Abstract
VB-111 is an engineered antiangiogenic adenovirus that expresses Fas-c in angiogenic blood vessels and has previously been shown to have significant antitumor activity in vitro and in vivo in Lewis lung carcinoma, melanoma, and glioblastoma models. To evaluate the efficacy of VB-111 in thyroid cancer, we conducted in vivo xenograft nude mouse studies using multiple thyroid cancer-derived cell lines models. VB-111 treatment resulted in 26.6% (P = 0.0596), 34.4% (P = 0.0046), and 37.6% (P = 0.0249) inhibition of tumor growth in follicular, papillary and anaplastic thyroid cancer models, respectively. No toxicity was observed in any model. All tumor types showed a consistent and significant reduction of CD-31 staining (P < 0.05), reflecting a reduction of angiogenic activity in the tumors, consistent with the intended targeting of the virus. A phase 2 clinical trial of VB-111 in patients with advanced differentiated thyroid cancer is ongoing.
Keywords: antiangiogenic agent, angiogenesis, thyroid cancer, virotherapy
The varied phenotypic characteristics and biological behaviors arising from a single cell type make thyroid cancers important oncogenic model systems. Follicular cell–derived thyroid cancers include follicular (FTC), papillary (PTC), and anaplastic (ATC) morphotypes. The American Cancer Society estimated that ~48,000 new cases of thyroid cancer with about 1,740 deaths would be seen in the year 2011.1 PTC accounts for about 80% of the disease, but FTC and ATC are the more aggressive morphotypes, accounting for about 40% of the total deaths associated with this disease. Thyroid cancer is nevertheless generally associated with good prognosis, particularly if detected early. Efforts continue to identify, develop, and validate effective novel therapies for those cancers that are not detected early, particularly in ATC wherein median survival regardless of stage is only about 5 months. Aggressive FTCs and all ATCs are resistant to routinely used therapies including surgery, radiotherapy, and chemotherapy,2,3 emphasizing the need for innovations in treatment modalities.
In particular, virus-mediated gene therapies for cancer have garnered much attention in the past decade. Tumor vasculature, being a critical contributor to tumor growth and progression, has been an especially attractive therapeutic target. VB-111 is a nonreplicative adenovirus engineered to express the chimerical receptor (Fas-c) gene under the regulation of a modified preproendothelin-1 (PPE-1) promoter (Ad-PPE-Fas-c).4,5 This modification results in specific expression of Fas-c transgene in endothelial cells (ECs) of angiogenic blood vessels, resulting in attenuation of tumor blood supply. In in vitro studies, infection of both bovine and human ECs (BAEC and HUVEC, respectively) with VB-111 at an MOI of 1,000 induced massive cell death by apoptosis.6 In contrast, a recombinant vector containing the Fas-C gene driven by the non–EC-specific CMV promoter induced apoptosis in both ECs and non-ECs,6 demonstrating that VB-111 is an efficient and specific tool for targeting ECs. In preclinical studies, VB-111 was previously shown to have significant antitumor activity in Lewis lung carcinoma and melanoma6 and glioblastoma models7 and has been demonstrated to result in growth retardation, reduction of tumor mass, and inhibition of metastasis.
During a Phase I trial of VB-111 to assess safety of the agent, a single case of differentiated thyroid cancer showed a positive response to treatment.8 To preliminarily evaluate the potential of VB-111 to treat the various morphotypes of thyroid cancer, including aggressive thyroid cancer, we evaluated the efficacy of VB-111 using validated follicular cell–derived thyroid cancer xenografts using a nude mouse model.
