Abstract
We tested the hypothesis that Crohn’s disease (CD)-related genetic polymorphisms involved in host innate immunity are associated with shifts in human ileum–associated microbial composition in a cross-sectional analysis of human ileal samples. Sanger sequencing of the bacterial 16S ribosomal RNA (rRNA) gene and 454 sequencing of 16S rRNA gene hypervariable regions (V1–V3 and V3–V5), were conducted on macroscopically disease-unaffected ileal biopsies collected from 52 ileal CD, 58 ulcerative colitis and 60 control patients without inflammatory bowel diseases (IBD) undergoing initial surgical resection. These subjects also were genotyped for the three major NOD2 risk alleles (Leu1007fs, R708W, G908R) and the ATG16L1 risk allele (T300A). The samples were linked to clinical metadata, including body mass index, smoking status and Clostridia difficile infection. The sequences were classified into seven phyla/subphyla categories using the Naïve Bayesian Classifier of the Ribosome Database Project. Centered log ratio transformation of six predominant categories was included as the dependent variable in the permutation based MANCOVA for the overall composition with stepwise variable selection. Polymerase chain reaction (PCR) assays were conducted to measure the relative frequencies of the Clostridium coccoides – Eubacterium rectales group and the Faecalibacterium prausnitzii spp. Empiric logit transformations of the relative frequencies of these two microbial groups were included in permutation-based ANCOVA. Regardless of sequencing method, IBD phenotype, Clostridia difficile and NOD2 genotype were selected as associated (FDR ≤0.05) with shifts in overall microbial composition. IBD phenotype and NOD2 genotype were also selected as associated with shifts in the relative frequency of the C. coccoides – E. rectales group. IBD phenotype, smoking and IBD medications were selected as associated with shifts in the relative frequency of F. prausnitzii spp. These results indicate that the effects of genetic and environmental factors on IBD are mediated at least in part by the enteric microbiota.
Introduction
Abnormal host-microbial interactions and genetic susceptibility are implicated in the pathogenesis of inflammatory bowel diseases (IBD) [1]–[4]. Culture-independent microbiological technologies coupled with high-throughput DNA sequencing have revolutionized the scale, speed, and economics of microbial ecological studies. When applied to IBD, these technologies have uncovered alterations in human intestine-associated microbial compositions (“dysbiosis”) in IBD patients compared with controls [5]–[12]. To further investigate mechanisms and the biological and clinical significance of dysbiosis in IBD, we have begun integrating metagenomic and phenotype data with genotype and additional clinical metadata.
We focused on the three prevalent risk alleles of the nucleotide oligomerization domain 2 (NOD2; Leu1007fs, R702W, and G908R) and the ATG16L1 T300A genotype out of the ∼100 IBD related genotypes identified thus far, because these loci have been linked to host innate immunity, particularly Paneth cell function, and ileal Crohn’s disease (CD) phenotype [13]–[27]. We recently conducted an exploratory study that integrated NOD2 and ATG16L1 genotype data with a previously published 16S rRNA sequence dataset [5], [11]. This analysis revealed potential associations between alterations in intestine-associated microbial composition and respectively disease phenotype, NOD2 and ATG16L1 genotype. One limitation was that the samples from IBD patients were collected from two separate anatomic sites (ileum and colon), and from both grossly disease-affected and disease unaffected regions. The CD patients were heterogeneous with respect to disease location and included patients with both ileal and colonic disease. There is evidence that patients with isolated colonic CD have distinct genetic characteristics from patients with ileal CD [28]. Genetic associations for Crohn’s colitis patients overlap extensively with UC patients and differ from ileal CD patients [29]. For example, the relative frequency of patients with at least one of the three major NOD2 risk alleles is only 16% in Crohn’s colitis patients, approaching the frequency observed in non-IBD control subjects [30]. Subphenotyping CD patients with respect to disease location would therefore facilitate biological interpretation of integrating metagenomic data with genotype data [31]–[33]. Another limitation of the previous study was that relatively limited clinical metadata was available for assessing the effects of potentially confounding variables, such as obesity [34].
In the current study, 16S rRNA sequence analysis was conducted on the proximal margins of resected ileum collected from a larger independent set of subjects with and without inflammatory disease to test the hypothesis that these genes affect ileum-associated microbiota in grossly disease-unaffected regions of the ileum, In contrast to the previous study, the subjects in the current study were restricted to three disease phenotypes that were unlikely to overlap with respect to disease location: 1.) Ileal CD patients undergoing ileocolic resection; 2.) colitis patients (without ileal disease) undergoing total colectomy or proctocolectomy; and 3.) control non-IBD patients undergoing either initial right hemicolectomy or total colectomy. Patients with ileocolic anastomoses from previous surgeries were excluded, because increased reflux of colonic luminal contents could potentially impact ileal mucosal microbial profiles. The samples were also linked to far more extensive clinical metadata than those used in the previous exploratory analysis [5], [11].
Because the previous dataset we analyzed was generated by amplifying the entire 16S rRNA gene followed by Sanger sequencing, this methodology was also applied in the current study. However, to increase depth of coverage and corroborate results derived from Sanger sequencing datasets, we also performed 454 sequencing of two regions of the 16S rRNA gene (V1–V3 and V3–V5) using primers adopted by the ongoing Human Microbiome Project [35]–[38]. These three parallel datasets provide a unique opportunity for comparing the results of these three sequencing methods in a disease setting.
Materials and Methods
Patients and Acquisition of Macroscopically Disease-Unaffected Proximal Margin Ileal Tissue Samples
This study was approved by the Institutional Review Boards of Washington University-St. Louis and Stony Brook University. The diagnosis of CD or UC was made ultimately on the basis of pathological criteria (surgical resection specimen) [31]–[33]. Ileal CD patients undergoing ileocolic resection, colitis patients undergoing total colectomy and control non-IBD patients undergoing either right hemicolectomy or total colectomy were prospectively enrolled in a consecutive fashion by the Washington University Digestive Diseases Research Core Center Tissue Procurement Facility to donate surgically resected tissue samples and clinical information between April 2005 and February 2010. The clinical information and patient samples were stripped of all identifying information and assigned a patient code and sample code.
The ileal CD patients were predominantly those falling within the Montreal classification of ileal disease with or without cecal disease (L1) [30]. Based on post-operative pathological diagnosis of the resected colon, 47 patients were diagnosed with UC, 9 patients were diagnosed with Crohn’s colitis and 2 patients were diagnosed with indeterminate colitis. Fifty-eight percent of the control non-IBD patients underwent resection for benign colonic diseases (colonic inertia, diverticulosis, adenomas, etc.) and the remaining 42% underwent resection for primary colonic adenocarcinomas. Patients who were unwilling or unable to give informed consent were excluded. Patients who had undergone previous resection of ileum as evidence by the presence of an ileocolonic anastomosis were excluded from this study. The number of subjects (n = 170) included in this cross-sectional study was designed to exceed the number of subjects studied previously (n = 125) [5], [11].
