Abstract
Brain plasticity can be conceptualized as nature’s invention to overcome limitations of the genome and adapt to a rapidly changing environment. As such, plasticity is an intrinsic property of the brain across the life-span. However, mechanisms of plasticity may vary with age. The combination of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) or functional magnetic resonance imaging (fMRI) enables clinicians and researchers to directly study local and network cortical plasticity, in humans in vivo, and characterize their changes across the age-span. Parallel, translational studies in animals can provide mechanistic insights. Here, we argue that, for each individual, the efficiency of neuronal plasticity declines throughout the age-span and may do so more or less prominently depending on variable ‘starting-points’ and different ‘slopes of change’ defined by genetic, biological, and environmental factors. Furthermore, aberrant, excessive, insufficient, or mistimed plasticity may represent the proximal pathogenic cause of neurodevelopmental and neurodegenerative disorders such as autism spectrum disorders or Alzheimer’s disease.
Keywords: Cortical brain plasticity, Transcranial magnetic stimulation, Electroencephalography, Functional magnetic resonance imaging, Lifespan
Plasticity as an Intrinsic Property of Human Brain
The world we live in changes rapidly. Afferent inputs and efferent demands to the brain shift quicker than the time needed to implement genetic or even epigenetic changes. Brain plasticity can be conceptualized as nature’s invention to overcome limitations of the genome and adapt to the rapidly changing environment (Fig. 1; Pascual-Leone et al. 2005). As such, plasticity represents an intrinsic property of the nervous system retained throughout life that enables modification of function and structure in response to environmental demands via the strengthening, weakening, pruning, or adding of synaptic connections and by promoting neurogenesis. This means that the brain does not remain static but, instead, continues to change as the obligatory consequence of each sensory input, motor act, association, reward signal, action plan, and awareness (Pascual-Leone et al. 2005).
Fig. 1.
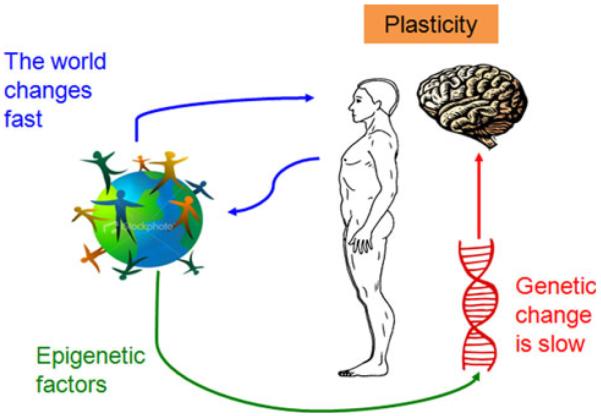
Schematic representation of the concept of plasticity. Brain plasticity allows for rapid adaptation to environmental changes that occur quicker than genetic or epigenetic response times
However, it is worth remembering William James who defined plasticity is the “possession of a structure weak enough to yield to an influence, but strong enough not to yield all at once”. Excess plasticity can be equally deleterious as insufficient plasticity (Fig. 2). Plasticity is essential to the establishment and maintenance of brain circuitry, it can be beneficial for the individual enabling acquisition of new skills and adaptation after an injury, but it can also account for the symptoms of disease. Normal plasticity mechanisms can serve to compound the pathological consequences of a specific genetic mutation or sustained environmental insult, and aberrant plasticity mechanisms can act on a previously normal brain to induce pathological manifestations of disease. Early altered or mistimed plasticity may set the stage for otherwise innocuous processes to become pathogenic (Gogolla et al. 2009). A deficit in plasticity will render the brain unable to adjust to changing demands. On the other hand, if the brain is too plastic, structural connections may become unstable and functional systems necessary for cognition and behavior may be compromised.
Fig. 2.
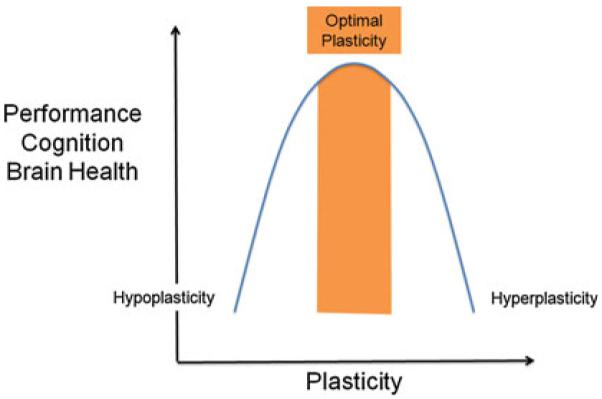
A bell-curve response to plasticity levels suggests that both too little and too much plastic response can hinder cognitive performance and overall brain health. “Optimal amounts of plasticity” will necessarily be different for different individuals, varying across brain regions and networks and changing across the lifespan
The brain is highly interconnected. Therefore, plasticity plays out across the multiple levels of nervous system complexity, from cellular through microcircuits to circuits and large-scale networks. Activity in lower levels may influence activity in higher levels, and vice versa. Changes in local plasticity can be compensated for by circuit and network adaptations in such a way that behavior may not deteriorate (in fact, some behaviors may even be paradoxically improved). Alternatively, local changes may be compounded by further maladaptive circuit and network dynamics giving rise to disability and the symptoms of disease. Thus, changes in local plasticity may constitute the first in a chain of events culminating in ‘circuitopathies’ in which symptoms are the consequence of dysfunctions of neural circuits and networks. If so, measures of cortical plasticity may provide very early local and network biomarkers of neuropsychiatric disease.
In the present article we discuss concepts of plasticity as they might evolve across the age-span and contribute to normal development, life-long cognitive abilities, and the manifestation of developmental or neurodegenerative disorders. We argue that, in all individuals, the efficacy of the mechanisms of plasticity changes over the lifespan, but that it does so from variable starting-points and with variable slopes depending on a number of genetic factors, environmental factors, and their complex interaction (Fig. 3). Empirical determination of each individual’s slope of changing brain plasticity across the lifespan is possible with real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) (Freitas et al. 2011a, b). Such methods might ultimately provide early predictors of individual risk for age-related cognitive decline, diagnostic biomarkers for neurodevelopmental and neurodegenerative disorders, and enable plasticity-based interventions to optimize outcomes for each individual.
Fig. 3.
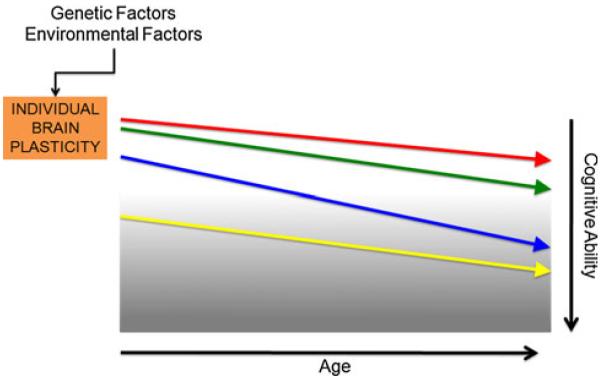
Schematic representation of individual plasticity across the lifespan. Although mechanisms of plasticity show a downward trend over the course of a typical lifetime, this trend will manifest differently according to initial “baseline” levels, genetic factors, and environmental influences. Therefore, one may conceptualize each individual has a unique “slope of plasticity” across the lifespan
Plasticity Changes Across the Lifespan: Animal Models and Indirect Evidence in Humans
Animal studies building on pioneering work from Barnes in the late 1970s (Barnes 1979) have demonstrated an age-associated decline in synaptic plasticity that correlates with neurocognitive impairments (Rosenzweig and Barnes 2003). For example, once induced, hippocampal long-term potentiation (LTP) decays faster in older rats, and this appears to be associated with a greater degree of forgetfulness (Barnes and McNaughton 1980; Kelly et al. 2006). Moreover, deficits in the balance between LTP and longterm depression (LTD) result in impaired learning and memory (Larson et al. 1986; Roman et al. 1987; Bliss et al. 2003).
