Abstract
While the death of retinal ganglion cells in glaucoma is frequently associated with an elevation of intraocular pressure (IOP), the mechanisms connecting the two processes remain unclear. Extracellular ATP is released throughout the body in response to mechanical deformations. We have previously shown that patients with an acute rise in IOP have an elevated concentration of ATP in the anterior chamber. In the present study we ask whether ATP levels remain increased in patients with chronic elevations of IOP. The concentration of ATP in samples of aqueous humor obtained from patients with primary chronic angle-closure glaucoma (PCACG) was compared with that from control cataract patients whose IOP was normal. The mean ATP concentration in aqueous humor was 14-fold higher for PCACG samples than for control. ATP levels were correlated with IOP and the cup-to-disk ratio (C/D ratio). Brief treatment of Timolol, Alphagan, Pilocarpine and/or Azopt did not affect the rise in ATP concentration. In conclusion, sustained elevations in extracellular ATP levels accompany the chronic elevation of IOP in chronic glaucoma. As numerous ocular tissues express purinergic receptors, an increased extracellular ATP may have diverse physiological and pathophysiological effects.
Keywords: Primary chronic angle closure glaucoma, intraocular pressure (IOP), ATP, aqueous humor, human patients, adenosine, mechanosensitivity
1. Introduction
Extracellular ATP acts as a transmitter throughout the body, stimulating both ionotropic P2X and metabotropic P2Y receptors (North 2002, Jacobson and Boeynaems 2010). While ATP is released from both neural and non-neural tissues in response to a range of stimuli (Schwiebert 2001; Corriden and Insel 2010), mechanical perturbation is one of the most effective triggers (Burnstock 1999; Burnstock 2009). Tissue distention (Knight et al. 2002), increased sheer stress (Burnstock 1999) and cell swelling (Mitchell et al. 1998, Li et al. 2011) all trigger ATP release from varied cell types.
Glaucoma is a complex multifactorial disease associated with the death of retinal ganglion cells, and elevated intraocular pressure (IOP) is its most common risk factor (AGIS 2000). The degree of mechanical strain on ocular tissues is a key risk factor for the death or retinal ganglion cells (Norman et al. 2010), although the mechanical effects of strains on posterior structures are better understood than on anterior structures. Mechanosensitive release of ATP has been proposed to contribute to the pathophysiology in glaucoma (Mitchell and Lu 2008; Mitchell et al. 2009). Several observations support a link between acute elevations in IOP and the release of ATP. Elevation of hydrostatic pressure in the bovine eyecup triggers an ATP release associated with pannexin hemichannels that is not linked to cell lysis (Reigada et al., 2008). Much of the damage to retinal ganglion cells in a rat model of acute IOP elevation is mediated by released ATP (Resta et al. 2007). In human patients with primary acute angle-closure glaucoma (PAACG) the ATP levels in aqueous humor are nine-fold higher than in controls (Zhang et al. 2007).
While ATP release is clearly triggered by acute increases in IOP, the vast majority of glaucoma patients experience chronic IOP elevation. We thus asked whether ATP concentrations in the aqueous humor in primary chronic angle-closure glaucoma (PCACG) are similarly elevated and examine key factors in this relationship.
2. Methods
2.1. Patient recruitment and aqueous humor collection
Aqueous humor was collected from 55 patients (56 eyes) at Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, P.R. China. For one patient, samples were taken from both eyes. All patients were first-time visitors with no previous ocular medication experience. Thirty-five were PCACG patients, and twenty were cataract patients undergoing routine cataract surgeries (phaco-emulsification plus intraocular lens implantation). All were members of the Han Chinese ethnic group. The PCACG group comprised 18 males and 17 females; while the control group had 13 males and 7 females. All procedures were performed with informed consent and with the approval of the Sun Yat-sen University Institutional Review Board, and followed the Declaration of Helsinki. The Instutional Review Board of the University of Pennsylvania granted Exempt Status to allow data analysis to be performed remotely.