A minimum of 4 biopsies were taken from the macroscopically disease unaffected proximal ileal margin of fresh pathological specimens using Radial Jaw4 large-capacity biopsy forceps (Boston Scientific, Natick, MA), immediately placed in RNA stabilization solution (RNAlater, Applied Biosystems/Ambion, Austin, TX) and archived at −80°C [39]. The designation of disease-unaffected was based on the macroscopic appearance of the mucosa and the surgical pathology report of the adjacent biopsies (“no histopathologic abnormality”). The samples were de-identified and linked to a detailed clinical database by a patient study code.
Information on potential confounding variables was obtained by reviewing the medical records, including the pathological report of the resected intestine by a gastroenterologist (EL). Preoperative mechanical bowel preparations were not routinely ordered, particularly for the IBD patients. Also, adherence to bowel preparations was quite varied among participating subjects. For this reason, preoperative bowel preparation was not included in the analysis. A smoker was defined as smoking ≥7 cigarettes a week for at least a year [40]–[42]. The body mass index (BMI) was recorded for all individuals [34]. Concomitant Clostridium difficile infection was recorded as the presence of a positive fecal C. difficile toxin [43], [44]. Most of these patients were treated with metronidazole or oral vancomycin [45]. All of the patients received intravenous antibiotic prophylaxis covering both aerobic and anerobic bacteria (e.g. ciprofloxacin and metronidazole, cefoxitin, cefotetan) within one hour of incision [46].
Genotyping of NOD2 and ATG16L1 Single Nucleotide Polymorphisms (SNPs)
Each patient was genotyped for the three major NOD2 SNPs, Leu1007fsInsC (rs2066847, SNP13), R702W (rs206884, SNP8) and G908R (rs2066845, SNP12) and for the autophagy like ATG16L1T300A SNP (rs2241880) by direct sequencing, and/or by a TaqMan MGB (Applied Biosystems, Foster City, CA) genotyping platform using genomic DNA prepared from peripheral blood and/or tissue by the Sequenom Technology Core within the Washington University Division of Human Genetics.(http://hg.wustl.edu/info/Sequenom_description.html) as previously described [39]. Because some combinations of individual NOD2 risk alleles, ATG16L1 risk alleles, and disease phenotype were not sampled in this study, the three NOD2 risk alleles were combined to form two composite categories: 1) NOD2NR, subjects harboring none of the three risk alleles (i.e., NOD2NR/NR); or 2) NOD2R, subjects harboring at least one of the three risk alleles (i.e., NOD2R/R + NOD2R/NR). The three ATG16L1 genotype categories were: 1.) ATG16L1NR/NR, no ATG16L1 risk allele; 2.) ATG16L1R/NR, a single ATG16L1 risk allele; 3.) ATG16L1R/R, two ATG16L1 risk alleles.
Library Construction and 16S rRNA Sequence Analysis
Parallel sequence datasets were generated for each of the samples at the Genome Institute at Washington University as previously described by 1.) broad-range PCR amplification of bacterial rRNA genes and Sanger sequencing and 2.) 454 sequencing of two separate segments of the 16S rRNA gene that encode either the V1 and V3 (V1–V3) or V3, V4, and V5 (V3–V5) hypervariable regions (see Methods S1) [35]–[37]. Of note, the 454 sequencing primers used in this current study were identical to the primers employed for characterizing the microbial communities in healthy individuals at different body sites, including the gastrointestinal tract by the Human Microbiome Project (http://hmpdacc.org/). The analysis software used to process the sequencing data consisted of established function specific tools that are described in greater detail in Methods S1. All sequences were screened for fidelity to a 16S rRNA bacterial covariance model (CM) based on secondary structure using the Infernal software package and were checked for chimerism with ChimeraSlayer [37], [47]. Potentially chimeric sequences and sequences lacking high fidelity to the CM were removed from subsequent analysis. Patient DNA samples with less than 100 total screened sequences were excluded from the analysis.
The sequences were classified into seven phyla/subphyla categories using the Naïve Bayesian Classifier of the Ribosome Database Project as described in Methods S1 [5], [11]: The seven categories were 1) Actinobacteria, 2) Bacteroidetes, 3) Firmicutes. Clostridium Group IV, 4) Firmicutes. Clostridium Group XIVa, 5.) Firmicutes. Bacillus, 6.) Proteobacteria, and 7.) Other taxa. The subdivisions of the Firmicutes phyla were based on concordance between the RDP classifier and the Greengenes 16S rRNA phylogenetic schema [5], [11], [48]–[51]. Six of these seven bacterial categories (Actinobacteria, Bacteroidetes, Firmicutes/Clostridium GroupIV, Firmicutes/Clostridium GroupXIVa, Firmicutes/Bacillus, and Proteobacteria) were selected as representing the overall microbial composition.
Assembled Sanger sequences were deposited in GenBank accession HQ739096-HQ821395. 454 V1–V3 and V3–V5 sequences were deposited in the Sequence Read Archive accession SRX021348-SRX021368, SRX037800-SRX037802. Clinical and genotyping data can be accessed through the dbGAP authorized access system. Request access to: phs000255. The study accession is SRP002479 “Effect of Crohn’s disease risk alleles on enteric microbiota”. In order to request access to any of the individual-level datasets within the controlled-access portions of the database, the Principal Investigator (PI) and the Signing Official (SO) at the investigator’s institution will need to co-sign a request for data access, which will be reviewed by an NIH Data Access Committee at the appropriate NIH Institute or Center https://dbgap.ncbi.nlm.nih.gov/aa/wga.cgi?page=login.
Quantitative PCR (qPCR)
QPCR assays were performed for the Clostridium coccoides – Eubacterium rectales Faecalibacterium prausnitzii and total bacteria using established primers (see Methods S1) [52], [53]. The assays were carried out in triplicate. Plasmid quantification standards were prepared from representative clones of the target organisms.