However, despite these and other findings in animal models, evidence in humans has remained largely indirect. Brain imaging studies with structural MRI, fMRI, diffusion tensor imaging (DTI), and positron emission tomography (PET) can provide evidence supporting the claim that plasticity changes across the lifespan. But, for the most part, these methods assess the consequences of plasticity and fail to directly assess the mechanisms of plasticity themselves.
Structural neuroimaging can assess the integrity of brain structures and provide indirect measures of plasticity phenomena. For example, cross-sectional studies have consistently identified age-associated morphometric brain changes encompassing regional cortical thinning, volumetric subcortical reductions, and ventricular enlargement (e.g., Fjell et al. 2009; Walhovd et al. 2005, 2009). Longitudinal studies have demonstrated annual atrophy rates for brain volume, hippocampus and entorhinal cortex (e.g., Scahill et al. 2003; Fotenos et al. 2005), and atrophy in cortical brain regions over different periods of time (Raz et al. 2005; Driscoll et al. 2009). Cortical thickness decreases over the lifespan are estimated at 0.5% a year (Thompson et al. 2007). These changes affect different neural systems differently: motor and visual cortices show regional thinning, whereas non-limbic temporal regions and parietal areas are relatively spared in normal aging (Salat et al. 2004; Raz et al. 2004). Furthermore, DTI can address structural changes in white matter structure (myelination) and connectivity. For example, DTI has demonstrated that white-matter connections, largely in fronto-striatal areas, have reduced myelination as age increases (Salat et al. 2005). It is assumed that such structural, morphometric MRI and DTI measures must be associated with cognitive and behavioral consequences (Fjell and Walhoyd 2010).
There are changes in cognitive task performance associated with aging and, as a corollary to this, changes in the neural activation resulting from these cognitive tasks. It has been widely established that normal human aging in humans is associated with decrements in cognitive performance across several domains including processing speed, working memory, episodic memory, attentional control, inhibitory control, and executive function (e.g., Gazzaley and D’Esposito 2007; Reuter-Lorenz and Park 2010; Park and Reuter-Lorenz 2009). In parallel, a host of neuroimaging investigations have described altered patterns of brain activation in healthy elders as they perform cognitive tasks. One such pattern is that of a reduction in prefrontal hemispheric asymmetry in elderly individuals, referred to as the HAROLD model (hemispheric asymmetry reduction in older adults) (Cabeza et al. 2002). According to the HAROLD model, the older brain displays less localizable and more bilateral activation during certain cognitive tasks. A second pattern is a shift in evoked neural activity from posterior to anterior cortex, a model referred to by Davis et al. as PASA (posterior-anterior shift in aging) (2008). The PASA model posits that the aging brain is more likely to recruit prefrontal, rather than occipito-temporal, cortex in the service of task execution.
In addition to life-span changes in task-related brain activation patterns, resting-state fMRI is revealing age-related differences in the functional connectivity across large-scale brain networks. One such large-scale brain functional network, the default mode network (DMN) has been shown to undergo notable modifications with advancing age in health and disease (Buckner et al. 2008). Older individuals reportedly exhibit significantly lower DMN activity in the posterior cingulate as well as a tendency toward lower activity in all other DMN regions as compared to younger subjects (Koch et al. 2010). Functional connectivity within the DMN also seems to be reduced in older adults (Grady et al. 2010). During performance of a working memory task, the pattern of deactivation of the DMN also seems to be affected by aging, with older individuals not only showing decreased connectivity but also decreased ability to suppress lowfrequency oscillations of the DMN (Sambataro et al. 2010). Age-specific changes in activation and connectivity are also seen in the task-positive network (TPN), though the functional significance of this remains uncertain (Grady et al. 2010; Sambataro et al. 2010). During memory encoding and recognition, age-related changes appear to occur mainly in the long-range connections with widespread reductions associated with aging in the fronto-temporal and temporo-parietal regions, and a few age-related increases in the posterior parietal regions (Wang et al. 2010). During developmental years, children and young adults appear to have similar patterns of functionally connected regions, but with differences in the size of functionally connected regions as well as in the strength of functional connectivity between brain regions (Jolles et al. 2010).
The apparent tendency for the aged brain to activate bifrontal cortex more anterior (as opposed to posterior) cortical regions during certain cognitive tasks as well as the observed changes in resting-state functional brain connectivity are often explained on the basis of compensatory strategies. It has been theorized that these changes represent plasticity-based modifications to compensate for deficits associated with normal aging. However, there is also increasing discussion that age-related changes in brain activation patterns may also reflect a break-down of optimized brain function, an age-related increase in neural noise that might ultimately be maladaptive. Further longitudinal studies are needed and, in addition, greater mechanistic understanding of the nature of age-related changes in brain activity and structure is warranted. In relation to plasticity, direct experimental measures of plasticity mechanisms in humans that might be translated to animal models are desirable.
Direct Empirical Measures of Mechanisms of Plasticity in Humans
Transcranial Magnetic Stimulation (TMS)
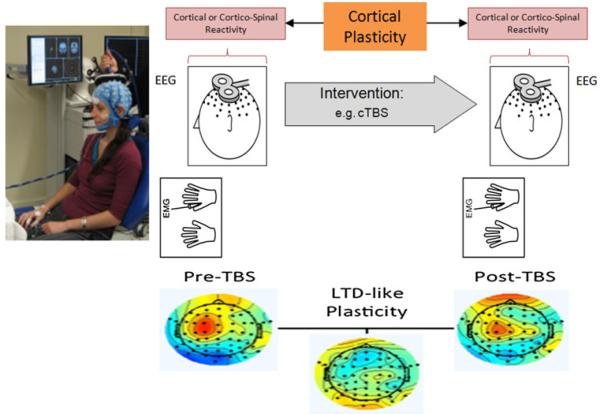
TMS is a non-invasive procedure used to create electric currents in discrete brain regions (Kobayashi and Pascual-Leone 2003; Wagner et al. 2007; Horvath et al. 2011). TMS is based on Faraday’s principle of electromagnetic induction and features application of rapidly changing magnetic field pulses to the scalp via a copper wire coil connected to a magnetic stimulator. These brief pulsed magnetic fields painlessly pass through the skull and can create electric currents of sufficient magnitude in discrete brain regions to depolarize neurons. When applied to the motor cortex, this depolarization results in a series of descending (direct and indirect) cortico-spinal waves that can sum-up at the spinal segmental level, depolarize alpha motor neurons, and lead to the contraction of contralateral muscles. This contraction, known as a motor evoked potential (MEP), can be measured using electromyography (EMG). Applied to non-motor cortical regions, TMS evokes a local field potential that can be recorded with EEG, and represents a measure of cortical reactivity to TMS (Fig. 4). In addition, beyond the targeted cortical brain region, TMS exerts transynaptic distributed network effects that can be revealed combining TMS with neuroimaging.