PCACG patients were diagnosed under standard guidelines (Foster et al., 2002); (1) anatomical abnormality confirmed by slit-lamp biomicroscopy and gonioscopy, namely a partially or totally closed anterior chamber angle [Numerical grade = 0 by Shaffer’s grading system (Van Herick et al., 1969)], peripheral anterior synechiae (PAS) > ½ circumference, and a shallow anterior chamber determined by Smith’s method (Smith, 1979); (2) IOP > 21 mmHg measured by Goldman applanation tonometry; (3) some degree of glaucomatous optic atrophy, including cup to disc ratio (C/D) ≥ 0.5, and a visual field defect as determined by Humphrey computerized automated perimetry (Carl Zeiss Meditec). Upon admittance, patients were screened to rule out any disease which would preclude suitability for ocular surgery .and were free of additional ophthalmic complications. The “History” of the disorder was self reported based upon glaucomatous symptoms perceived by the patients (e.g. a feeling of fullness in the eye, blurred vision, eye pain), and is related to the magnitude of elevated IOP, the tolerance of individual patients, the severity of symptoms, as well as the accessibility to medication, and the economic status of patients. Furthermore, due to the latency of chronic angle closure glaucoma as well as atypical symptoms in the early stage, it is not feasible to get a completely accurate record of duration of PCACG. Although these qualifications make the parameter of limited value, this “History” is perhaps the only currently available way to estimate the duration of PCACG in one-time visitors.
The first goal of treatment was to administer drugs to lower IOP. If the IOP levels did not fall to below 21 mm Hg within 72 hrs, surgery was performed to reduce IOP. Patients had IOP measured when initially examined (T0) and after drug treatment was completed (T). Only patients with both T0 and T greater than 21 mmHg were schedules for surgery and sample acquisition. As a group, IOP did not respond to treatment(s) in these patients, with a mean decrease of 1.0 ± 1.2 mm Hg.
Aqueous humor samples were collected by a procedure described previously (Zhang et al. 2007). An anterior chamber paracentesis was performed in order to relieve the IOP before trabeculectomy surgery as this procedure has previously been found effective (Carnahan and Platt 2002; Lam et al. 2002). A 50–100 µL sample of aqueous humor was removed before trabeculectomy surgery. Samples were frozen at −80°C for later analysis. Using the same method, samples of aqueous humor were also obtained from the anterior chamber of 20 routine cataract patients by the same ophthalmic surgeon (Dr. Zhang). The IOP of control cataract patients was within the normal range and patients were free of other ocular or systemic disease. Since cataract patients were not treated with antiglaucomatous drugs, their IOP was measured only once, and T was thereby regarded to be commensurate with T0. In all patients, recorded IOP was the mean of three measurements using Goldmann applanation tonometry. The cup-to-disk ratio (C/D) was determined by a glaucoma specialist (Dr. Zhang) using indirect ophthalmoscopy.
2.2 ATP measurements
Sample ATP concentration was determined by the chemiluminescent luciferin-luciferase reaction based on a previously-published method with some modification (Reigada and Mitchell, 2005; Reigada et al., 2006; Zhang et al., 2007). In brief, 5 ml of distilled water was added to a vial of ATP Assay Mix (Sigma-Aldrich Co., St Louis, MO). Measurements were made by combining 50 µL diluted assay mix with 50 µL sample per well of a white 96-well microplate, and the luminescence intensity read by a microplate luminometer (Orion II Luminometer, Berthold Detection Systems, TN) was then converted into ATP concentration using a standard curve constructed daily. This modified method provided adequate sensitivity to detect ATP in aqueous humor. All samples were obtained under sterile conditions, stored at −80°C and thawed on ice just before measurement to minimize ATP degradation.
2.3. Data analysis
All data are shown as means ± SEM, and n is the number of eyes studied. Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL). Wilcoxon test and Mann-Whitney U test were utilized to compare two sets of paired or unpaired data, respectively; Kruskal Wallis test was applied in comparing three or more sets of data. The relationship between parameters was determined using Spearman’s Rank Order Correlation (SROC). All data at p < 0.05 are defined as significant.
3. Results
3.1. Basic characteristics of PCACG and Control Groups
The clinical manifestations for PCACG and control patients are listed in Table 1. PCACG patients had a mean age of 54.3±1.6 years. The initial IOP levels measured upon admission, T0, ranged from 22 to 56 mmHg with a mean pressure of 35.1±1.6 mmHg (n=36). After being hospitalized, they were treated with multiple drugs for 1–3 days. Surgery and sample collection was performed only on patients who did not significantly respond to IOP-lowering drugs; the IOP of glaucoma patients shortly before surgery, T, was 34.5±1.3 mmHg (Fig. 1A). The average anterior chamber depth in these PCACG patients was 1.75±0.06 mm, largely similar to previous reports (Delmarcelle et al., 1969; Lowe, 1970). All patients had a self-reported history of ocular discomfort and/or decrease in vision lasting an average of 23.5±5.1 months before presentation at the clinic.