Statistical Analysis
Genotype and clinical categorical variables (e.g. disease phenotype, smoking, etc.) were compared between disease phenotypes using chi-square test for contingency tables. Clinical continuous variables, such as age and body mass index (BMI) were compared between disease phenotypes using the Kruskal-Wallis test. The relative frequencies of six of seven (excluding Other Taxa) categories selected to represent the overall microbial composition, were adjusted by adding 0.5 to all raw sequence counts in order to avoid 0% frequencies, and then subjected to centered log ratio transformation for the analysis of compositional data (see Methods S1) [54]. The effect of the independent variables and all first order interactions on these six bacterial categories (represented as a single vector), was analyzed in parallel for each sequencing platform by permutation based multivariate analysis of covariance (MANCOVA) with stepwise variable selection using the adonis function in R software (Version 2.12.1) package vegan (Version 1.17-2), Euclidean distances and a threshold significance level of 0.05 [55], [56]. To address the multiple comparison issue, we applied the Benjamini-Hochberg method to adjust P-values to the false discovery rate (FDR) [57]. The effect of these independent variables and their first order interactions on individual bacteria categories was further analyzed by permutation based analysis of covariance (ANCOVA) with stepwise variable selection and a threshold significance of 0.05 [32], [55], [56]. Repeated measures ANCOVA was then used to assess the union of the variables and first order interactions selected by the parallel ANCOVAs conducted on the three sequencing platforms separately, in an effort to utilize all three data sets simultaneously [58], [59]. The empirical logit transformations of the relative frequencies of the C. coccoides – E. rectales and the F. prausnitzii groups (measured by targeted qPCR) was used in the permutation-based ANCOVA. The Benjamini-Hochberg method was used to adjust P-values to the false discovery rate (FDR) [57]. The R codes are provided in Methods S1.
Results
Distribution of NOD2 and ATG16L1 Genotypes and Clinical Characteristics of Ileal CD, Colitis, and Non-IBD Control Subjects
As shown in Table 1, with the exception of race and gender, there were differences (FDR ≤0.05) in the distribution of the 11 remaining variables between the three disease phenotypes. For example, subjects who harbored at least one NOD2 risk allele, NOD2R, were more prevalent among ileal CD patients [16], [17], [39]. Only two (4%) ileal CD patients were ATG16L1NR/NR Ileal CD patients were younger than the control patients at the time of surgery [23]. Actively smoking subjects were less prevalent in colitis patients [39]–[41]. The median BMI and age were lower in ileal CD subjects. C. difficile was more prevalent among subjects with colitis [43], [45]. None of the control subjects were treated with5-ASA, immunomodulators, and/or anti-TNFα biologics. All of the patients received intravenous antibiotic prophylaxis that covered both aerobic and anaerobic bacteria within one hour prior to incision [46].
Table 1. Distribution of NOD2 composite and ATG16L1 genotype and clinical characteristics of ileal CD, colitis and control non-IBD patients.
| Variables | Ileal CD (n = 52) | Colitis (n = 58) | Control (n = 60) | P-value | FDR |
| NOD2R (R/R + R/NR) | 38% | 15% | 12% | 0.003 | 0.004 |
| ATG16L1T300A (NR/NR) | 4% | 28% | 23% | 0.006 | 0.007 |
| Gender (male) | 48% | 57% | 38% | 0.130 | 0.14 |
| Race (Caucasian) | 92% | 90% | 83% | 0.316 | 0.32 |
| Median age (range) y | 33 (18–72) | 42 (17–68) | 60 (32–64) | <0.001 | <0.001 |
| Current smoker | 33% | 3% | 25% | 0.003 | 0.004 |
| Positive fecal C. difficile toxin | 6% | 28% | 0% | <0.001 | <0.001 |
| Median BMI (range) kg/m2 | 24 (16–41) | 25 (16–45) | 28 (18–47) | 0.006 | 0.007 |
| 5-ASA | 52% | 59% | 0% | <0.001 | <0.001 |
| Steroids | 54% | 72% | 0% | <0.001 | <0.001 |
| Immunomodulators | 44% | 72% | 0% | <0.001 | <0.001 |
| Anti-TNFα biologics | <0.001 | <0.001 | |||
| Current (≤8 weeks of surgery) | 29% | 29% | 0% | ||
| Past (>8 weeks of surgery) | 6% | 7% | 0% | ||
| Never | 65% | 64% | 0% |
The variables shown above are included in the subsequent MANCOVA and ANCOVA analyses. Chi-square test for contingency table was used for categorical data and Kruskal-Wallis test was used for age and BMI. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR).
Comparison of the Relative Frequencies of Phyla/Subphyla Bacterial Categories between Ileal CD, Colitis and Control Disease-Unaffected Ileal Samples
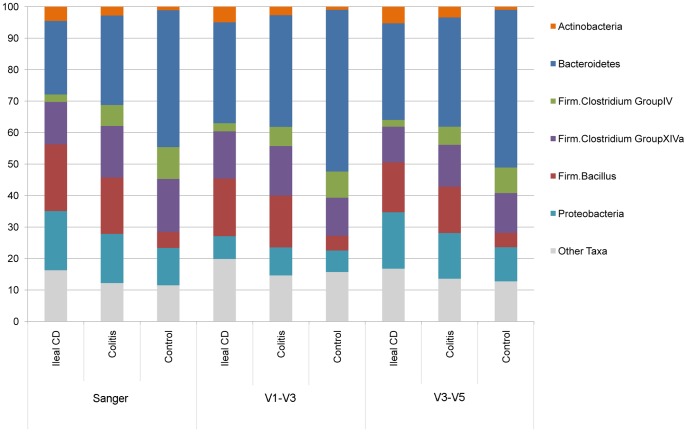
Using the Sanger method, a total of 81,644 near full length 16S rRNA sequences with acceptable quality were obtained with an average of 500 reads/sample. A total of 1,191,278 454 V1–V3 sequences (mean 7260 reads/sample) and a total of 917,900 454 V3–V5 sequences (mean 5400 reads/sample) were obtained from disease-unaffected samples. Greater than 90% of the sequences were binned into six of seven phyla/subphyla categories as shown in Figure 1. The Clostridium Group IV and Group XIVa taxa correspond to two prominent subsets of the “Lachnospiraceae” taxonomic group previously discussed by Frank et al. [5], [11]. As shown in Figure 1, and Table S1, the distribution of relative frequencies of the phyla/subphyla bacterial categories between the three disease phenotypes (ileal CD, colitis, control non-IBD) were very similar between the three datasets.
Figure 1. Phyla/subphyla comparison of three disease phenotypes (ileal CD, colitis, using the Sanger, 454 V1–V3 and 454 V3–V5 data sets.
The average relative frequency of each taxa is shown for ileal CD, colitis and control subjects for each of the three sequencing data sets, (see also Table S1 for means ± standard deviations).