Fig. 4.
TMS-based measures of cortical reactivity and plasticity. As recorded using either electromyography (EMG) or electroencephalography (EEG), brain responses to TMS can be measured as motor evoked potentials (when TMS is applied to motor cortex) or localized evoked field potentials. Such responses reflect cortical or corticospinal reactivity. These measures can be obtained before and following a given intervention, for example controlled modulation of cortical excitability by repetitive TMS in the form of theta burst stimulation (TBS). The TBS-induced change in EMG or EEG responses to TMS provides a measure of local cortical plasticity. An example of TMS-EEG measures before and after continuous TBS illustrates the measurement of LTD-like plasticity
Trains of repeated TMS pulses (rTMS) at various stimulation frequencies and patterns can induce a lasting modification of activity in the targeted brain region which can outlast the effects of the stimulation itself. Such lasting changes presumably represent alterations in plasticity mechanisms. One rTMS protocol, known as theta burst stimulation (TBS), mimics paradigms used to induce LTP and LTD in animal models (Huang et al. 2005). TBS consists of bursts of 3 pulses at 50 Hz repeated at intervals of 200 ms. When applied to the motor cortex, this protocol can induce robust and consistent responses across subjects, and lead to enhancement or depression of MEP amplitudes depending on stimulation parameters. After TBS is applied to the motor cortex in an intermittent fashion (iTBS), TMS-evoked potentials show increased amplitude. Conversely, after TBS is applied continuously (cTBS), TMS-evoked potentials show a marked decrease in amplitude.
TBS is safe if appropriate safety guidelines are followed (Oberman et al. 2011) and various lines of evidence support the notion that such TMS protocols can indeed provide a measure of synaptic plasticity in humans (Cárdenas-Morales et al. 2010). Physiologic and pharmacologic studies of TBS in humans show involvement of glutamatergic and GABAergic mediators (Huang et al. 2008; Stagg et al. 2009), and the effects and their time-course are consistent with LTP- or LTD-phenomena. Furthermore, Tokay et al. (2009) demonstrated that high-frequency magnetic stimulation induces LTP in rat hippocampal slices and that stimulation-induced LTP was prevented by the presence of a selective N-methyl-d-aspartate receptor (NMDAR) blocker. These findings further suggested that presynaptic involvement of the induced LTP may be excluded based on the lack of changes in paired-pulse ratio and the afferent fiber volleys. However, studies to date are limited and further understanding of the mechanistic effects of TMS is of obvious and crucial importance.
Combining TMS with EEG and fMRI
Real-time integration of single- and paired-pulse TMS with EEG can provide information about local cortical reactivity and distributed network dynamics in health and in disease (Thut et al. 2005; Thut and Pascual-Leone 2010). Contrasting such measures before and serial following various rTMS protocols (for example TBS) can provide insights into LTP- and LTD-like cortical plasticity and network dynamics in humans in vivo across cortical region.
Using EEG as the outcome measure enables the implementation of a variety of sophisticated techniques to identify and characterize connectivity networks between different brain regions (Sameshima and Baccala 1999; Kaminski et al. 2001; Stam and van Dijk 2002; Kramer et al. 2009). Graph theoretic techniques can be utilized to derive an understanding of some of the important properties of these networks (Bullmore and Sporns 2009). Several studies have shown that these network architectures vary during normal aging (Micheloyannis et al. 2009; Boersma et al. 2011), and may be abnormal in patients with a variety of neuropsychiatric conditions including schizophrenia (Micheloyannis et al. 2006; Medkour et al. 2010), depression (Fingelkurts et al. 2007; Leistedt et al. 2009), ASD (Murias et al. 2007), epilepsy (Bettus et al. 2008; van Dellen et al. 2009; Douw et al. 2010), TBI (Cao and Slobounov 2010; Sponheim et al. 2011), and AD (Stam et al. 2007; Jelles et al. 2008; de Haan et al. 2009). The combination of such EEG approaches with TMS allows clinicians and researchers to fully characterize dynamic properties of local and distributed brain cortical activity by offering a behaviorally independent input of quantifiable and parametrically scalable magnitude applicable across ages, individuals, and neural states.
Esser et al. (2006) demonstrated that after trains of high-frequency (5 Hz) rTMS, EEG responses to single TMS pulses—TMS-evoked potentials (TEP)—are significantly potentiated. Conversely, amplitude of TEPs is significantly decreased after low-frequency (0.6 Hz) rTMS (Van der Werf and Paus 2006). These findings are consistent with the differential effects of high and low frequency rTMS on cortical excitability and plasticity (Valero-Cabre et al. 2007). A number of studies have shown that the cortical responses to various sensory stimuli are also altered by different rTMS protocols; for example, Ishikawa et al. (2007) demonstrated that the amplitude of somato-sensory evoked potentials is altered after TBS, with the effects persisting for up to 50 min. Repetitive TMS protocols also induce quantifiable changes in various EEG metrics of intrinsic cortical activity, such as changes in EEG power in various frequency bands (Okamura et al. 2001; Schutter et al. 2001; Griskova et al. 2007, 2009; Brignani et al. 2008; Fuggetta et al. 2009), or synchrony and coherence between different cortical regions (Jing and Takigawa 2000; Strens et al. 2002; Oliviero et al. 2003; Schindler et al. 2008). Julkunen et al. (2008, 2011) assessed navigated TMS-evoked EEG responses in patients with AD, and showed prominent changes in functional connectivity (and reactivity) in AD subjects. In particular, TMS-evoked response at 30-50 ms decreased significantly in AD patients over widespread brain regions, thus suggesting dysfunction of a large-scale sensorimotor network or less network synchronization in patients with AD.
TMS-EEG local cortical and network plasticity measures can be further elucidated by obtaining simultaneous neuroimaging with fMRI. Functional MRI allows for the investigation of whole brain response to and recovery from patterned stimulation. For example, combining TMS with resting-state fMRI allows clinicians and researchers to experimentally manipulate local cortical responses with patterned stimulation and observing change in network responses (Halko et al. 2010).
Direct Evidence of Changing Plasticity Mechanisms in Humans Across the Life-Span
Direct evidence of brain plasticity changes in humans throughout the lifespan in health and disease can be measured via real-time combination of TMS with EMG, EEG, and/or fMRI (Fig. 4). Moreover, measures of TMS before and serially following cTBS or iTBS can provide measurements of LTP- or LTD-like plasticity.
Preliminary experimental evidence reveals a decline in corticomotor plasticity across the human lifespan (Freitas et al. 2011a, b). In a cross-sectional study of 36 healthy volunteers throughout the adult age-span ranging from 19 to 81 years, we found the duration and magnitude of corticospinal excitability modulation by cTBS was inversely and significantly correlated with age (Fig. 5). These data provide direct experimental evidence that, in humans, LTD-like plasticity becomes increasingly less efficient with advancing age. Such decreasing plasticity in the motor cortex with advancing age may be associated with the decrement of hand motor function (e.g., longer reaction time) observed during normal aging in both men and women (e.g., Carmeli et al. 2003) and to the age-related deficits in motor learning (e.g., Brown et al. 2009). TMS studies using EEG instead of EMG measures will enable assessment of non-motor cortical regions and provide direct experimental assessments of age-related changes in mechanisms of plasticity.