Table 1.
Characteristics and Comparison between PCACG and Control Patients
| Case | Male | Female | Age (Y) |
T0 (mmHg) |
T (mmHg) |
C/D Ratio |
Duration (D) |
Numbers | |
|---|---|---|---|---|---|---|---|---|---|
| PCACG | 36 | 18 | 17 | 54.3±1.6 | 35.1±1.6 | 34.5±1.3* | 0.82±0.03 | 1.9±0.2 | 2.0±0.2 |
| Control | 20 | 13 | 7 | 67.7±1.4 | 14.6±0.6 | 14.6±0.6# | 0.33±0.02 | 0 | 0 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
Notes: Case: eyes recruited into this study; Age: Y, years; History: M, months between onset of symptom to hospitalization; T0: initial IOP measured upon hospitalization; T: IOP measured before operation; C/D ratio: the cup to disk ratio; Duration: D, days between start of drug treatment and sample collection; Numbers: numbers of drugs applied before operation. The comparison of the same parameter between PCACG and Control was performed using nonparametric Mann-Whitney U test.
p=0.909 vs. T0 of PCACG Group by Wilcoxon test for the paired, non-normally distributed data.
T of Control group was considered the same as the corresponding T0, because control patients with normal IOP received no treatment.
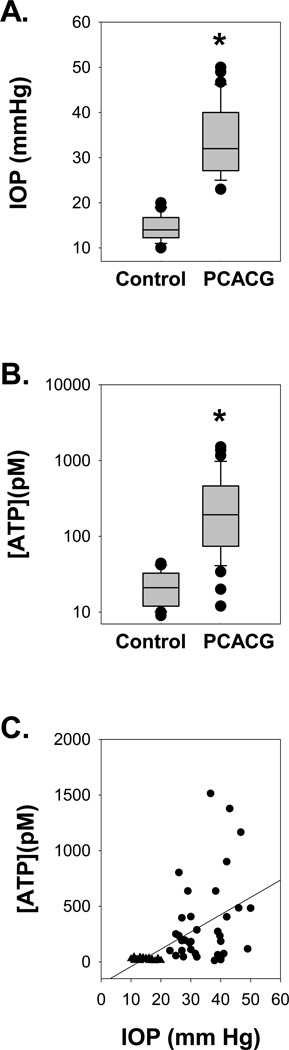
Figure 1. ATP Concentrations in the Aqueous Humor Link to IOP.
A. IOP levels from all Control and PCACG patients. The mean level of IOP from PCACG patients (n=36) measured shortly before surgery was significantly higher than that from Control patients (n=20). Whiskers represent 90th and 10th percentile with upper and lower dots representing all outliers. * p<0.001 vs. control by Mann-Whitney test.
B. The mean level of ATP from PCACG patients (n=36) was 14-fold higher than that from Control patients (n=20). Whiskers represent 90th and 10th percentile with upper and lower dots representing all outliers. * p<0.001 vs. Control by Mann-Whitney U test.
C. The concentration of ATP in aqueous humor was correlated to IOP, with a Spearman’s Rank Order Correlation coefficient of 0.668 (p<0.001). Circles represent data from individual PACCG patients and triangles are from Control.
Control patients had a greater average age of (67.7±1.4 years, n=20, p< 0.001 vs. PCACG group). The mean IOP of control patients was 14.6±0.6 mmHg (Fig. 1A).
3.2. ATP concentration rises with IOP
In control eyes, the concentration of ATP in aqueous humor ranged from 9 to 44 pM with a mean of 23±3 pM (n=20). This is consistent with values reported previously (Zhang et al., 2007). In contrast, ATP concentration in PCACG patients varied broadly from 12 to 1510 pM, with a mean of 342±64 pM (n=36). On average, these values were 14-fold higher than in control (p<0.001, Fig. 1B).