Disease Phenotype, C. difficile and NOD2 Genotype are Associated with Shifts in Overall Disease-Unaffected Ileum-Associated Microbial Composition (MANCOVA)
The three sequence datasets were analyzed in parallel using a vector that combined the relative iesof the six most prevalent of the seven phyla/subphyla categories (see Figure 1) as the dependent variable. Exploratory permutation based MANCOVA with stepwise variable selection including all first order interactions, was conducted with each of the datasets. The parallel analyses with each dataset selected disease phenotype among 13 variables, as associated (FDR ≤0.05) with overall changes in the composition of mucosal bacterial communities (see Table 2). In addition, C. difficile and NOD2 genotype were selected (FDR ≤0.05) as associated with shifts in microbial composition. These two variables had smaller effects (R2) compared to the effect size of disease phenotype. Repeating the analysis with the 150 Caucasian subjects yielded similar results (see Table S2). Repeating the analysis after excluding subjects diagnosed with Crohn’s colitis and indeterminate colitis also yielded similar results (see Table S3).
Table 2. Permutation based MANCOVA with stepwise variable selection results for Sanger, 454 V1–V3 and 454 V3–V5 sequencing.
| Sequencing | Sanger (n = 164) | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.151 | 0.001 | 0.008 |
| C. difficile | 0.019 | 0.019 | 0.04 | |
| NOD2 | 0.018 | 0.011 | 0.03 | |
| Interactions | Disease phenotype * 5-ASA | 0.022 | 0.012 | 0.03 |
| Steroids * Immunomodulators | 0.022 | 0.006 | 0.03 | |
| Disease phenotype * Age | 0.033 | 0.008 | 0.03 | |
| Sequencing | 454 V1–V3 (n = 164) | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.126 | 0.001 | 0.008 |
| C. difficile | 0.019 | 0.016 | 0.03 | |
| NOD2 | 0.017 | 0.029 | 0.05 | |
| Interactions | Disease phenotype * 5-ASA | 0.022 | 0.011 | 0.03 |
| Steroids * Immunomodulators | 0.022 | 0.006 | 0.03 | |
| Disease phenotype * Age | 0.032 | 0.009 | 0.03 | |
| Sequencing | 454 V3–V5 (n = 169) | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.119 | 0.001 | 0.008 |
| C. difficile | 0.020 | 0.014 | 0.03 | |
| NOD2 | 0.029 | 0.004 | 0.02 | |
| Interactions | Disease phenotype * 5-ASA | 0.020 | 0.015 | 0.03 |
| Steroids * Immunomodulators | 0.030 | 0.001 | 0.008 | |
| NOD2 * ATG16L1 | 0.024 | 0.028 | 0.05 | |
| 5-ASA * ATG16L1 | 0.024 | 0.040 | 0.06 |
The dependent variable was the vector generated by the centered log ratio of the relative frequencies of six phyla/subphyla categories (see text). The significant main effects and first order interactions selected by analysis of each of the three data sets as well as the R2, P values are listed here. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The number of samples (total = 170 samples) that yielded results suitable for analysis is listed for each method.
Disease Phenotype Is Associated with Shifts in the Relative Frequencies of Actinobacteria, Bacteroidetes, Firmicutes. Clostridium GroupIV and Firmicutes. Bacillus Categories
In order to explore whether these variables were associated with particular microbial groups, permutation based ANCOVA with stepwise variable selection was carried out in parallel for each of the three datasets for each of the six phyla/subphyla categories (see Table S4). The union of the significant independent variables and first order interactions identified by these parallel analyses was then reanalyzed by permutation based repeated measures ANCOVA, in which the data from each sequence dataset was treated as a repeated measure (see Table 3). Analyzing the three datasets in parallel and as repeated measures (See Table 3 and Table S4), disease phenotype was selected as associated (FDR ≤0.05) with shifts in the relative frequencies of four of the six phyla/subphyla categories (Actinobacteria, Bacteroidetes, Firmicutes. Clostridium GroupIV and Firmicutes.Bacillus). Repeated measures ANCOVA also provided a means of comparing the results of the three sequencing methods. The three sequencing methods demonstrated good agreement (FDR>0.05) for the Actinobacteria and Bacteroidetes categories. Differences between the three sequencing methods may have the biggest effect (R2 = 0.040) on assessing the relative frequency of Proteobacteria.
Table 3. Permutation-based repeated measures ANCOVA results for each of the six phyla/subphyla bacterial categories (Clade).
| Category/Clade | ACTINOBACTERIA | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.126 | 0.001 | 0.01 |
| Steroids | 0.021 | 0.029 | 0.08 | |
| Interactions | Age of Surgery * ATG16L1 | 0.041 | 0.014 | 0.07 |
| Measurements | 0.001 | 0.328 | 0.47 | |
| Category/Clade | BACTEROIDETES | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.117 | 0.001 | 0.01 |
| Smoking | 0.018 | 0.034 | 0.09 | |
| 5-ASA | 0.021 | 0.025 | 0.08 | |
| Steroids | 0.023 | 0.013 | 0.07 | |
| Interactions | Steroids * Immunomodulators | 0.040 | 0.006 | 0.04 |
| Disease phenotype * 5-ASA | 0.019 | 0.023 | 0.08 | |
| 5-ASA * Age | 0.016 | 0.050 | 0.12 | |
| 5-ASA * C. difficile | 0.019 | 0.023 | 0.08 | |
| Measurements | 0.002 | 0.047 | 0.12 | |
| Category/Clade | FIRM.CLOSTRIDIUM GROUPIV | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.161 | 0.001 | 0.01 |
| Gender | 0.038 | 0.003 | 0.02 | |
| Smoking | 0.025 | 0.015 | 0.07 | |
| NOD2 | 0.021 | 0.027 | 0.08 | |
| Interactions | Disease phenotype * Age | 0.040 | 0.003 | 0.02 |
| BMI * 5-ASA | 0.025 | 0.010 | 0.06 | |
| Measurements | 0.008 | 0.001 | 0.01 | |
| Category/Clade | FIRM.CLOSTRIDIUM GROUPXIVa | R2 | P value | FDR |
| Main effects | Gender | 0.023 | 0.019 | 0.08 |
| Interactions | Disease phenotype * Age | 0.049 | 0.004 | 0.03 |
| Steroids * BMI | 0.030 | 0.011 | 0.06 | |
| Measurements | 0.006 | 0.006 | 0.04 | |
| Category/Clade | FIRM.BACILLUS | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.175 | 0.001 | 0.01 |
| NOD2 | 0.016 | 0.046 | 0.12 | |
| Interactions | Disease phenotype * Steroids | 0.040 | 0.002 | 0.02 |
| Disease phenotype * Age | 0.027 | 0.032 | 0.09 | |
| 5-ASA * ATG16L1 | 0.025 | 0.025 | 0.08 | |
| Steroids * Immunomodulators | 0.019 | 0.027 | 0.08 | |
| Measurements | 0.008 | 0.001 | 0.01 | |
| Category/Clade | PROTEOBACTERIA | R2 | P value | FDR |
| Main effects | NOD2 | 0.027 | 0.016 | 0.07 |
| Interactions | Steroids * NOD2 | 0.040 | 0.002 | 0.02 |
| 5-ASA * Race | 0.032 | 0.007 | 0.04 | |
| Steroids * Immunomodulators | 0.021 | 0.030 | 0.08 | |
| NOD2 * ATG16L1 | 0.056 | 0.019 | 0.08 | |
| Measurements | 0.040 | 0.001 | 0.01 | |
Sequencing results for all three platforms were available for 157 samples (44 ileal CD, 53 colitis and 60 control non-IBD). The variables and first order interactions with P≤0.05 are listed above. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The variables and first order interactions with FDR ≤0.05 are bolded.