Fig. 5.
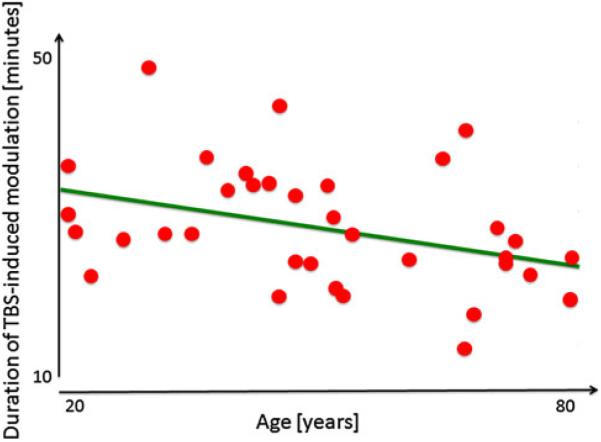
Modified from Freitas et al. (2011a, b). Correlation between age and duration of the modulation of cortico-spinal responses to TMS after cTBS in cognitively intact, healthy adults ranging in age from 19 to 81 years. This measure of plasticity shows a significant decrease with age
Advantage of Translation of Method to Animals
Through the use of TMS in controlled animal models of healthy and diseased states, the underlying mechanisms and the role of TMS in evaluating neural plasticity may be more thoroughly defined. Animal models allow for more precise pharmacologic manipulation, improving our ability to correlate specific neurochemical changes with TMS effects. In addition, the use of rodent models provides for the potential to evaluate the role of TMS under specific genetic backgrounds, i.e., transgenic mice. In turn, TMS may also improve animal studies by allowing a noninvasive quantitative neurophysiologic measure that can be obtained in real-time and evaluated serially in longitudinal studies within the same subjects. In translational animal research, such as in neuroprotective stroke research, failure to bring the successes of animal studies to clinical fruition may be, in part, the result of different outcome markers between animal and human investigations (Gladstone et al. 2002). TMS measures of neuronal excitability and plasticity may serve as a unifying outcome measure amongst animal and human research to better facilitate clinical translation.
The use of TMS in animal studies has been met with some difficulty. Namely, conventional TMS coils, designed for human application, generate relatively broad volumes of electrical current which may activate multiple cortical and subcortical regions when applied to the rat. As such, the focality of TMS in animals is brought into question and obscures the origin and interpretation of resultant TMS metrics. Furthermore, TMS animal studies require anesthesia to both minimize animal discomfort and suppress spontaneous muscle activation. The presence of neuro-chemically-active anesthetics may further obscure TMS findings (Luft et al. 2001; Rotenberg et al. 2010).
Yet, rat TMS studies have made important advancements in recent years. TMS in rats and mice has been shown to elicit stable motor evoked potentials (MEPs) with similar recruitment curves as seen in humans (Rotenberg et al. 2010). In addition, when placed over the scalp in an eccentric orientation, TMS is able to evoke MEPs lateralized to the contralateral limb. These findings suggest a resolution of rodent TMS to at least one hemisphere. Further, the longer latency of TMS-evoked MEPs when compared with MEPs from cervical electrical stimulation, as well as the similarities in latency and morphology with MEPs from focal cortical electrical stimulation, favors a presumed cortical origin of TMS (Luft et al. 2001, 2002; Rotenberg et al. 2010; Schlag et al. 2001; Zandieh et al. 2003). Studies have also begun to demonstrate the role of animal TMS as a marker of neuronal plasticity. Luft and colleagues demonstrated the effect of motor learning, simulated by somato-sensory afferent stimulation, on the enhancement of cortical motor excitability (Luft et al. 2002). More recent research has translated paired-pulse TMS techniques to murine models, providing measures of cortical inhibition as an indicator of neuronal plasticity (Vahabzadeh-Hagh et al. 2010). TMS-EEG studies in animal models can also parallel those conducted in humans (Ives et al. 2006). Although many variables remain, rodent TMS research is proving to be a viable model through which many TMS questions may be further investigated.
Individual Differences in Plasticity
TMS-based experimental methods to examine cortical plasticity mechanisms in humans enable longitudinal studies that are critically needed to further examine brain changes across the lifespan. Longitudinal studies are particularly critical as individual differences in brain plasticity are likely quite large, and understanding the factors that condition such individual differences will offer unique and novel targets to promote individual brain health and wellbeing across the lifespan.
Important factors to consider that likely contribute to differences in mechanisms of plasticity include genetic and epigenetic mechanisms (e.g., polymorphisms, genetic expression), hormonal factors (e.g., gender, menstrual cycle), impact of morbidities (e.g. diabetes, cancer, or infections), and lifetime experiences (e.g., traumatic brain injury, exposure to toxins, stress, sleep deprivation, substance abuse, poor cognitive reserve, poor nutrition, sedentariness, etc.). Therefore, dissimilar ‘starting points’ for different individuals and distinct lifelong ‘slopes of change’ in plasticity might be postulated (Fig. 3). Beyond that, Fig. 6 schematically illustrates how different genetic and environmental factors may impact each individual’s brain plasticity ‘slope’ throughout the age-span, inducing distributed neural network responses that may prove adaptive or maladaptive, and might determine age-related cognitive abilities and even the risk of late-life dementia.
Fig. 6.
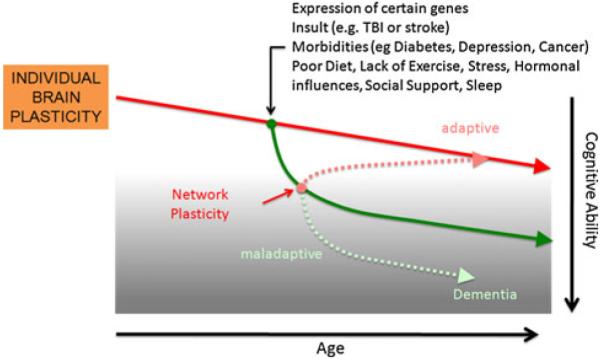
Schematic representation of the influence of genetic or environmental impacts on brain plasticity. Alteration of local plasticity will trigger secondary adaptive responses across diffuse neural networks that may prove ultimately adaptive or maladaptive for the individual. Depending upon the amount and scope of such secondary responses, initial insult effects may be alleviated or heightened
The Role of Genetic Differences
A number of genetic factors have been identified that relate to regulation of human brain plasticity (Pearson-Fuhrhop et al. 2009). For example, the brain derived neurotrophic factor (BDNF) gene plays an important role in neuroplasticity. The BDNF gene contains a functional single-nucleotide polymorphism (rs6265) that results in the substitution of valine to methionine (Val66Met), leading to reduced mature BDNF expression. The BDNF Val66Met polymorphism has been shown to differentially modulate human cortical plasticity and the response to training (Kleim et al. 2006), brain stimulation (Cheeran et al. 2008) and motor learning (Fritsch et al. 2010). Thus, the BDNF example illustrates the fact that individuals with a certain genetic predisposition may show a different response to interventions that modulate brain plasticity. Furthermore, the BDNF Val66Met polymorphism has also been shown to distinctively influence both brain structure and function by exerting a more global, rather than local, effect. For instance, brain allometry shows that BDNF 66Met carriers display larger total lobar volumes, while regional differences might only reflect the proportion of the volumes occupied by different brain regions relative to the total lobar volumes (Toro et al. 2009). Similarly, the BDNF Val66Met polymorphism appears to differently influence whole-brain—but not regional-activation patterns (McHughen et al. 2010). The functional relevance of such effects remains unclear. Yet, it is conceivable that the presence of the Val66Met polymorphism may have distinct repercussions on the mechanisms of plasticity during normal development and aging. Accordingly, individuals carrying this polymorphism may have distinct starting points and plasticity slopes throughout the age-span. Such differences may, eventually, contribute to late-life neuro-degeneration and dementia. Indeed, it has been suggested that the presence of the BDNF Val66Met polymorphism renders higher susceptibility to AD, although conflicting results have been found (e.g., Desai et al. 2005; Matsushita et al. 2005; Saarela et al. 2006; Tsai et al. 2006; Fehér et al. 2009). Recently, the low-activity Met66 allele was shown to be an additional risk factor for rapid disease progression during the preclinical period of AD (Hashimoto et al. 2009) and may also constitute a risk factor for the development of psychotic symptoms in AD (Pivac et al. 2010).