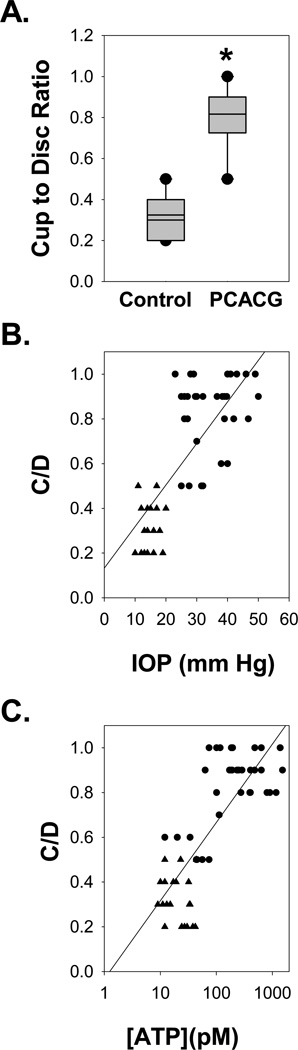
The connection between IOP and extracellular ATP concentration was further probed by correlating the two parameters. ATP levels rose as a function of IOP, with a Spearman Rank Order Correlation (SROC) coefficient of 0.668 (p<0.001; Fig. 1C). The cup to disk ratio (C/D) was also significantly higher in PCACG patients than control, with a mean value of 0.82±0.03 in glaucomatous patients and only 0.33±0.02 in control (p< 0.001, Fig. 2A). The C/D levels were correlated both with IOP measurements (SROC= 0.738, p<0.001; Fig. 2B) and extracellular ATP levels (SROC = 0.762, p<0.001; Fig. 2C).
Figure 2. The Cup to Disc Ratio.
A. The cup to disc ratio (C/D) was significantly higher in PCACG patients (n=36) than Control (n=20; p<0.001; plot definitions as in Fig. 1).
B. The C/D levels changed in proportion to IOP Spearman’s Rank Order Correlation coefficient of 0.738 (p<0.001).
C. The C/D levels also rose with the concentration of ATP; Spearman’s Rank Order Correlation coefficients = 0.762, p<0.001. Circles represent data from individual PCACG patients and triangles are from Control patients.
Collecting aqueous humor samples by anterior chamber paracentesis transiently reduced the volume of the intraocular fluid and decreased the IOP, which potentially induced a mechanical perturbation. To exclude the possibility that the increased ATP concentrations of PCACG were due to collecting distinct amounts of aqueous humor, all of the samples were obtained by the same ophthalmic surgeon observing a fixed procedure, and carefully quantified before storage. The average volume of aqueous humor collected from PCACG patients was 72.7 ± 2.6 µL (n=36), which was not significantly different from that obtained from Control (77.2 ± 3.4 µL, n=20, p=0.221). Moreover, the ATP level was not correlated with the collected liquid amount (SROC=−0.220, p=0.103). This suggests that ATP levels were not influenced by sampling.
3.3 ATP levels did not change with duration of symptom history, age or drug treatment
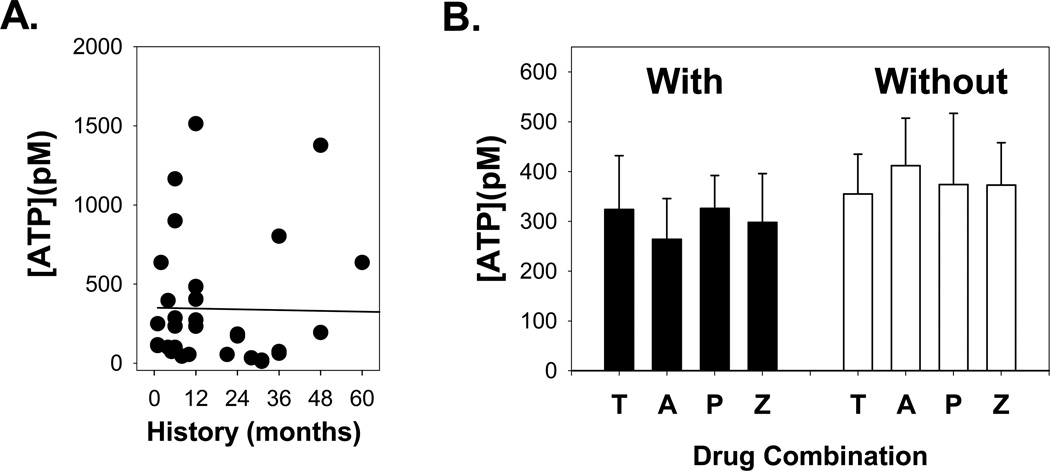
The elevation of ATP in the aqueous humor of patients suffering from a chronic increase in IOP implies that the release of ATP is a sustained event. However, the release of transmitters can also be phasic, with release greatest during the initial manifestation of disease. As such, the relationship between ATP concentration and the history of symptoms was examined (Table 2). There was no correlation between duration of symptoms and ATP levels (SROC = − 0.05, p=0.75; Fig. 3A). Further, although PCACG patients were significantly younger than control patients, there was no correlation between age and ATP levels (Table 2).