Quantification of Clostridium Coccoides – Eubacterium Rectales and Fecalibacterium Prausnitzii by qPCR
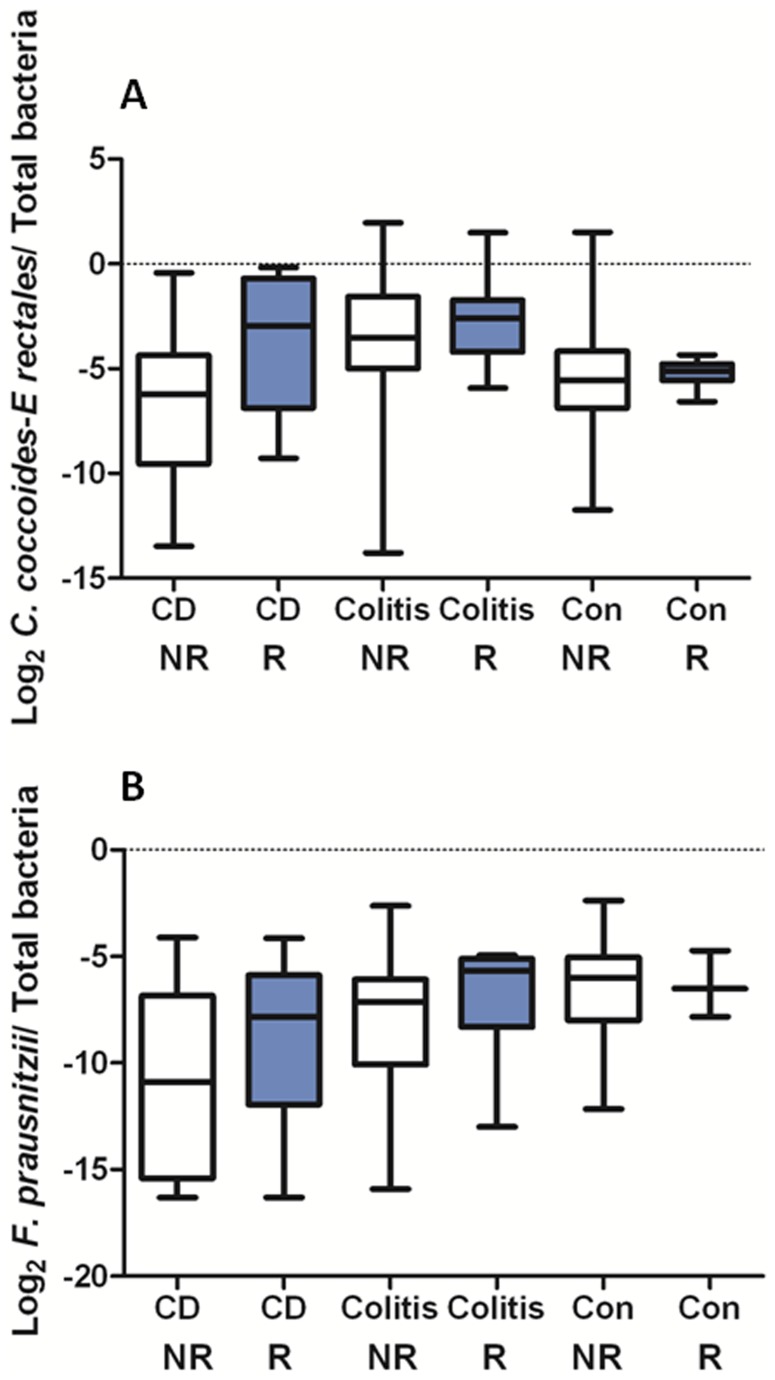
QPCR analyses were conducted on total bacteria, the C. coccoides-E. rectales group and F. prausnitzii using previously established primers (see Figure 2).52–53 The relative frequency of the C. coccoides-E. rectales group, which overlaps the “Lachnospiraceae” taxonomic group (Clostridium GroupIV and XIVa are prominent subsets) was previously shown to be reduced in a subset of IBD subjects.5 F. prausnitzii is a major species within the Clostridium Group IV category. Low relative frequency of F. prausnitzii has been reported to be reduced in patients with ileal CD and has been associated with an increased risk of ileocolonoscopic recurrence of ileal CD [8], [9], [12].
Figure 2. Targeted qPCR results for the C. coccoides-E. rectales group and for F. prausnitzii spp.
Boxplots of (panel A) the log2 C. coccoides-E. rectales group/total bacteria and (panel B) the log2 F. prausnitzii/total bacteria as a function of disease phenotype and NOD2 genotype assayed using qPCR are shown. The middle line represents the median, and the lower edge and the upper edge of the box represent the 25% and 75% quartiles. The bottom and top lines represent the minimum and maximum values, respectively. For the C. coccoides-rectales group, all 170 samples were assayed. For F. prausnitzii, 157 of 170 samples were assayed.
As shown in Figure 2, the relative frequency of the C. coccoides-E. rectales group was significantly higher in NOD2R ileal CD subjects than in NOD2NR ileal CD subjects. In contrast, the relative frequency of the C. coccoides-E. rectales group was lower in ileal CD subjects compared to control non-IBD subjects, consistent with previous analysis of an independent set of tissues [5]. As shown in Table 4, IBD phenotype, NOD2 genotype and IBD medications were selected by ANCOVA with stepwise variable selection as associated (FDR ≤0.05) with shifts in the relative frequency of C. coccoides-E. rectales group. First order interactions between IBD phenotype and race, and between NOD2 genotype and steroids, were also selected. Unfortunately 13 DNA samples were exhausted before the qPCR assays for F. prausnitzii were conducted, reducing the total samples analyzed from 170 to 157. IBD phenotype, smoking and steroids were selected by ANCOVA with stepwise variable selection as associated (FDR ≤0.05) with shifts in the relative frequency of F. prausnitzii (see Table 4). First order interactions between NOD2 and smoking, and between age and gender were also selected.