Another common genetic mutation that illustrates the impact of genetic polymorphisms on distinct aspects of brain plasticity is the apolipoprotein E (APOE) susceptibility gene located on chromosome 19 and its ε4 allele, which is thought to raise the risk of AD (e.g., Corder et al. 1993) in a dose-dependent manner (Bertram et al. 2007; Bertram and Tanzi 2009). Wolk and Dickerson (2010) have shown that the presence of APOE-ε4 differentially influences large-scale brain networks and this contributes to the clinical phenotype of AD. Thus, it seems that the presence of APOE-ε4 may influence network plasticity and the slope of plasticity throughout the lifespan.
In addition, mutations in a number of other genes involved in synaptic plasticity have been shown to confer an increased risk for certain mental disorders that are associated with changes in cortical local and network plasticity. Examples can be found in candidate genes for autism spectrum disorders (ASD), including Neuroligin 3 and 4, which are implicated in synaptogenesis (Jamain et al. 2003), CNTNAP2, which contributes to neural migration (Durand et al. 2007), SHANK3, which encodes a protein involved in dendritic development (Durand et al. 2007), and, more recently, c3orf58, NHE9, and PCDH10, also critically involved in synaptic development and plasticity (Morrow et al. 2008).
The interface between genes and environment may translate into differential genetic expression between individuals and also throughout the lifespan. For instance, Lu et al. (2004) used transcriptional profiling to identify a set of genes whose expression decreases with increasing age; among the genes most affected were those involved with synaptic plasticity, including the gene coding for a subunit of the Glu R1 AMPA receptor and genes coding for subunits of the NMDA and GABAA receptors. Additionally, these authors found that genes involved with the synaptic calcium signaling system (which is critical for LTP) were downregulated with advancing age, including calmodulin 1 and CAM kinase IIα. Moreover, as argued by some (Bray 2008), individual differences in gene expression are likely to be responsible for much of the human diversity.
The Role of Environmental and Other Personal Differences
Beyond genetic factors, and certainly contributing to genetic expression via epigenetic mechanisms or interactions with susceptible genes, environmental factors also contribute to shape differences in the efficacy and functional consequences of plasticity mechanisms across the lifespan. This includes such diverse factors as educational experience, family upbringing and other social interactions, cultural background, hormonal factors (e.g., gender), morbidities (e.g. diabetes, cancer, infections, or depression), nutritional factors, exposure to toxins, stress, sleep deprivation, substance abuse, physical exercise, as well as unexpected insults (e.g., traumatic brain injuries). Physical exercise is increasingly recognized as a powerful influence on plasticity across the lifespan (e.g. Voss et al. 2011). Nutrition also has a significant influence on the aging process (Woo 2011), possibly due in part to an impact on brain plasticity slopes across the lifespan. The richness of one’s environment (Volkers and Scherder 2011) as well as cultural influences (Park and Gutchess 2002) impact the aging process, perhaps via modified cortical plasticity mechanisms.
Another important factor potentially contributing to inter-individual variability in efficacy of plasticity and its lifespan slope is intelligence. Indeed, brain plasticity and intellectual ability both seem to be partly driven by shared genes (Brans et al. 2010). In their longitudinal study of adult twins, Brans et al. (2010) showed that increased thickening in the medial temporal lobe and attenuated thinning of the frontal cortex, until age 35, were associated with higher IQ scores and further showed that genes implicated in cortical thickness overlapped with those involved in the level of intelligence. This may be related to early life experience and contribute to the construct of cognitive reserve.
Epidemiological studies suggest that early-life abuse and neglect increase susceptibility to psychopathology. Reviews of large-scale research studies conclude that there is clearly an excess of early traumatic events in individuals who experience psychosis, schizophrenia, and other psychiatric conditions in adulthood (Larkin and Read 2008; Read et al. 2005). Indeed, the quality of each individual’s social environment can have profound influences on the development and activity of neural systems, with repercussions on a variety of behavioral and physiological responses (Curley et al. 2011). If so, changes in brain plasticity in individuals living in dysfunctional environments may be distinct from the changes of those in protective and supportive ones.
At the opposite end of the lifespan, one might consider the construct of cognitive reserve (Stern 2003, 2009), which allows cognitive function to be maintained—or minimally disrupted—in older age and can enable individuals to sustain a greater amount of neuropathological insults before they manifest signs and symptoms of cognitive decline. A number of lifestyle factors including education, work complexity, social network, and leisure activities seem to contribute to this reserve (Fratiglioni and Wang 2007; Scarmeas and Stern 2003). For instance, highly-educated individuals, even those with neuropathologic Alzheimer’s disease (AD), seem to have a reduced risk of clinical manifestation of dementia (Roe et al. 2007), perhaps related to greater white matter integrity (Teipel et al. 2010). In mild AD patients with the same degree of cognitive deterioration, highly educated patients have been found to have more advanced pathological and functional brain changes (Kemppainen et al. 2008). This suggests that the clinical manifestation of advancing AD pathology is delayed in individuals with higher educational attainment (Stern et al. 1992; Alexander et al. 1997), presumably due to more efficient compensatory, plasticity-based mechanisms against the underlying pathology. In the proposed model of plasticity across the lifestyle, adaptive network plasticity might represent the neurobiological substrate of cognitive reserve.
Link to Pathology
Acquired brain insults, such as traumatic brain injury, or certain systemic diseases like diabetes, depression, or cancer may impact the capacity for plasticity and the slopes of change in plasticity thereafter. For instance, former athletes with a history of sports concussion show altered cognitive and physiological responses more than three decades post-concussion (De Beaumont et al. 2009). These findings suggest that the efficiency of plasticity mechanisms may become impaired and the slope of decline in brain plasticity may be more pronounced in those having sustained a sports concussion. Indeed, recent evidence demonstrates reorganization of functional connectivity after acquired brain injury (Castellanos et al. 2010) that, while initially adaptive, may ultimately limit compensatory adaptations for age-related changes in brain plasticity mechanisms.