Table 2.
Spearman Rank Order Correlation Analysis between ATP Levels and Key Variables
| PCACG | Control | |||
|---|---|---|---|---|
| Key Variable | SROC | p | SROC | p |
| Age | 0.259 | 0.128 | 0.176 | 0.459 |
| History | −0.055 | 0.751 | 0.219 | 0.354 |
| T0 | 0.339 | 0.043 | −0.304 | 0.193 |
| Duration | −0.004 | 0.981 | n/a | |
| Numbers | −0.162 | 0.345 | n/a | |
Notes: SROC: Spearman rank order correlation coefficient. Age: Y, years; History: M, months between onset of symptom to hospitalization; T0: IOP measured at hospitalization showed the mean of three measurements; Duration: D, days between start of drug treatment and sample collection; Numbers: numbers of drugs applied before operation.
Figure 3. Effect of Duration of Symptom History and Drug Treatment.
A. The ATP concentration in aqueous humor did not change with length of symptom history. Data are fit with a first-order regression (line). The data are not significantly correlated with a Spearmans Rank Order of Correlation of − 0.05, p=0.75.
B. As more than one drug was typically given in the 2–3 days between admittance and surgery, ATP levels were grouped by the presence (left) or absence (right) of a drug. Drugs given were Timolol – T, Alphagan – A, Pilocarpine – P, Azopt- Z. “n” values are detailed in Table 3; numbers total more than 36 because of the use of multiple drugs on a single patient. For each drug, ATP levels with vs. without drug were not significantly different (p>0.05). In addition, none of the groups were significantly different from one another.
All PCACG patients were given one or more drugs to lower their IOP between their initial examination upon admittance to hospital and surgery 2–3 days later. Although the patients in this study were selected for surgery because the drugs failed to bring about a substantial reduction of IOP, it is possible that treatment might have affected ATP levels in the aqueous humor. It is particularly important to determine if treatments affected ATP levels as control patients did not receive any of the administered drugs. Four topical anti-glaucomatous drugs [Timolol, Alphagan (Brimonidine), Pilocarpine and Azopt (Brinzolamide)] with disparate mechanisms of action to lower IOP were applied. As most patients received multiple drugs, their effect on ATP levels was determined by comparing levels in the presence and absence of an individual drug. ATP concentrations in the eyes receiving a specific a particular drug treatment (+) were not statistically different from those not receiving that specific drug (−) (p>0.05, Table 3; Fig. 3B). Moreover, ATP concentration of an experimental group with a designated drug treatment did not differ from other three groups treated with different drugs. (p=0.644, Table 3). Of the drugs used, Timolol, Alphagan, and Azopt act as aqueous humor suppressants, while pilocarpine does not. However, Table 3 indicates that there is no significant effect on ATP concentration between eyes treated with pilocarpine, suggesting changes in ATP level are not due to alterations in aqueous dynamics. Of note, no drug interfered with the luciferase assay used to measure ATP levels. Therefore, the increased ATP levels detected in the aqueous humor of PCACG patients is unlikely to have been influenced by drug treatment.
Table 3.
Lack of Effect of Different Topical Agents
| Group | Specified Drug (+) ATP (pM) |
n | Specified Drug (−) ATP (pM) |
n | p* |
|---|---|---|---|---|---|
| Alphagan | 264±82 | 17 | 412±95 | 19 | 0.199 |
| Azopt | 298±98 | 15 | 373±85 | 21 | 0.413 |
| Timolol | 324±108 | 15 | 355±80 | 21 | 0.360 |
| Pilocarpine | 326±66 | 24 | 374±143 | 12 | 0.411 ψ |
| p# | 0.644 | ||||
The analysis compared the ATP levels in eyes either treated (+) or not treated (−) with a designated drug by Mann-Whitney U test.
The result indicated that the ATP level in eyes treated with pilocarpine was not different from those treated with aqueous humor suppressants (Alphagan, Azopt and Timolol).
The analysis compared the ATP level of the group treated with the indicated drug versus the rest three groups treated with other specified drugs by Kruskal Wallis Test.
