M, Datar A, et al. Knockdown of Hoxa11 in vivo in the uterosacral ligament and uterus of mice results in altered collagen and matrix metalloproteinase activity. Biol. Reprod. 2011 doi: 10.1095/biolreprod.111.093245. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 53.Strinic T, Vulic M, Tomic S, Capkun V, Stipic I, Alujevic I. Increased expression of matrix metalloproteinase-1 in uterosacral ligament tissue from women with pelvic organ prolapse. Acta Obstet. Gynecol. Scand. 2010;89(6):832–834. doi: 10.3109/00016341003592545. [DOI] [PubMed] [Google Scholar]
- 54.McNanley AR, Johnson AM, Flynn MK, Wood RW, Kennedy SD, Reeder JE. Inherited pelvic organ prolapse in the mouse: preliminary evaluation of a new murine model. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009;20(1):19–25. doi: 10.1007/s00192-008-0723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ennen S, Kloss S, Scheiner-Bobis G, Failing K, Wehrend A. Histological, hormonal and biomolecular analysis of the pathogenesis of ovine prolapsus vaginae ante partum. Theriogenology. 2011;75(2):212–219. doi: 10.1016/j.theriogenology.2010.08.007. •• Landmark paper comparing pathophysiology of pelvic organ prolapse in sheep to humans. This was the first paper investigating the relation between MMPs and pelvic organ prolapse in sheep.
- 56. Blaney BJ, Bloomfield RC, Moore CJ. Zearalenone intoxication of pigs. Aust. Vet. J. 1984;61(1):24–27. doi: 10.1111/j.1751-0813.1984.tb07126.x. • These authors discovered a potentially valuable animal model that prolapses at a young age following exposure to the potent estrogenic metabolite, zearalenone.
- 57.Scott P, Gessert M. Management of ovine vaginal prolapse. In Practice. 1998;20(1):28–34. [Google Scholar]
- 58.Sobiraj A. Ante partum vaginal prolapse in sheep – an unsolved problem. Tierarztl. Prax. 1990;18(1):9–12. [PubMed] [Google Scholar]
- 59.Low JC, Sutherland HK. A census of the prevalence of vaginal prolapse in sheep flocks in the Borders region of Scotland. Vet. Rec. 1987;120(24):571–575. doi: 10.1136/vr.120.24.571. [DOI] [PubMed] [Google Scholar]
- 60.Bennetts HW. Metaplasia in the sex organs of castrated male sheep maintained on early subterranean clover pastures. Aust. Vet. J. 1946;22(3):70–78. doi: 10.1111/j.1751-0813.1946.tb06451.x. [DOI] [PubMed] [Google Scholar]
- 61.Adams NR. Pathological changes in the tissue of infertile ewes with clover disease. J. Comp. Pathol. 1976;86(1):29–35. doi: 10.1016/0021-9975(76)90024-4. [DOI] [PubMed] [Google Scholar]
- 62.Hosie BD, Low JC, Bradley HK, Robb J. Nutritional factors associated with vaginal prolapse in ewes. Vet. Rec. 1991;128(9):204–208. doi: 10.1136/vr.128.9.204. [DOI] [PubMed] [Google Scholar]
- 63.McLean JW. Vaginal prolapse in ewes part III: the effect of topography on incidence. N. Z. Vet. J. 1957;5(3):93–97. [Google Scholar]
- 64.McLean JW, Claxton JH. Vaginal prolapse in sheep Part IV: cyclic changes in the vulva, vestibule, and vagina during the year. N. Z. Vet. J. 1958;6(5):133–137. [Google Scholar]
- 65.Scott PR, Gessert ME. Ultrasonographic examination of 12 ovine vaginal prolapses. Vet. J. 1998;155(3):323–324. doi: 10.1016/s1090-0233(05)80031-0. [DOI] [PubMed] [Google Scholar]