In addition to such instances, though, dissimilar starting points and different slopes of change in brain plasticity across the lifespan may represent the proximal cause for neuropsychiatric diseases, either during early developmental or aging years. Consistent with such notion, early findings suggest that the mechanisms of plasticity are aberrantly altered in neurodevelopmental and neurodegenerative disorders such as ASD and AD, respectively.
Autism Spectrum Disorders (ASD)
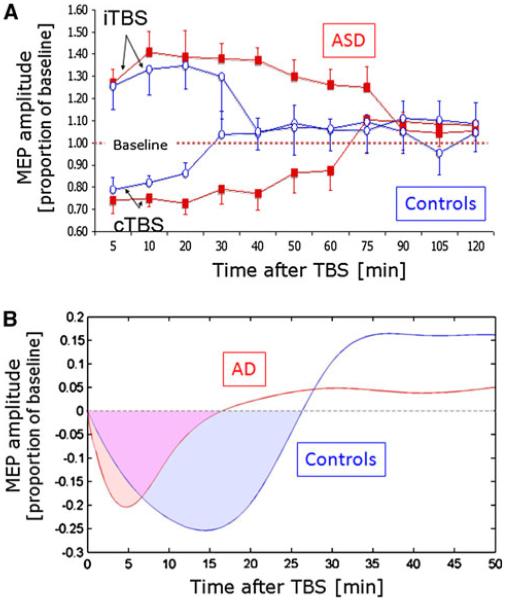
In a recent study, we have found compelling direct evidence for abnormal plasticity in individuals with fragile X and ASD (Oberman et al. 2010). As compared with neurotypical, age-, gender- and IQ-matched controls, adult subjects with ASD showed greater and longer-lasting modulation of MEPs following TBS (Fig. 7a). The return to baseline latency following TBS was on average between 80 and 90 min in the ASD group compared to 25–30 min in the control group. Additionally, the return to baseline latency was significantly correlated with performance on behavioral motor learning tasks. This finding was replicated in a second cohort of individuals and suggests that ASD is associated with a hyperplastic state.
Fig. 7.
Alterations of TMS-based measures of plasticity in nervous system pathology exemplified by autism spectrum disorder (ASD; a) and Alzheimer’s diseases (AD; b). a As compared with age-, gender- and IQ-matched controls, subjects with ASD show stronger and longer-lasting modulatory responses to continuous theta burst stimulation suggesting abnormal, hyper-plastic mechanisms. This is true for both, LTP- and LTD-like plasticity. b As compared with age-, gender- and IQ-matched controls, subjects with mild AD show weaker and shorter-lasting modulatory responses to continuous theta burst stimulation suggesting abnormal, hypo-plastic mechanisms
Alzheimer’s Disease (AD)
Recently, we collected proof-of-principle data supporting the existence of aberrant mechanisms of plasticity in patients in early stages of AD as compared with cognitively normal, age-matched elderly individuals. Results show that the duration and magnitude of the modulation of corticospinal excitability by cTBS is significantly shorter in individuals with early AD than in controls (Fig. 7b; unpublished data). This finding suggests that a hypoplastic state may underlay the cognitive and behavioral decline in AD. Our findings corroborate and extend those of others (for review, Freitas et al. 2011b). For example, Battaglia et al. (2007) studied neocortical (motor) LTP-like plasticity in AD and healthy individuals using a paired associative stimulation (PAS) protocol and found it to be significantly reduced in AD patients. Furthermore, Inghilleri et al. (2006) tested the effects of cortical motor modulation induced by suprathreshold high-frequency (5 Hz) rTMS and found the amplitude of MEPs progressively decreased in patients while increasing in controls. This suggests impaired LTP-like plasticity.
Conclusions
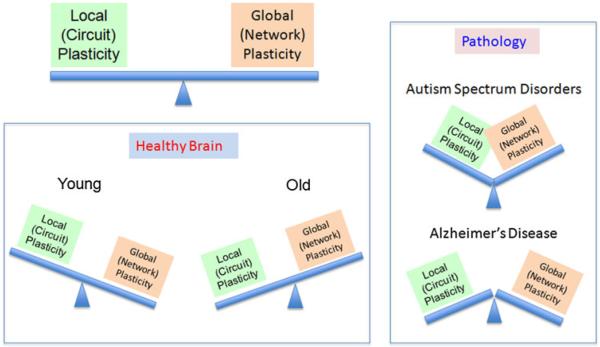
The integrity of the neurophysiological mechanisms underlying brain plasticity play an important role throughout the lifespan—during developmental and aging years—in health and also in disease. In health, local cortical and network plasticity might keep a fine tuned balance which optimizes functionality. The mechanisms of plasticity, and thus the balance between local and network plasticity, change over the lifespan (Fig. 8). In the young brain, local cortical plasticity appears to be higher, and cross-sectional studies suggest that it decays with age. Longitudinal studies are critically needed as individual factors are presumed to be critical. On the other hand, in the older healthy brain, network dynamics appears to incorporate non-functionally connected regions and may provide a critical neurobiological substrate to cognitive reserve. Hypo- or hyperplastic mechanisms may set the stage for abnormal circuit development, network compensation, and, accordingly, pathological behavior. Mismatched balance between local cortical plasticity and network plasticity is also likely to be an important contributor to pathophysiology in developmental (e.g., ASD) and neurodegenerative diseases (e.g., AD). Likewise, a progressive dampening in the efficiency of plasticity mechanisms that may be induced by a number of genetic, lifestyle, and environmental factors may trigger for a cascade of events potentially leading to cognitive deterioration and even dementia in the absence of successful network compensatory strategies.
Fig. 8.
Schematic representation of the hypothesized balance between local and network plasticity, its change across the lifespan, and its alterations by pathology
Empirically measuring plasticity may provide critical predictors and early biomarkers of disease. Additionally, in the setting of longitudinal studies, plasticity measures, as those proposed here, may allow for the assessment of the impact of genetic, biological, and environmental factors on changes in brain plasticity throughout the lifespan. Ultimately, modulating brain plasticity may minimize, delay or even prevent symptoms of brain disease. Such measures of cortical brain plasticity may thus serve to guide plasticity-based therapeutic interventions aimed at promoting brain health and cognitive function across the age-span.
Acknowledgments
Work on this study was supported by grants from the National Center for Research Resources: Harvard-Thorndike General Clinical Research Center at BIDMC (NCRR MO1 RR01032) and Harvard Clinical and Translational Science Center (UL1 RR025758), NIH grant K24 RR018875, Center for Integration of Medicine and Innovative Technology (CIMIT), Neuronix and Nexstim to APL. CF was supported by a post-doctoral grant from the Foundation for Science and Technology, Portugal (SFRH/BPD/ 66846/2009), co-funded by the European Social Fund. LO was supported by NIH fellowship F32MH080493 and 1KL2RR025757-01. APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and is an inventor on patents and patent applications related to noninvasive brain stimulation and the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
Footnotes
This is one of several papers published together in Brain Topography on the “Special Issue: Brain Imaging across the Lifespan”.
