Abstract
We report on the preparation of the first material for therapeutic delivery of CO. A peptide amphiphile was synthesized with a covalently attached ruthenium tricarbonyl. Self-assembled nanofiber gels containing this peptide spontaneously released CO with prolonged release kinetics compared to soluble CO donors. Oxidatively stressed cardiomyocytes had improved viability when treated with this peptide, demonstrating its potential as a biodegradable gel for localized therapeutic CO delivery.
Biological signalling gases, including nitric oxide (NO) and carbon monoxide (CO), are known to be important for cell signal transduction and govern a variety of cellular processes. Although much more is known about the mechanism of NO activity, CO has also been identified as an important signaling gas over the past four decades.1 CO is produced by every cell in the body through heme catabolism by the enzyme heme oxygenase-1. Additionally, CO is known to play a protective role in tissue through its anti-inflammatory, anti-apoptotic, and anti-proliferative functions; it is also known to inhibit the formation of reactive oxygen species.2–4 These functions are thought to occur by modulating the redox state of the cell and by reactions with metal-containing proteins. As a result, preclinical studies show that CO is likely to have therapeutic implications in cardiovascular disease, intestinal inflammation, pulmonary hypertension, organ transplantation, and also acute lung, kidney, and liver diseases, among others.5 As such, CO inhalation therapy has been evaluated preclinically in many of these scenarios.6
An interesting alternative to inhaled gaseous CO therapy is the use of synthetic CO-releasing molecules (CORMs).7 Small molecule CORMs circumvent the risks of systemic overexposure and CO poisoning from inhaled delivery by providing more precise control over the concentration, dose, kinetics, and localization of therapeutic CO delivery. Metal carbonyls, including those of Ru, Fe, Mn, W, and others, are the most commonly used method for CO delivery.8 Several groups have studied ligand effects on CO release rate from various metal centres, though most of the CORMs developed to date require light activation.9–14 The most extensively evaluated CORM is Ru(CO)3Cl(glycinate), termed CORM-3, which spontaneously releases CO in aqueous solution.15 This water-soluble ruthenium tricarbonyl releases one equivalent of CO with a reported half-life on the order of a few minutes under physiological conditions.16 Despite its short half-life of release, the therapeutic effects of CORM-3 persist long past the depletion of free CO.9 CORM-3 has shown efficacy in a wide variety of animal models and was demonstrated to be non-toxic at doses of 4 mg/kg in primates over 30 days.17
Although CORM-based therapies provide greater control over delivery than inhaled CO gas, these small molecules are expected to diffuse quickly after administration, thus limiting their ability to localize within a specific tissue. To address this problem, Hubbell recently developed CO-releasing polymeric micelles, which could potentially be systemically directed through the use of surface ligands that bind specific targets.18 Though CO-releasing micelles may be useful for delivering CO to endothelial cells, lymph nodes, or tumours, CO-releasing materials that can be precisely localized are likely better suited for injectable therapies into specific tissues or complete coating of an organ prior to transplantation.
We report here on a self-assembling peptide amphiphile (PA) designed to deliver CO. PAs are short peptide sequences covalently bound to an alkyl tail,19,20 and they belong to a group of self-assembling peptide-based materials that have been evaluated for use in medicine over the past decade.21 PAs can be designed to self-assemble into long, one-dimensional nanofibers through the incorporation of a β-sheet-forming component in the peptide sequence.22 Nanofiber-forming PAs also include one or more charged residues, and upon charge screening the supramolecular nanofibers can form physically crosslinked gels.23 These peptide nanofiber materials are easily injectable in the sol state and are biodegradable by composition, making them less invasive alternatives to traditional hydrogels based on covalent polymers.24 Current therapeutic targets for self-assembling peptides include immune system programming,25 drug and protein delivery,26–29 tissue regeneration,30,31 and cancer therapeutics.32,33
Our laboratory has studied PAs as scaffolds for regenerative medicine extensively over the past decade,34 and we have shown that injectable PAs can be designed to release both small molecule drugs and proteins in vitro and in vivo.28,35–37 In one particularly relevant example, NO was released by sequestering small molecule diazeniumdiolates in PA nanofiber gels.38 The therapeutic NO was shown to effectively inhibit neointimal hyperplasia when delivered using PA nanofiber gels following arterial injury.
Two reports of metal carbonyl complexes attached to peptides have recently been published, but both required light activation for efficient CO release.39,40 CORM-3 spontaneously releases CO, which is preferable in biological applications where UV light may harm tissues and cells. Because of its spontaneous CO release and lack of toxicity, we envisioned a PA that contained a Ru(CO)3Cl(glycinate) motif reminiscent of CORM-3. First, a PA of the sequence C16V3A3E3K(βD) (1) was synthesized under standard solid phase synthesis conditions and purified by HPLC (here C16 = palmitic acid and βD indicates that the Asp residue was attached through its side chain carboxylic acid). PA 1 was designed to include a β-Asp residue to afford a NH2CHRCOOH unit similar to the Gly component in CORM-3. Reaction of PA 1 with [Ru(CO)3Cl2]2 in the presence of sodium methoxide at room temperature generated CO-releasing PA 2 (Scheme 1). Functionalization reactions consistently achieved yields of 70–75% conversion, as determined by LCMS (Fig. S1). We attribute the modest conversions to the limited solubility of PA 1 in MeOH. The product identity was confirmed by mass spectrometry and IR spectroscopy (Fig. S2). Exhaustive efforts were undertaken to purify PA 2, but the product was found to decompose under the HPLC conditions necessary for removal of unreacted PA 1. As a result, all structural, kinetics and biological studies were carried out on a mixture containing of 70% PA 2 and 30% PA 1. It should be noted that the previously reported polymer functionalization reactions with [Ru(CO)3Cl2]2 also showed modest conversions and were studied as synthesized.18 PA 2 was found to assemble into short nanofibers by TEM, and this nanostructure was confirmed by small angle X-ray scattering (SAXS) (Fig. S3). Fitting of the SAXS data to a polydisperse core-shell cylinder model gave an average nanofiber diameter of 8.2 nm, which is consistent with the TEM images.