- 66.Sobiraj A, Busse G, Gips H, Bostedt H. Investigations into the blood plasma profiles of electrolytes, 17 beta-oestradiol and progesterone in sheep suffering from vaginal inversion and prolapse ante partum. Br. Vet. J. 1986;142(3):218–223. doi: 10.1016/0007-1935(86)90063-1. [DOI] [PubMed] [Google Scholar]
- 67.Stubbings DP. Observations on serum calcium levels in ewes in North Lincolnshire in relation to prolapse of the vagina and incomplete cervical dilatation. Vet. Rec. 1971;89(11):296–300. doi: 10.1136/vr.89.11.296. [DOI] [PubMed] [Google Scholar]
- 68.Vipond JE, Lewis M, Horgan G, Noble RC. Malt distillers grains as a component of diets for ewes and lambs and its effects on carcass tissue lipid composition. An. Feed Sci. Technol. 1995;54(1):65–79. [Google Scholar]
- 69.Rubod C, Boukerrou M, Brieu M, Dubois P, Cosson M. Biomechanical properties of vaginal tissue. Part 1: New experimental protocol. J. Urol. 2007;178(1):320–325. doi: 10.1016/j.juro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 70.Alcalay M, Livne M, Shidlovsky D, Hod E. Pullout force of polypropylene mesh deployed by EndoFast Reliant fastener – a comparative study in a sheep model. Presented at: International Continence Society; 1 October 2009; San Francisco, CA, USA. [Google Scholar]
- 71.De Tayrac R, Alves A, Therin M. Collagen-coated vs noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007;18(5):513–520. doi: 10.1007/s00192-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 72.Deprest J, Zheng F, Konstantinovic M, et al. The biology behind fascial defects and the use of implants in pelvic organ prolapse repair. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006;17(Suppl 1):S16–S25. doi: 10.1007/s00192-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 73.Krause H, Goh J. Sheep and rabbit genital tracts and abdominal wall as an implantation model for the study of surgical mesh. J. Obstet. Gynaecol. Res. 2009;35(2):219–224. doi: 10.1111/j.1447-0756.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 74.Rezapour M, Novara G, Meier PA, Holste J, Landgrebe S, Artibani W. A 3-month preclinical trial to assess the performance of a new TVT-like mesh (TVTx) in a sheep model. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007;18(2):183–187. doi: 10.1007/s00192-006-0130-x. [DOI] [PubMed] [Google Scholar]
- 75.Bosse P, Grimand B, Mialot J. Vaginal prolapse in ewe. Rec. Med. Vet. Ec. Alfort. 1989;165:355. [Google Scholar]
- 76.Baden WF, Walker TA. Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin. Obstet. Gynecol. 1972;15(4):1048–1054. doi: 10.1097/00003081-197212000-00020. [DOI] [PubMed] [Google Scholar]
- 77.Ayen E, Noakes DE. Displacement of the tubular genital tract of the ewe during pregnancy. Vet. Rec. 1997;141(20):509–512. doi: 10.1136/vr.141.20.509. [DOI] [PubMed] [Google Scholar]
- 78.Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum. Reprod. 2006;21(6):1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 79.McSporran KD, Fielden ED. Studies on dystocia in sheep. II: Pelvic measurements of ewes with histories of dystocia and eutocia. N. Z. Vet. J. 1979;27(4):75–78. doi: 10.1080/00480169.1979.34603. [DOI] [PubMed] [Google Scholar]