Table 4. ANCOVA with stepwise variable selection results for relative frequencies of the C. coccoides-E. rectales microbial group and F. prausnitzii spp. based on targeted qPCR assays.
| Category | C. coccoides-E. rectales n = 170 | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.06368 | 0.001 | 0.006 |
| NOD2 genotype | 0.08250 | 0.001 | 0.006 | |
| Anti-TNFα | 0.08216 | 0.001 | 0.006 | |
| ASA | 0.02904 | 0.009 | 0.02 | |
| Immunomodulator | 0.02548 | 0.019 | 0.03 | |
| Race | 0.01050 | 0.125 | 0.16 | |
| Steroids | 0.00002 | 0.954 | 0.95 | |
| Interactions | Disease phenotype * Race | 0.05563 | 0.003 | 0.01 |
| NOD2 * steroids | 0.02605 | 0.056 | 0.03 | |
| Category | F. prausnitzii n = 157 | R2 | P value | FDR |
| Main effects | Disease phenotype | 0.0662 | 0.002 | 0.008 |
| Smoking | 0.0284 | 0.018 | 0.03 | |
| Steroids | 0.0270 | 0.017 | 0.03 | |
| NOD2 | 0.0033 | 0.394 | 0.48 | |
| Age | 0.0020 | 0.500 | 0.51 | |
| Gender | 0.0025 | 0.456 | 0.52 | |
| Interactions | Smoking * NOD2 | 0.0528 | 0.004 | 0.01 |
| Age * Gender | 0.0274 | 0.019 | 0.03 |
See Materials and Methods. The variables and first order interactions with significant P values (≤0.05) as well as the variables in the first order interactions are listed above. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The variables and first order interactions with FDR ≤0.05 are bolded.
Comparison of Non-IBD Control Samples from Subjects with and without Primary Colon Adenocarcinoma
Because a major proportion (42%) of the non-IBD control subjects underwent surgery for resection of right sided primary colon adenocarcinoma, we performed exploratory analyses comparing the relative frequencies of the seven phyla/subphyla clades as well as other clinical variables. As shown in Table S5, the only variable that was different (FDR ≤0.05) between the two groups of patients was the age of surgery, which is consistent with the observation that the incidence of colon cancer increases with patient age [60].
Discussion
In this study we report the results of multiple regression analysis of the largest 16S rRNA sequence datasets reported thus far on ileal tissues collected from IBD subjects. This analysis demonstrates that IBD phenotype has a predominant effect on microbial composition associated with the macroscopically normal appearing proximal margin of resected ileum. The changes in intestinal microbiota that were observed in ileal CD may have occurred early in the pathogenic process before overt disease was manifest. Alternatively, chronic enteric dysbiosis that arises as a consequence of pathologic inflammation at one location within the GI tract may be propagated to unaffected sites. The observed alterations in ileal microbiota could have an impact on regional inflammation or metabolic properties (e.g., butyrate metabolism), however the functional implications of these shifts remain to be determined.
NOD2 genotype was also selected albeit with more modest effect for all three 16S rRNA sequence datasets and for targeted qPCR assays of the C. coccoides- E. rectales group. The bacterial 16S rRNA sequences assayed by the C. coccoides – E. rectales qPCR assay likely overlap with some but not all the 16S rRNA sequences included in Group XIVa and IV. The observation that ileal CD phenotype and NOD2 risk alleles, which presumably contribute to this phenotype, had opposite effects on this microbial group, suggests that the effect of the NOD2 risk allele on the relative frequency of the C. coccoides-E. rectales group is not simply mediated through an association with ileal CD phenotype. Thus the results of targeted QPCR assays and 16S rRNA sequence analysis both demonstrate a significant effect of NOD2 genotype on ileum associated microbial composition. Our results in disease –unaffected ileal tissues support our previous analysis of an independent set of intestinal tissues that were more heterogeneous with respect to anatomic location and inflammation. Our findings are consistent with the report that the relative frequency of Firmicutes spp., as determined by targeted qPCR, is higher in NOD2 knockout mice than in wild type mice [20], [21]. However, it is important to note that the patient-based studies are not directly comparable to the mouse studies because 1) the NOD2 knockout mouse may differ phenotypically from the NOD2Leu1007fs knock-in mouse [61], and 2) only 4% of the ileal CD patients compared to 28% of colitis and 23% of control non-IBD patients, were homozygous for the ATG16L1 T300A nonrisk allele (ATG16L1NR/NR). These findings are also consistent with the recent report that the relative frequency of Firmicutes measured by targeted qPCR is higher in three CD patients that were homozygous for the Leu1007fs (SNP13) compared to 11 CD patients that were homozygous for the wild type allele [21]. Since only two of the ileal CD patients were homozygous for the Leu1007fs allele in our dataset, a parallel comparison could not be made (see Table S6).
C. difficile infections, particularly recurrent C. difficile infections have been associated with altered fecal microbial composition [62], [63]. Patients with inflammatory bowel diseases are more likely to develop C. difficile infections, which are also associated with clinical exacerbation of their disease [43], [44]. It is possible that the shifts in microbial composition associated with IBD contribute to these patients’ susceptibility to C. difficile and other infections that may exacerbate inflammation. Alternatively antibiotic treatment of subjects with C. difficile could contribute to the observed shifts in microbial composition [64], [65].
Sanger sequencing of the entire 16S rRNA gene permits accurate phylogenetic identification of bacteria, whereas 454 pyrosequencing generates much greater depth of coverage, but with lesser phylogenetic resolution. While the results of all three sequencing methodologies demonstrated associations of disease phenotype, C. difficile infection and NOD2 genotype to the overall microbial composition in parallel analyses, they differed with respect to the main effects and first order interactions associated with individual phyla/subphyla categories. Methodological differences may reflect biases introduced during the initial PCR amplification step, as noted by Olsen and coworkers for the 27F forward primer [66]. In addition, there are likely differences in the phylogenetic resolution/assignment of the complete 16S sequence as opposed to different hypervariable regions (V1–V3 and V3–V5) of the gene sequence. Although the results from each sequencing method may converge with increasing the sample size, differences in the microbial composition data generated by different sequencing methods will make it challenging to compare results from studies using different primers for the initial PCR amplification.