Conflict of interest None.
Contributor Information
Alvaro Pascual-Leone, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA; Institut Universitari de Neurorehabilitació Guttmann, Universidad Autónoma de Barcelona, Barcelona, Spain.
Catarina Freitas, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Lindsay Oberman, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Jared C. Horvath, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA
Mark Halko, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Mark Eldaief, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Shahid Bashir, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Marine Vernet, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Mouhshin Shafi, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA; Department of Neurology, Massachusetts General Hospital; Partner’s Neurology Program, Harvard Medical School, Boston, MA, USA.
Brandon Westover, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA.
Andrew M. Vahabzadeh-Hagh, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, USA; Department of Neurology, Children’s Hospital, Boston, Harvard Medical School, Boston, MA, USA
Alexander Rotenberg, Department of Neurology, Children’s Hospital, Boston, Harvard Medical School, Boston, MA, USA.
References
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154(2):165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, et al. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer’s disease. Hum Mol Genet. 2009;18(R2):R137–R145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81(1):58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Introduction. Long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma M, Smit DJ, de Bie HM, Van Baal GC, Boomsma DI, de Geus EJ, Delemarre-van de Waal HA, Stam CJ. Network analysis of resting state EEG in the developing young brain: structure comes with maturation. Hum Brain Mapp. 2011;32(3):413–425. doi: 10.1002/hbm.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, Kahn RS, Schnack HG, van Baal GC, Posthuma D, van Haren NE, Lepage C, Lerch JP, Collins DL, Evans AC, Boomsma DI, Hulshoff Pol HE. Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci. 2010;30(16):5519–5524. doi: 10.1523/JNEUROSCI.5841-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NJ. Gene expression in the etiology of schizophrenia. Schizophr Bull. 2008;34(3):412–418. doi: 10.1093/schbul/sbn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp. 2008;29(5):603–612. doi: 10.1002/hbm.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. 2009;4(8):e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;24:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(4):312. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA, and sLORETA analyses of EEG signals. IEEE Trans Neural Syst Rehabil Eng. 2010;18(1):11–19. doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas-Morales L, Nowak DA, Kammer T, Wolf RC, SchönfeldtLecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22(4):294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci. 2003;58(2):146–152. doi: 10.1093/gerona/58.2.m146. [DOI] [PubMed] [Google Scholar]
- Castellanos NP, Paúl N, Ordóñez VE, Demuynck O, Bajo R, Campo P, Bilbao A, Ortiz T, del-Pozo F, Maestú F. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain. 2010;133(Pt. 8):2365–2381. doi: 10.1093/brain/awq174. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Mashoodh R, Champagne FA. Social influences on neurobiology and behavior: epigenetic effects during development. Psychoneuroendocrinology. 2011;36(3):352–371. doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cort. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132(Pt. 3):695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- de Haan W, Pijnenburg YA, Strijers RL, van der Made Y, van der Flier WM, Scheltens P, Stam CJ. Functional neural network analysis in frontotemporal dementia and Alzheimer’s disease using EEG and graph theory. BMC Neurosci. 2009;10:101. doi: 10.1186/1471-2202-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Nebes R, DeKosky ST, Kamboh MI. Investigation of the effect of brain-derived neurotrophic factor (BDNF) polymorphisms on the risk of late-onset alzheimer’s disease (AD) and quantitative measures of AD progression. Neurosci Lett. 2005;379(3):229–234. doi: 10.1016/j.neulet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Douw L, van Dellen E, de Groot M, Heimans JJ, Klein M, Stam CJ, Reijneveld JC. Epilepsy is related to theta band brain connectivity and network topology in brain tumor patients. BMC Neurosci. 2010;11:103. doi: 10.1186/1471-2202-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Rogé B, Héron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):90–98. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69(1):86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fehér A, Juhász A, Rimanóczy A, Kálmán J, Janka Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord. 2009;23(3):224–228. doi: 10.1097/WAD.0b013e318199dd7d. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007;28(3):247–261. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhoyd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19(9):2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, Bashir S, Vernet M, Peña-Gómez C, Pascual-Leone A. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011a;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Mondragón-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: Systematic review and perspectives for the future. Exp Gerontol. 2011b;46(8):611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggetta G, Rizzo S, Pobric G, Lavidor M, Walsh V. Functional representation of living and nonliving domains across the cerebral hemispheres: a combined event-related potential/transcranial magnetic stimulation study. J Cogn Neurosci. 2009;21(2):403–414. doi: 10.1162/jocn.2008.21030. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Esposito M. Top-down modulation and normal aging. In: DeLeon MJ, Snider DA, Federoff H, editors. Imaging and the Aging Brain. Ann NY Acad Sci. New York: 2007. pp. 67–83. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62(4):510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griskova I, Ruksenas O, Dapsys K, Herpertz S, Höppner J. The effects of 10 Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neurosci Lett. 2007;419(2):162–167. doi: 10.1016/j.neulet.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Grossheinrich N, Rau A, Pogarell O, Hennig-Fast K, Reinl M, Karch S, Dieler A, Leicht G, Mulert C, Sterr A, Padberg F. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry. 2009;65(9):778–784. doi: 10.1016/j.biopsych.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Halko M, Eldaief MC, Horvath JC, Pascual-Leone A. Combining transcranial magnetic stimulation and fMRI to examine the default mode network. J Vis Exp. 2010;46 doi: 10.3791/2271. doi:10.3791/2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Hirata Y, Asada T, Yamashita F, Nemoto K, Mori T, Moriguchi Y, Kunugi H, Arima K, Ohnishi T. Effect of the brain-derived neurotrophic factor and the apolipoprotein E polymorphisms on disease progression in preclinical Alzheimer’s disease. Genes Brain Behav. 2009;8(1):43–52. doi: 10.1111/j.1601-183X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Perez JM, Forrow L, Fregni F, Pascual-Leone A. Transcranial magnetic stimulation: a historical review and future prognosis of therapeutically relevant ethical concerns. J Med Ethics. 2011;37(3):137–143. doi: 10.1136/jme.2010.039966. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18(3):563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Frasca V, Scaldaferri N, Gilio F, Santini M, et al. Altered response to rTMS in patients with Alzheimer’s disease. Clin Neurophysiol. 2006;117:103–109. doi: 10.1016/j.clinph.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effects of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol. 2007;118(5):1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clin Neurophysiol. 2006;117(8):1870–1875. doi: 10.1016/j.clinph.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelles B, Scheltens P, van der Flier WM, Jonkman EJ, da Silva FH, Stam CJ. Global dynamical analysis of the EEG in Alzheimer’s disease: frequency-specific changes in functional interactions. Clin Neurophysiol. 2008;119(4):837–841. doi: 10.1016/j.clinph.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Kleibeuker SW, Rombouts SA, Crone EA. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Dev Sci. 2010;14(4):713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Westeren-Punnonen S, Pirinen E, Soininen H, Kononen M, et al. Navigated TMS combined with EEG in mild cognitive impairment and Alzheimer’s disease: a pilot study. J Neurosci Methods. 2008;172:270–276. doi: 10.1016/j.jneumeth.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Könönen M, Pääkkönen A, Karhu J, Soininen H. Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer’s disease. Int J Alzheimers Dis. 2011:654794. doi: 10.4061/2011/654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85:145–157. doi: 10.1007/s004220000235. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res. 2006;68(Suppl 1):S5–S20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63(1):112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde ALW, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter. Neuroimage. 2010;15(1):280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Cash SS, Kolaczyk ED. Network inference with confidence from multivariate time series. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:061916. doi: 10.1103/PhysRevE.79.061916. [DOI] [PubMed] [Google Scholar]