4. Discussion
This study demonstrates that ATP levels in the aqueous humor of patients with primary chronic angle closure glaucoma are elevated. Our prior data from patients with primary acute angle closure glaucoma demonstrated a strong association between high IOP and extracellular ATP concentration in the aqueous humor (Zhang et al., 2007). The current observation that extracellular ATP is raised in patients with chronic elevation of IOP suggests that excess levels of extracellular ATP may be a common occurrence in glaucoma patients.
Several observations strengthen the relationship between elevated IOP and increased ATP levels. The patients identified as suffering from PCACG had a 14-fold increase in ATP concentration in their aqueous humor as compared with Controls (Fig 1B). ATP levels were significantly correlated with both IOP (Fig 1C) and the cup to disc ratio (Fig 2C). ATP levels were not influenced either by the duration of the symptoms (Fig. 3A) or the short term treatment with anti-glaucoma drugs (Fig. 3B). Together, these observations imply that extracellular ATP levels are likely increased in human patients with a chronic elevation of IOP.
The lack of a correlation between ATP levels and the length of symptom history suggests that extracellular ATP levels are elevated regardless of the length of IOP elevation. In other words, elevation in ATP levels appears to be sustained. The history information obtained by a self report is somewhat unreliable, although given the variable onset of chronic angle-closure glaucoma can be the only estimate of the onset of the disorder. However, the complete lack of correlation between this “History” and ATP levels suggests the parameters are unlikely to be linked with more accurate estimates of the disease onset. Regardless, the close correlations between the cup to disk ratio and IOP, and the cup to disk ratio and ATP levels suggest that IOP levels were raised for a sufficiently long time period to produce a substantial loss of ganglion cell axons. The cup to disc ratio is commonly accepted in the clinic as an indicator of both the magnitude of IOP elevation and its duration (Tanito et al. 2003), and is closely related to the severity of visual field loss in PCACG patients (Gazzard et al. 2003). On this basis it is reasonable to conclude that the IOP in these patients was elevated for some time, and given their 14-fold ATP increase in levels, conclude that the rise in ATP can be sustained in chronic glaucoma.
From a mechanistic standpoint, it is unclear whether sustained increase or oscillations in IOP are responsible for the release in ATP. An in vitro model indicated that modest elevations in pressure gave only transient release of ATP, while larger pressures triggered a more sustained release (Reigada et al. 2008). The normal circadian variation in IOP, with highest levels in the morning, may be exaggerated in PCACG patients. The fluctuation in IOP is larger in eyes with elevated IOP in general (Bengtsson and Heijl 2005) and specifically in PCACG patients (Baskaran et al. 2009). Some, but not all, studies suggest this fluctuation may itself be a risk factor for chronic glaucoma progression (David et al. 1992, Gazzard et al. 2003; Sihota et al. 2005; Caprioli 2007; Hong et al. 2007). It is thus possible likely that the cyclical deformation that results from these fluctuations in IOP contributes to the increased concentration of ATP in the aqueous humor of PCACG patients.
The elevated cup to disc ratio and self reported history suggests that the patients in this study went for a considerable time without pharmacological treatment, and patients were only given drugs for a brief time as part of this study. As such, the confounding effects of drug treatment were relatively easy to rule out. Neither the presence nor the absence of treatment with Timolol, Alphagan, Pilocarpine and/or Azopt altered ATP levels (Fig. 3B).