- 80.McLean JW, Claxton JH. Vaginal prolapse in ewes: part VII: the measurement and effect of intra-abdominal pressure. N. Z. Vet. J. 1960;8(3):51–61. [Google Scholar]
- 81.Nygaard I, Kreder K, Mueller E, et al. Does urethral competence affect urodynamic voiding parameters in women with prolapse? Neurourol. Urodyn. 2007;26(7):1030–1035. doi: 10.1002/nau.20436. [DOI] [PubMed] [Google Scholar]
- 82.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am. J. Obstet. Gynecol. 2002;186(6):1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 83.Bai S, Chung D, Yoon J, Shin J, Kim S, Park K. Roles of estrogen receptor, progesterone receptor, p53 and p21 in pathogenesis of pelvic organ prolapse. Int. Urogynecol. J. 2005;16(6):492–496. doi: 10.1007/s00192-005-1310-9. [DOI] [PubMed] [Google Scholar]
- 84.Chen B, Wen Y, Yu X, Polan ML. Elastin metabolism in pelvic tissues: Is it modulated by reproductive hormones? Am. J. Obstet. Gynecol. 2005;192(5):1605–1613. doi: 10.1016/j.ajog.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 85.Chung DJ, Bai SW. Roles of sex steroid receptors and cell cycle regulation in pathogenesis of pelvic organ prolapse. Curr. Opin. Obstet. Gynecol. 2006;18(5):551–554. doi: 10.1097/01.gco.0000242959.63362.1e. [DOI] [PubMed] [Google Scholar]
- 86.Adams NR, Hearnshaw H, Oldham CM. Abnormal function of the corpus luteum in some ewes with phyto-oestrogenic infertility. Aust. J. Biol. Sci. 1981;34(1):61–65. [PubMed] [Google Scholar]
- 87.Adams NR. Morphogenic change in the cervix of the ewe after prolonged exposure to oestradiol-17 beta. J. Reprod. Fertil. 1986;76(2):727–733. doi: 10.1530/jrf.0.0760727. [DOI] [PubMed] [Google Scholar]
- 88.Adams NR. Morphological changes in the organs of ewes grazing oestrogenic subterranean clover. Res. Vet. Sci. 1977;22(2):216–221. [PubMed] [Google Scholar]
- 89.Obst JM, Seamark RF. Hormone studies on ewes grazing an oestrogenic (Yarloop clover) pasture during the reproductive cycle. Aust. J. Biol. Sci. 1975;28(3):279–290. doi: 10.1071/bi9750279. [DOI] [PubMed] [Google Scholar]
- 90.Shackell G, Wylie J, Kelly R. Effects of prolonged exposure of ewes to oestrogenic pasture 2. Occurence of abnormalities of the external genitalia and altered mating performance. NZJ. Agric. Res. 1993;36:459–464. [Google Scholar]
- 91.Underwood EJ, Shier FL. The permanence of the oestrogenic effects of subterranean clover grazing on the ewe. Aust. Vet. J. 1951;27(3):63–67. doi: 10.1111/j.1751-0813.1951.tb05008.x. [DOI] [PubMed] [Google Scholar]
- 92.Shepherd PR. Vaginal prolapse in ewes. Vet. Rec. 1992;130(25):564. doi: 10.1136/vr.130.25.564. [DOI] [PubMed] [Google Scholar]
- 93.Gruber DD, Warner WB, Lombardini ED, Zahn CM, Buller JL. Anatomical and histological examination of the porcine vagina and supportive structures: in search of an ideal model for pelvic floor disorder evaluation and management. Fem. Pelv. Med. Recon. Surg. 2011;17(3):110–114. doi: 10.1097/SPV.0b013e318214b1a6. [DOI] [PubMed] [Google Scholar]
- 94. Otto LN, Slayden OD, Clark AL, Brenner RM. The rhesus macaque as an animal model for pelvic organ prolapse. Am. J. Obstet. Gynecol. 2002;186(3):416–421. doi: 10.1067/mob.2002.121723. • Characterizes the rhesus macaque as a possible animal model for pelvic organ prolapse.