All studies were conducted in accordance with accepted standards of animal care and with approval by the Mayo Institutional Committee on Animal Care. None of the mice in this study showed evidence of morbidity. The FTC-derived FTC-133 cells (a DNA fingerprint-verified thyroid cancer cell line from Dr. Matthew Ringel, Ohio State University, Columbus, OH) were grown in DMEM with 10% FBS. The PTC-derived Lam-1 cell line and the ATC-derived KTC-3 cell line (DNA fingerprint-verified thyroid cancer cell lines from Dr. John A Copland, Mayo Clinic, Jacksonville, FL) were cultured in RPMI-1640 containing 10% FBS (Biomeda Corporation, Foster City, CA) with sodium pyruvate and HEPES buffer. All media contained nonessential amino acids and penicillin–streptomycin antibiotics. All cell lines were grown at 37°C in a humidified atmosphere of 5% CO2. All cell lines in this study have been examined by DNA profiling to ensure that they conform to bona fide thyroid cell lines per Schweppe et al.9
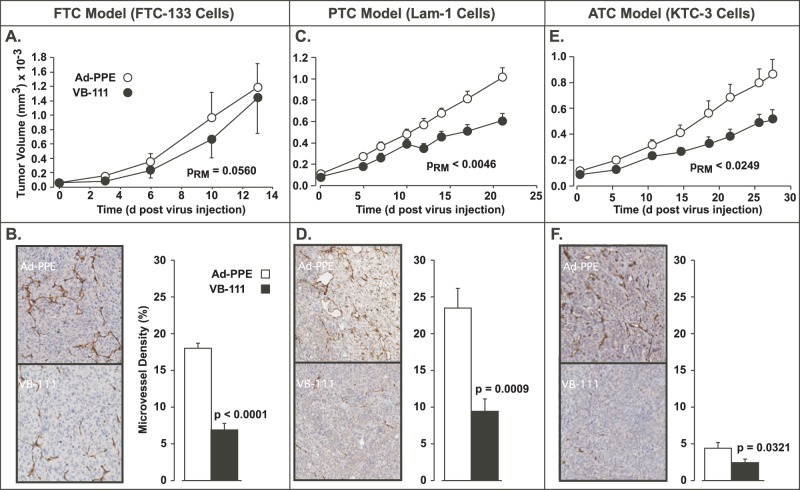
For each cell line, 10 × 106 cells were injected subcutaneously into the right flank of 3- to 4-week-old athymic nude mice (Harlan Sprague Dawley Company, Indianapolis, IN). Tumor nodules were allowed to grow subcutaneously to approximately 50 to 100 mm3 in size. The mice were randomly divided into 2 groups of 10 tumors each that received a single intravenous tail vein injection of 1011 virus particles (original virus stocks were supplied by Vascular Biogenics Ltd, Or Yehuda, Israel), in a total volume of 100 µL, per tumor of either the VB-111 or the control Ad-PPE virus. Tumor size was measured every 3 days via caliper, and tumor volume was calculated according to the following formula: Vtumor = 0.5236 (l·w·h), where l, w, and h represent length, width, and height (Fig. 1). As shown in Fig. 1, A, C, and E, VB-111 therapy produced inhibition of tumor growth in all 3 cell line xenografts (FTC, 26.6%; PTC, 34.4%; and ATC, 37.6%) that was statistically significant for PTC and ATC xenografts (P = 0.0046 and 0.0249, respectively) as assessed by repeated-measures ANOVA (SAS 9.1, SAS Institute Inc, Cary, NC).
Figure 1.
Efficacy of VB-111 on thyroid cancer–derived xenograft tumors. Intravenous administration of VB-111 to nude mice carrying xenograft tumors using the FTC-derived cells FTC-133 (A), PTC-derived Lam-1 cells (C), or ATC-derived KTC-3 cells (E) resulted in a significant inhibition of tumor growth. Reduction in tumor growth was associated with 2.6-, 2.4-, and 1.8-fold decreased vasculature in FTC-derived (B), PTC-derived (D), and ATC-derived (F) xenografts, respectively, as determined by CD-31 staining. The values in A, C, and E represent means ± SE from 10, 10, and 9 independent tumors each for VB-111 or Ad-PPE treatment. Values in B, D, and F are combined from 15 independent images selected from 3 tumors per group treated with either VB-111 or Ad-PPE, respectively.
To evaluate whether the observed reduction in tumor growth by VB-111 treatment in the xenograft model was due to inhibition of emerging vasculature, 3 tumors per cell line per virus treatment (VB-111 or Ad-PPE) were harvested at the end of the study (~1,000 mm3), formalin fixed, and paraffin embedded. Blocks were sectioned in phase into 5-µm slices and stained for microvessel density using CD-31 antibody (sc-1506-R, Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 dilution) for 30 to 60 minutes and counterstained with hematoxylin. Antigen retrieval for CD-31 was done in EDTA at 37°C for 30 minutes after deparaffinization. Sections were blocked with peroxidase and protein for 5 minutes, respectively. Antibody staining was visualized using Dako Envision plus System (Dako North America, Inc., Carpinteria, CA) for 15 minutes followed by DAB chromogen for 10 minutes. Separate tumor sections were stained with H&E to visualize tumor histology.