Physiological source and implications of elevated ATP
ATP is rapidly degraded by ubiquitous nucleotidases and thus the turnover is high (Joseph et al., 2003; Robson et al., 2006). As such, the sustained elevation in extracellular ATP levels implies that ATP is released continually. The precise origin of the ATP measured in the anterior chamber is unknown. The ciliary epithelium is the most likely source given its role in secreting aqueous humor, and its ability to release ATP upon swelling (Mitchell et al. 1998, Li et al. 2010). However, mechanical perturbations are known to release ATP from a variety of ocular cells including the corneal endothelium (Gomes et al. 2005a), lens (Eldred et al. 2003, Shahidullah et al. 2011), trabecular meshwork (Fleischhauer et al. 2003, Leung et al. 2009, Luna et al. 2009), retinal pigmented epithelium (Mitchell 2001), and whole retina (Reigada et al. 2008). Preliminary data also suggest release from retinal ganglion cells (Xia et al. 2010) and retinal astrocytes (Beckel et al. 2011). It is thus likely that the elevated ATP in aqueous humor observed in the present study reflects release primarily from the ciliary epithelium with additional contributions possible from numerous other cell types. Of note, the pressure-dependent release of ATP from the retina was not accompanied by an elevation in lactose dehydrogenase, implying the release can be a physiological response to stretch and not necessarily a result of cell lysis (Reigada et al. 2008). It is of course possible that the increased levels of ATP result from a decreased activity of ecto-ATPases, although preliminary data suggest an increase in ecto-ATPase levels in the glaucomatous eye (Lu et al., 2009)
An elevated concentration of ATP may have a variety of actions in the anterior eye. The ATP could stimulate purinergic receptors directly or indirectly via its metabolites ADP and adenosine. In the anterior eye, ATP could stimulate multiple P2Y receptors on trabecular meshwork cells (Crosson et al. 2004) or corneal endothelial cells (Gomes et al. 2005b). As stimulation of P2 receptors has been linked to pain signaling (Burnstock 2006, Donnelly-Roberts and Jarvis 2007), it is possible that an increased level of ATP could explain the severe ocular pain experienced during acute elevations in IOP. Further, following ATP dephosphorylation, adenosine could act at receptors in the trabecular meshwork to modulate outflow facility (Fleischhauer et al., 2003; Crosson et al. 2005), or stimulate A3 receptors on the non-pigmented ciliary epithelial cells to decrease inflow (Mitchell et al. 1999). It is not clear whether ATP in the anterior eye targets only anterior receptors or whether it can also stimulate receptors in the retina. In this regard, the ability of P2X7 receptor stimulation to kill retinal ganglion cells in vitro (Zhang et al. 2005) and in vivo (Hu et al. 2010), and of the pressure-induced ATP release to impede the transmission of the visual signal to the brain (Resta et al. 2007), is of particular relevance as these cells are on the front edge of the retina. Regardless of the source of ATP activating the receptors in the posterior and anterior eye, it should be emphasized that although the absolute concentrations of ATP measured in this study were low, released ATP can be orders of magnitude higher near cell membranes than that sampled in the bath solution (Beigi et al., 1999). As such, ATP may well be able to act on purinergic receptors. Whether such action is ultimately protective or detrimental to ocular health remains to be determined given the complexities of purinergic signaling in the eye.
Conclusions
This study demonstrates that the concentration of extracellular ATP in the aqueous humor of patients with PCACG is elevated 14 fold over controls. This implies that purinergic signals can potentially contribute to the pathologies in chronic glaucoma.
Highlights.
-
●
Extracellular ATP level in aqueous humor is increased 14 fold in patients with PCACG
-
●
Extracellular ATP level correlates to the magnitude of IOP increase and the C/D ratio
-
●
Extracellular ATP level is unrelated to other factors (e.g., age, medication)
-
●
Released ATP and metabolite adenosine may modulate the aqueous humor dynamics
Acknowledgements
Supported by Grants #30872831 from National Natural Science Foundation of China (XZ), #20100171110077 from Doctoral Fund of Ministry of Education of China (RFDP), #2007CB512200 from the National Basic Research Program of China (973 Project, GJ), and NIH grant EY 015537 (CHM). The authors thank Lan Luo and Huiling Hu for technique assistance, Shida Chen for manuscript preparation, and Mortimer Civan and Richard Stone for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Baskaran M, Kumar RS, Govindasamy CV, Htoon HM, Wong CY, Perera SA, Wong TT, Aung T. Diurnal intraocular pressure fluctuation and associated risk factors in eyes with angle closure. Ophthalmology. 2009;116:2300–2304. doi: 10.1016/j.ophtha.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Argall AJ, Lim JC, Shahidullah M, Macarak EJ, Laties AM, Delamere NA, Mitchell CH. Optic Nerve Astrocytes Release ATP upon Mechanical Strain. Invest Ophthalmol Vis Sci. 2011 ARVO E-Abstract 1819. [Google Scholar]