- 95.Pierce LM, Reyes M, Thor KB, et al. Innervation of the levator ani muscles in the female squirrel monkey. Am. J. Obstet. Gynecol. 2003;188(5):1141–1147. doi: 10.1067/mob.2003.329. [DOI] [PubMed] [Google Scholar]
- 96.Rosenberg K, Trevathan W. Birth, obstetrics and human evolution. Br. J. Obstet. Gynecol. 2002;109(11):1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 97.Adams RJ, Rock JA, Swindle MM, Garnett NL, Porter WP. Surgical correction of genital prolapse in three rhesus monkeys. Lab. Anim. Sci. 1985;35(4):405–408. [PubMed] [Google Scholar]
- 98.Hartwig WC. Perinatal life history traits in new world monkeys. Am. J. Primat. 1996;40:99–130. [Google Scholar]
- 99.Coates KW, Galan HL, Shull BL, Kuehl TJ. The squirrel monkey: an animal model of pelvic relaxation. Am. J. Obstet. Gynecol. 1995;172(2 Pt 1):588–593. doi: 10.1016/0002-9378(95)90577-4. [DOI] [PubMed] [Google Scholar]
- 100.Barber MD, Bremer RE, Thor KB, Dolber PC, Kuehl TJ, Coates KW. Innervation of the female levator ani muscles. Am. J. Obstet. Gynecol. 2002;187(1):64–71. doi: 10.1067/mob.2002.124844. [DOI] [PubMed] [Google Scholar]
- 101.Pierce LM, Baumann S, Rankin MR, et al. Levator ani muscle and connective tissue changes associated with pelvic organ prolapse, parity, and aging in the squirrel monkey: a histologic study. Am. J. Obstet. Gynecol. 2007;197(1):60–69. doi: 10.1016/j.ajog.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 102.Konstantinovic ML, Pille E, Malinowska M, Verbeken E, De Ridder D, Deprest J. Tensile strength and host response towards different polypropylene implant materials used for augmentation of fascial repair in a rat model. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007;18(6):619–626. doi: 10.1007/s00192-006-0202-y. [DOI] [PubMed] [Google Scholar]
- 103.Krause HG, Galloway SJ, Khoo SK, Lourie R, Goh JT. Biocompatible properties of surgical mesh using an animal model. Aust. N. Z. J. Obstet. Gynaecol. 2006;46(1):42–45. doi: 10.1111/j.1479-828X.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 104.Sergent F, Desilles N, Lacoume Y, Bunel C, Marie JP, Marpeau L. Mechanical evaluation of synthetic biomaterials used in the correction of pelvic floor disorders – experimental study in rabbits. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;147(1):106–110. doi: 10.1016/j.ejogrb.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 105.Zhang K, Han J, Yao Y, Yang J, Qiao J. Local reaction to the different meshes at the vesicovaginal space in rabbit model. Int. Urogynecol. J. 2012 doi: 10.1007/s00192-011-1612-z. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 106.Ho MH, Heydarkhan S, Vernet D, et al. Stimulating vaginal repair in rats through skeletal muscle-derived stem cells seeded on small intestinal submucosal scaffolds. Obstet. Gynecol. 2009;114(2 Pt 1):300–309. doi: 10.1097/AOG.0b013e3181af6abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen JN, Jakus-Waldman SM, Walter AJ, White T, Menefee SA. Perioperative complications and reoperations after incontinence and prolapse surgeries using prosthetic implants. Obstet. Gynecol. 2012;119(3):539–546. doi: 10.1097/AOG.0b013e3182479283. [DOI] [PubMed] [Google Scholar]
- 108.Scheidbach H, Tamme C, Tannapfel A, Lippert H, Kockerling F. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg. Endosc. 2004;18(2):211–220. doi: 10.1007/s00464-003-8113-1. [DOI] [PubMed] [Google Scholar]
- 109.De Filippo RE, Bishop CE, Filho LF, Yoo JJ, Atala A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation. 2008;86(2):208–214. doi: 10.1097/TP.0b013e31817f1686. [DOI] [PubMed] [Google Scholar]
- 110.Couri BM, Lenis AT, Kinley B, Balog BM, Kuang M, Damaser MS. Post-partum upregulation of stem cell homing cytokines in lysyl oxidase like-1 knock out model of pelvic organ prolapse. Neurourol. Urodyn. 2012;31:231. [Google Scholar]
- 111.Clark AL, Gregory WT, Pearson JM, Lou JS, Nixon R, Slayden OD. Poster 2: spontaneous Grade III pelvic organ prolapse (POP) in the rhesus macaque. Fem. Pelvic Med. Reconstr. Surg. 2005;11(Suppl. 1):S26. [Google Scholar]