The F. prausnitzii 16 S rRNA sequences assayed by targeted qPCR form a major subset of all the sequences grouped within the Firmicutes.Clostridium Group IV clade. The selection of smoking as a potentially significant covariate is intriguing, since smoking has been associated with ileal CD phenotype and with early postoperative recurrence in ileal CD patients, and is consistent with the observations of Sokol and coworkers that low ileal mucosal concentrations of F. prausnitzii is associated with early postoperative ileocolonoscopic recurrence of CD [8], [34]–[42]. Smoking was also selected by analysis of the Sanger and 454V1–V3 datasets as significantly associated with shifts in relative frequency of the Firmicutes.Clostridium Group IV clade (Table S4). Smoking cessation has clearly been linked to altering the subgingival microbial profile [67], but has not been previously linked to altering the ileum associated microbial profile.
Although pathologic review of the resected tissues provided rigorous phenotyping of the samples, the use of surgically resected tissues may bias the results by sampling of IBD patients with relatively severe disease who have been treated for various lengths of time with antibiotics and different IBD medications. Although the focus of this study was ileal CD, it is likely that other human diseases will exhibit similar links between genetically determined defects in mucosal immunity and alterations of resident microbiota. As we further expand these datasets by analyzing more samples, we anticipate that further associations between microbial composition, IBD subphenotypes, IBD polymorphisms and environmental factors will emerge.
Supporting Information
A. Relative frequencies of the six phyla/subphyla categories selected to represent overall microbial composition based on the Sanger dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD. B. Relative frequencies of the six phyla/subphyla categories selected to represent the overall microbial composition based on the 454 V1–V3 dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD. C. Relative frequencies of the six phyla/subphyla categories selected to represent the overall microbial composition based on the 454 V3–V5 dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD.
(DOCX)
Permutation-based MANCOVA with stepwise variable selection results for Caucasian patients. Because NOD2 risk alleles are rarely observed in subjects of Asian and African descent, the analysis was repeated for the 150 Caucasian subjects in the study (48 ileal CD, 52 colitis, 50 non-IBD control subjects). The dependent variable was the vector generated by the centered log ratio of the relative frequencies of six phyla/subphyla categories (see text). The significant main effects and first order interactions selected by analysis of each of the three data sets as well as the R2, P values are listed below. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The number of samples (total = 150 samples) that yielded results suitable for analysis is listed for each method.
(DOCX)
Permutation-based MANCOVA with stepwise variable selection results for Sanger, 454 V1–V3 and 454 V3–V5 sequencing. Samples with Crohn’s colitis and indeterminate colitis were excluded in this analysis. The dependent variable was the vector generated by the centered log ratio of the relative frequencies of six phyla/subphyla categories (see text). The significant main effects and first order interactions selected by analysis of each of the three data sets as well as the R2, P values are listed below. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The number of samples (around 150 samples) that yielded results suitable for analysis is listed for each method.
(DOCX)
Permutation based ANCOVA with stepwise variable selection results for each of the six individual phyla/subphyla categories. Permutation based ANCOVA with step wise variable selection was carried out for the individual phyla/subphyla categories. in parallel for each of the datasets. A total of 164 samples were analyzed for the Sanger and the 454 V1–V3 datasets respectively. A total of 169 samples were analyzed for the 454 V3–V5 dataset. Listed below are the main effects, first order interactions with P-values ≤0.05, as well as the main effects of first order interactions with P values ≤0.05. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The main effects and first order interactions with FDR ≤0.05 are bolded.
(DOCX)
Comparison between nonIBD control subjects with primary colon adenocarcinoma with non-IBD subjects without primary colon adenocarcinoma. Continuous variables (e.g. age, BMI, relative frequency of bacterial groups) were compared using the Wilcoxon rank sum test and categorical variables (e.g. genotype, smoking, race) were compared using the chi-square test. Note that none of the non-IBD control subjects had a positive C. difficile toxin or were taking any IBD medications. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). Variables with FDR ≤0.05 are bolded.
(DOCX)
Distribution of the three common NOD2 genotypes in ileal CD, colitis and non-IBD control subjects. The three major NOD2 risk alleles that account for 80% of the NOD2 variants, are Leu1007fs (SNP13, rs2066847), R702W (SNP8, rs2066844), and G908W (SNP12, rs2066845).
(DOCX)
(DOC)
Acknowledgments
The authors thank the patients who have contributed their medical information, blood and tissue samples to the Digestive Diseases Research Core Center (DDRCC) Clinical Database, the faculty of the Section of Colon and Rectal Surgery and the Division of Gastroenterology at Washington University. We thank Yanjiao Zhou in the Genome Center at Washington University for facilitating the 16S rRNA sequencing of the samples. We thank Drs. William Shannon and Phillip Tarr at Washington University and Drs. Kenny Ye and Tao Wang at Albert Einstein College of Medicine for many helpful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported partially by National Institutes of Health (NIH) UH2DK083994, the Crohn’s and Colitis Foundation of America, the Simons Foundation, and the Leona M. and Harry B. Helmsley charitable trust through the Sinai-Helmsley Alliance for Research Excellence Network and NIH R21HG005964. We acknowledge use of the Washington University Digestive Diseases Research Core Center Tissue Procurement Facility (P30 DK52574). No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19:427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Eckburg PB, Relman DA. The role of microbes in Crohn’s disease. Clin Infect. Dis. 2007;454:256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 4.Abraham C, Cho J. Mechanisms of disease: inflammatory bowel disease. New Engl. J Medicine. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson DA, Frank DN, Pace N, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: What has it revealed? Inflamm Bowel Dis. 2008;14:858–867. doi: 10.1002/ibd.20392. [DOI] [PubMed] [Google Scholar]
- 8.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, et al. Faecalibacterium prausnitzii is an anti inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willing B, Halfvarson WB, Dicksved J, Rosenquist M, Järnerot G, et al. Twin studies reveal specific imbalances in the mucosal-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- 10.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2009;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, et al. Disease phenotype and genotype are associated with shifts in intestinal-microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:179–84. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willing B, Dicksved J, Halfvarson J, Andersson AF, Lucio M, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 15.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 16.Cuthbert A, Fisher S, Croucher PJ, King K, Hampe J, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–74. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 17.Lesage S, Zouali H, Cézard JP the EPWG-IBD group, Colombel JF, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Human Genet. 2002;70:845–57. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Seminars in Immunology. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. PNAS. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 22.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, et al. A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn’s disease and is independent of CARD15 and IBD5. Gastroenterology. 2007;132:1665–1671. doi: 10.1053/j.gastro.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadwell K, Patel KK, Maloney NS, Liu TC, NG ACY, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in the intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. [PubMed] [Google Scholar]