- Bengtsson B, Heij A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243:513–518. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J. Intraocular pressure fluctuation: an independent risk factor for glaucoma? Arch Ophthalmol. 2007;125:1124–1125. doi: 10.1001/archopht.125.8.1124. [DOI] [PubMed] [Google Scholar]
- Carnahan MC, Platt LW. Serial paracenteses in the management of acute elevations of intraocular pressure. Ophthalmol. 2002;109:1604–1606. doi: 10.1016/s0161-6420(02)01126-0. [DOI] [PubMed] [Google Scholar]
- Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson CE, Sloan CF, Yates PW. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Yates PW, Bhat AN, Mukhin YV, Husain S. Evidence for multiple P2Y receptors in trabecular meshwork cells. J Pharmacol Exp Ther. 2004;309:484–489. doi: 10.1124/jpet.103.060319. [DOI] [PubMed] [Google Scholar]
- David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–283. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred JA, Sanderson J, Wormstone M, Reddan JR, Duncan G. Stress-induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans. 2003;31:1213–1215. doi: 10.1042/bst0311213. [DOI] [PubMed] [Google Scholar]
- Fleischhauer JC, Mitchell CH, Stamer WD, Karl MO, Peterson-Yantorno K, Civan MM. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membr Biol. 2003;193:121–136. doi: 10.1007/s00232-002-2013-5. [DOI] [PubMed] [Google Scholar]
- Gazzard G, Foster PJ, Devereux JG, Oen F, Chew P, Khaw PT, Seah S. Intraocular pressure and visual field loss in primary angle closure and primary open angle glaucomas. Br J Ophthalmol. 2003;87:720–725. doi: 10.1136/bjo.87.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005a;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Vereecke J, Himpens B. ATP-dependent paracrine intercellular communication in cultured bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005b;46:104–113. doi: 10.1167/iovs.04-0846. [DOI] [PubMed] [Google Scholar]
- Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;125:1010–1013. doi: 10.1001/archopht.125.8.1010. [DOI] [PubMed] [Google Scholar]
- Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Lam DS, Chua JK, Tham CC, Lai JS. Efficacy and safety of immediate anterior chamber paracentesis in the treatment of acute primary angle-closure glaucoma: a pilot study. Ophthalmol. 2002;109:64–70. doi: 10.1016/s0161-6420(01)00857-0. [DOI] [PubMed] [Google Scholar]
- Leung CT, Li A, Peterson-Yantorno K, Civan MM. Molecular Identification of ATP-Permeable Channels in Human Trabecular Meshwork Cells. Invest. Ophthalmol. Vis. Sci. 2009;50:1483. (Abstract) [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol - Cell Physiol. 2010;299:C1308–C1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2011 Mar 4; doi: 10.1002/jcp.22715. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Hu H, Laties AM, Sévigny J, Mitchell CH. Increased expression of extracellular ATP marker NTPDase 1 in the optic nerve of rats with experimental glaucoma. Invest Ophthalmol Vis Sci. 2009 ARVO E-Abstract. [Google Scholar]
- Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001;534:193–202. doi: 10.1111/j.1469-7793.2001.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Nat Acad Sci USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Lu W. Retinal ganglion cells and glaucoma: Traditional patterns and new possibilities. Curr Topics Membr. 2008;62:301–322. [Google Scholar]
- Mitchell CH, Lu W, Hu H, Zhang X, Reigada D, Zhang M. The P2X(7) receptor in retinal ganglion cells: A neuronal model of pressure-induced damage and protection by a shifting purinergic balance. Purinergic Signal. 2009;5:241–249. doi: 10.1007/s11302-009-9142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Peterson-Yantorno K, Carre DA, McGlinn AM, Coca-Prados M, Stone RA, Civan MM. A3 adenosine receptors regulate Cl- channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276:C659–C666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: Influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.09.014. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharm Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Beimgraben C, Delamere N. Hyposmotic stress causes ATP release and stimulates Na, K-ATPase activity in porcine lens. J Cell Physiol. 2011 doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- Sihota R, Saxena R, Gogoi M, Sood A, Gulati V, Pandey RM. A comparison of the circadian rhythm of intraocular pressure in primary phronic angle closure glaucoma, primary open angle glaucoma and normal eyes. Indian J Ophthalmol. 2005;53:243–247. doi: 10.4103/0301-4738.18905. [DOI] [PubMed] [Google Scholar]
- Tanito M, Itai N, Dong J, Ohira A, Chihara E. Correlation between intraocular pressure level and optic disc changes in high-tension glaucoma suspects. Ophthalmology. 2003;110:915–921. doi: 10.1016/S0161-6420(03)00101-5. [DOI] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Laties AM, Mitchell CH. Mechanosensitive Release of ATP via Pannexins Autostimulates P2X7 Receptors on Retinal Ganglion Cells. Invest. Ophthalmol. Vis. Sci. 2010;51 ARVO E-Abstract 3317. [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties A, Mitchell C. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]