Stained slides were scanned using the Hamamatsu NanoZoomer (Bacus Laboratories, Inc., Chicago, IL). Digital images composed of multiple tiles (4,096 × 64 pixels) were captured at 20× magnification. Image analysis and determination of color threshold values for CD-31 signal and background were conducted according to Monahan et al.10 For each tumor section, the relative signal intensity from 15 randomly chosen fields (205 × 300 pixels) was quantified by histogram analysis (Adobe Photoshop). Data were expressed as signal intensity (CD-31 signal pixel count divided by total image pixel count). As shown in Fig.1, B, D, and F, VB-111 treatment demonstrated 2.6-, 2.4- and 1.8-fold less staining intensity compared with the control Ad-PPE treated FTC-, PTC- or ATC-derived tumors, respectively. The reduction of CD-31 signal intensity was statistically significant for all 3 models (P < 0.05) as assessed by Student’s t test (SAS 9.1), consistent with the targeting of endothelial cells by VB-111. Whereas vasculature was reduced in the FTC-133 cell line–derived tumors, VB-111 treatment only resulted in a trend (P = 0.056) for suppression of tumor growth. This may be due to a decreased need for these cells to have an efficient blood supply. It has been shown that FTC-133 cells and other ATC cell lines express high levels of HIF-1α,11 a factor associated with utilization of the glycolytic pathway. This result suggests that some form of combinatorial therapy may be required to enhance the action of VB-111. For example, bevacizumab has been shown to increase viral distribution and efficacy of replicative adenoviral vectors in human anaplastic thyroid carcinoma xenografts.12 Alternatively, combinatorial strategies that target the tumor cells directly in conjunction with VB-111 may provide additional effectiveness for aggressive tumors.
In conclusion, nude mice xenograft experiments validate the tumoricidal efficacy of VB-111 and the associated decreased neovasculogenesis in vivo in multiple thyroid cancer model systems. These data demonstrate the potential of VB-111 to be used as a novel therapy for treatment of all morphotypes of follicular cell–derived thyroid cancer, including follicular, papillary, and anaplastic cancers, expanding potential targets for VB-111 virotherapy beyond the earlier observations with the Lewis lung carcinoma and melanoma models6 and glioblastoma models.7 VB-111 is currently being tested in a Phase 2 clinical trial for differentiated thyroid cancer.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The intellectual property for VB-111 is held by Vascular Biogenics Ltd, Or Yehuda, Israel.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by funding from Vascular Biogenics Ltd, Or Yehuda, Israel.
References
- 1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-36 [DOI] [PubMed] [Google Scholar]
- 2. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453-64 [DOI] [PubMed] [Google Scholar]
- 3. Haigh PI. Anaplastic thyroid carcinoma. Curr Treat Options Oncol. 2000;1:353-7 [DOI] [PubMed] [Google Scholar]
- 4. Harats D, Kurihara H, Belloni P, Oakley H, Ziober A, Ackley D, et al. Targeting gene expression to the vascular wall in transgenic mice using the murine preproendothelin-1 promoter. J Clin Invest. 1995;95:1335-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varda-Bloom N, Shaish A, Gonen A, Levanon K, Greenbereger S, Ferber S, et al. Tissue-specific gene therapy directed to tumor angiogenesis. Gene Ther. 2001;8:819-27 [DOI] [PubMed] [Google Scholar]
- 6. Greenberger S, Shaish A, Varda-Bloom N, Levanon K, Breitbart E, Goldberg I, et al. Transcription-controlled gene therapy against tumor angiogenesis. J Clin Invest. 2004;113:1017-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner AJ, Rogge J, Cohen YC, Breitbart E, Bangio L. Antiangiogenic gene therapy with VB111 is active in glioblastoma xenografts. 102nd Annual Meeting of the American Association of Cancer Research; April 2-6, 2011; Orlando, FL [Google Scholar]
- 8. Triozzi PL, Borden EC. VB-111 for cancer. Expert Opin Biol Ther. 2011;11:1669-76 [DOI] [PubMed] [Google Scholar]
- 9. Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monahan P, Rybak S, Raetzman LT. The notch target gene Hes1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burrows N, Resch J, Cowen RL, von Wasielewski R, Hoang-Vu C, West CM, et al. Expression of hypoxia-inducible factor 1 alpha in thyroid carcinomas. Endocr Relat Cancer. 2010;17:61-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libertini S, Iacuzzo I, Perruolo G, Scala S, Ierano C, Franco R, et al. Bevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947. Clin Cancer Res. 2008;14:6505-14 [DOI] [PubMed] [Google Scholar]