- Larkin W, Read J. Childhood trauma and psychosis: evidence, pathways, and implications. J Postgrad Med. 2008;54(4):287–293. doi: 10.4103/0022-3859.41437. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Leistedt SJ, Coumans N, Dumont M, Lanquart JP, Stam CJ, Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp. 2009;30(7):2207–2219. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Cohen LG, Thakor NV, Hanley DF. Transcranial magnetic stimulation in the rat. Exp Brain Res. 2001;140(1):112–121. doi: 10.1007/s002210100805. [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, Cohen LG. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142(4):562–569. doi: 10.1007/s00221-001-0952-1. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Arai H, Matsui T, Yuzuriha T, Urakami K, Masaki T, Higuchi S. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer’s disease. J Neural Transm. 2005;112(5):703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20(5):1254–1262. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkour T, Walden AT, Burgess AP, Strelets VB. Brain connectivity in positive and negative syndrome schizophrenia. Neurosci. 2010;169(4):1779–1788. doi: 10.1016/j.neuroscience.2010.05.060. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–66. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Vourkas M, Tsirka V, Karakonstantaki E, Kanatsouli K, Stam CJ. The influence of ageing on complex brain networks: a graph theoretical analysis. Hum Brain Mapp. 2009;30(1):200–208. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, Picker J, Rotenberg A, Pascual-Leone A. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2010;2:26. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J Clin Neurophysiol. 2011 doi: 10.1097/WNP.0b013e318205135f. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Jing H, Takigawa M. EEG modification induced by repetitive transcranial magnetic stimulation. J Clin Neurophysiol. 2001;18(4):318–325. doi: 10.1097/00004691-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Strens LH, Di Lazzaro V, Tonali PA, Brown P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res. 2003;49:107–113. doi: 10.1007/s00221-002-1344-x. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Aging, cognition, and culture: a neuroscientific perspective. Neurosci Biobehav Rev. 2002;26(7):859–867. doi: 10.1016/s0149-7634(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev of Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil. 2009;16(4):282–299. doi: 10.1310/tsr1604-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivac N, Nikolac M, Nedic G, Mustapic M, Borovecki F, Hajnsek S, et al. Brain derived neurotrophic factor Val66Met polymorphism and psychotic symptoms in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010;35(2):356–362. doi: 10.1016/j.pnpbp.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe—a study of fiveyear change. Neurol. 2004;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis, and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Pschol Sci Soc Sci. 2010;65(4):405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimers disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68(3):223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418(2):221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Muller PA, Vahabzadeh-Hagh AM, Navarro X, López-Vales R, Pascual-Leone A, Jensen F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela MS, Lehtimaki T, Rinne JO, Huhtala H, Rontu R, Hervonen A, Roytta M, Ahonen JP, Mattila KM. No association between the brain-derived neurotrophic factor 196G>A or 270C>T polymorphisms and Alzheimer’s or Parkinson’s disease. Folia Neuropathol. 2006;44(1):12–16. [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischi B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, Van Der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameshima K, Baccala LA. Using partial directed coherence to describe neuronal ensemble interactions. J Neurosci Methods. 1999;94:93–103. doi: 10.1016/s0165-0270(99)00128-4. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60(7):989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Nyffeler T, Wiest R, Hauf M, Mathis J, Hess ChW, et al. Theta burst transcranial magnetic stimulation is associated with increased EEG synchronization in the stimulated relative to unstimulated cerebral hemisphere. Neurosci Lett. 2008;436:31–34. doi: 10.1016/j.neulet.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Schlag MG, Hopf R, Redl H. Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in the rat. Somatosens Mot Res. 2001;18(2):106–116. doi: 10.1080/135578501012006219. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d’Alfonso AA, Postma A, de Haan EH. Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport. 2001;12(3):445–447. doi: 10.1097/00001756-200103050-00005. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, Lim KO. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage. 2011;54(Suppl 1):S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101(6):2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van Dijk BW. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D: Nonlinear Phenomena. 2002;163(3):236–251. [Google Scholar]
- Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Eur J Clin Invest. 2007;32(Suppl 1):79–83. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol. 2003;25(5):589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32(3):371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, Herpertz SC, Möller HJ, Hampel H. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49(3):2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, DeZubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW. Tracking Alzheimer’s disease. In: DeLeon MJ, Snider DA, Federoff H, editors. Imaging and the aging brain. Ann NY Acad Sci. New York: 2007. pp. 183–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Tokay T, Holl N, Kirschstein T, Zschorlich V, Köhling R. High-frequency magnetic stimulation induces long-term potentiation in rat hippocampal slices. Neurosci Lett. 2009;461(2):150–154. doi: 10.1016/j.neulet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Toro R, Chupin M, Garnero L, Leonard G, Perron M, Pike B, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Brain volumes and Val66Met polymorphism of the BDNF gene: local or global effects? Brain Struct Funct. 2009;213(6):501–509. doi: 10.1007/s00429-009-0203-y. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liu HC, Liu TY, Liou YJ. The brain-derived neurotrophic factor gene as a possible susceptibility candidate for Alzheimer’s disease in a chinese population. Dement Geriatr Cogn Disord. 2006;21(3):139–143. doi: 10.1159/000090673. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh-Hagh AM, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Measures of cortical inhibition by pairedpulse transcranial magnetic stimulation in anesthetized rats. J Neurophysiol. 2010;105(2):615–624. doi: 10.1152/jn.00660.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176(4):603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- van Dellen E, Douw L, Baayen JC, Heimans JJ, Ponten SC, Vandertop WP, Velis DN, Stam CJ, Reijneveld JC. Long-term effects of temporal lobe epilepsy on local neural networks: a graph theoretical analysis of corticography recordings. PLoS One. 2009;4(11):e8081. doi: 10.1371/journal.pone.0008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex: intracortical and cortico-cortical contributions. Exp Brain Res. 2006;175(2):231–245. doi: 10.1007/s00221-006-0551-2. [DOI] [PubMed] [Google Scholar]
- Volkers KM, Scherder EJ. Impoverished environment, cognition, aging and dementia. Rev Neurosci. 2011;22(3):259–266. doi: 10.1515/RNS.2011.026. [DOI] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the lifespan. J Appl Physiol. 2011 doi: 10.1152/japplphysiol.00210.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.013. doi:10.1016/j.neurobiolaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Y, Metzak P, He Y, Woodward TS. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 2010;50(3):862–872. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci USA. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J. Nutritional strategies for successful aging. Med Clin North Am. 2011;95(3):477–493. doi: 10.1016/j.mcna.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Zandieh S, Hopf R, Redl H, Schlag MG. The effects of ketamine/xylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord. 2003;41(1):16–22. doi: 10.1038/sj.sc.3101400. [DOI] [PubMed] [Google Scholar]