- 27.Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, et al. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 2008;57:903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 28.Hancock L, Beckly J, Geremia A, Cooney R, Cummings F, et al. Clinical and molecular characteristics of isolated colonic Crohn’s disease. Inflamm Bowel Dis. 2008;14:1667–1677. doi: 10.1002/ibd.20517. [DOI] [PubMed] [Google Scholar]
- 29.Waterman M, Xu W, Stempak JM, Milgrom R, Bernstein CN, et al. Distinct and overlapping genetic loci in Crohn’s disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17:1936–1942. doi: 10.1002/ibd.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Lee A, Bowcock A, Zhu W, Li E, et al. Influence of Crohn’s disease and smoking on disease location. Dis Colon Rectum. 2011;54:1020–1025. doi: 10.1007/DCR.0b013e31821b94b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stange EF, Travis SP, Vermeire S, Colombel JF. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55:i1–i15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geboes K, Van Eyken. Inflammatory bowel disease unclassified and indeterminate colitis: the role of the pathologist. J. Clin Pathol. 2009;62:201–205. doi: 10.1136/jcp.2008.059311. [DOI] [PubMed] [Google Scholar]
- 34.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 35.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas BJ, Gevers D, Earl A, Feldgarden M, Ward DV, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamm C, Reimers M, McCullough C, Gorbe EB, Lu J, et al. NOD2 status and human ileal gene expression. Inflamm Bowel Dis. 2010;16:1649–1657. doi: 10.1002/ibd.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott ID, et al. Does cigarette smoking influence the phenotype of Crohn’s disease? Analysis using the Montreal classification. Am J Gastroenterol. 2007;102:577–588. doi: 10.1111/j.1572-0241.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 41.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 42.Unkart JT, Anderson L, Li E, Miller C, Yan Y, et al. Risk factors for surgical recurrence after ileocolic resection of Crohn’s disease. Dis Colon Rectum. 2008;51:1211–1216. doi: 10.1007/s10350-008-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Issa M, Vijaypal A, Graham MB, Beaulieu DB, Otterson MF, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 44.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. The incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:339–44. doi: 10.1016/j.cgh.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–555. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 46.Nelson RL, Glenny AM, Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2009;(1):CD001181. doi: 10.1002/14651858.CD001181.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Nawrocki EP, Kolbe DL, Eddy SR. Infernal 1.0: Inference of RNA alignments. Bioinformatics. 2009;25:1335–7. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernanadez-Garayzabal J, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 52.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primer for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 53.Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokequchi S, et al. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. (2003) FEMS Immunol Med Microbiol. 2003;39:81–86. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 54.Aitchison J. The statistical analysis of compositional data, Monographs on Statistics and Applied Probability. London (UK): Chapman and Hall Ltd. 1986.
- 55.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- 56.McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- 57.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 58.Anderson MJ, Legendre P. An empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. J Stat Comput Simul. 1999;62:271–303. [Google Scholar]
- 59.Manly BFJ. London (UK): Chapman and Hall Ltd. 1997. Randomization, bootstrap, and Monte Carlo methods in biology, 2nd ed.
- 60.Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999–2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 61.Maeda S, Hsu LC, Liu H, Bankston H, Iimura LA, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 62.Hopkins MJ, MacFarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- 63.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 64.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, et al. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008;74:2461–70. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delima SL, McBride RK, Preshaw PM, Heasman PA, Kumar PS. Response of subgingival bacteria to smoking cessation. J Clin Microbiol. 2010;48:2344–2349. doi: 10.1128/JCM.01821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Relative frequencies of the six phyla/subphyla categories selected to represent overall microbial composition based on the Sanger dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD. B. Relative frequencies of the six phyla/subphyla categories selected to represent the overall microbial composition based on the 454 V1–V3 dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD. C. Relative frequencies of the six phyla/subphyla categories selected to represent the overall microbial composition based on the 454 V3–V5 dataset. The mean value ± standard deviation is shown for each of the three disease phenotypes, ileal CD, colitis and control non-IBD.
(DOCX)
Permutation-based MANCOVA with stepwise variable selection results for Caucasian patients. Because NOD2 risk alleles are rarely observed in subjects of Asian and African descent, the analysis was repeated for the 150 Caucasian subjects in the study (48 ileal CD, 52 colitis, 50 non-IBD control subjects). The dependent variable was the vector generated by the centered log ratio of the relative frequencies of six phyla/subphyla categories (see text). The significant main effects and first order interactions selected by analysis of each of the three data sets as well as the R2, P values are listed below. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The number of samples (total = 150 samples) that yielded results suitable for analysis is listed for each method.
(DOCX)
Permutation-based MANCOVA with stepwise variable selection results for Sanger, 454 V1–V3 and 454 V3–V5 sequencing. Samples with Crohn’s colitis and indeterminate colitis were excluded in this analysis. The dependent variable was the vector generated by the centered log ratio of the relative frequencies of six phyla/subphyla categories (see text). The significant main effects and first order interactions selected by analysis of each of the three data sets as well as the R2, P values are listed below. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The number of samples (around 150 samples) that yielded results suitable for analysis is listed for each method.
(DOCX)
Permutation based ANCOVA with stepwise variable selection results for each of the six individual phyla/subphyla categories. Permutation based ANCOVA with step wise variable selection was carried out for the individual phyla/subphyla categories. in parallel for each of the datasets. A total of 164 samples were analyzed for the Sanger and the 454 V1–V3 datasets respectively. A total of 169 samples were analyzed for the 454 V3–V5 dataset. Listed below are the main effects, first order interactions with P-values ≤0.05, as well as the main effects of first order interactions with P values ≤0.05. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). The main effects and first order interactions with FDR ≤0.05 are bolded.
(DOCX)
Comparison between nonIBD control subjects with primary colon adenocarcinoma with non-IBD subjects without primary colon adenocarcinoma. Continuous variables (e.g. age, BMI, relative frequency of bacterial groups) were compared using the Wilcoxon rank sum test and categorical variables (e.g. genotype, smoking, race) were compared using the chi-square test. Note that none of the non-IBD control subjects had a positive C. difficile toxin or were taking any IBD medications. To address multiple comparison issues, the Benjamini-Hochberg method was applied to adjust P-values to the false discovery rate (FDR). Variables with FDR ≤0.05 are bolded.
(DOCX)
Distribution of the three common NOD2 genotypes in ileal CD, colitis and non-IBD control subjects. The three major NOD2 risk alleles that account for 80% of the NOD2 variants, are Leu1007fs (SNP13, rs2066847), R702W (SNP8, rs2066844), and G908W (SNP12, rs2066845).
(DOCX)
(DOC)