Scheme 1.
Synthesis of CO-releasing PA 2.
The kinetics of CO release from soluble PA 2 were studied spectroscopically by monitoring the conversion of deoxy-myoglobin (dMb) to carbon monoxy-myoglobin (COMb). This assay is commonly used in the study of CORMs and provided a convenient method to establish the CO release profile of PA 2.9 Fig. 1A shows the visible spectrum of the myoglobin Q-band during conversion from dMb to COMb. In these experiments, the dMb peak at 556 nm decreased while the CoMb peaks at 542 nm and 578 nm increased. Quantification of conversion from dMb to COMb was measured by monitoring absorption at 542 nm against the isosbestic point at 550 nm, as has been previously established.18 As expected, PA 1 did not convert dMb to COMb (Fig. S5).
Fig. 1.
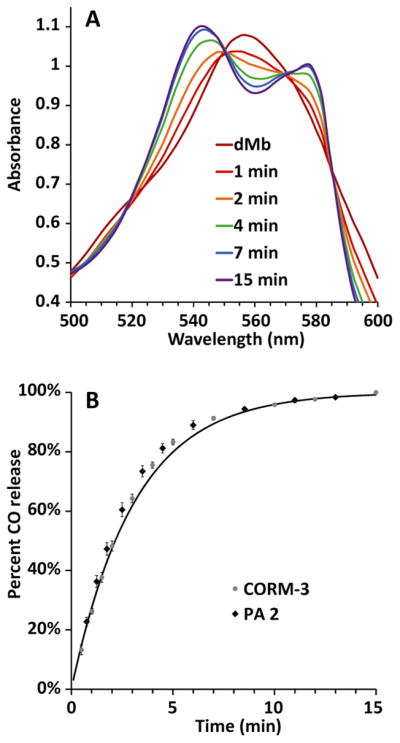
(A) Absorbance spectra showing the conversion of dMb to COMb for CO-releasing PA 2. (B) Kinetics plot derived from these spectra for PA 2 and for CORM-3. The half-lives of release are the same within error, and the solid line is the fit to the PA 2 kinetics.
Kinetic plots of CORM-3 and CO-releasing PA 2 in solution demonstrated first-order release with similar rate constants (Fig. 1B). The half-life of CO release was found to be 2.14 ± 0.17 min in the case of CORM-3 and 2.16 ± 0.05 min for PA 2. The similar half-lives are likely due to the placement of the Ru-carbonyl component in the solvent-exposed, charged region of the PA. Placement of the Ru-carbonyl closer to the PA core may alter release kinetics due to changes in nanostructure and peptide mobility.41
We also studied the effects of CO delivery on cells cultured in vitro to probe the therapeutic capacity of PA 2. Like NO, CO gas is freely diffusible through membranes and thus bypasses receptors and transporters to rapidly induce intracellular signaling. One model to evaluate the protective effects of CO on cells in culture is by using H2O2 to stress the cells, which enhances the production of reactive oxygen species (ROS) and promotes apoptosis. The protective effects of delivered CO are quantified in this assay through measurements of cell viability.42,43 CORM-3 has demonstrated cardioprotective capacity both in vitro and in vivo,15 so we used this model to determine whether PA 2 could also induce a similar cardioprotective effect on oxidatively stressed H9c2 rat cardiomyocytes (Fig. 2). Cells were treated with H2O2 for 2 hours to induce oxidative stress and were subsequently treated with 25 μM CO-releasing therapy or controls for 24 hours, at which point viability was assessed. This dose was chosen based on previously published cardioprotective studies for CORM-3.15 Treatment with CO-releasing PA 2 following H2O2 treatment was found to significantly (P<0.001) improve cardiomyocyte viability (50.8%) compared to an H2O2 control (35.6%). Treatment with PA 2 also significantly increased cell viability compared with a control sample utilizing non-CO-releasing PA 1 (37.5%). Treatment with CORM-3 as a CO-releasing control similarly resulted in a significant (P<0.001) improvement in cell viability (47.9%) compared to both the H2O2 and the PA 1 controls. Importantly, we found that PA 2 performed as well as CORM-3, which is the benchmark for CO-releasing molecules. In the absence of H2O2 treatment, CORM-3, PA 1 and PA 2 had no effect on cell viability (Fig. S6), confirming reports that ruthenium-containing CORMs have minimal toxicity at therapeutic concentrations.17 These experiments indicate that CO-releasing PA 2 exhibits similar cardioprotective activity to that previously established for CORM-3. Therefore, we can anticipate that PA 2 will be effective in many of the models for which CORM-3 has already shown therapeutic efficacy.5
Fig. 2.

Viability of cardiomyocytes after treatment with H2O2 and exposure to CO-releasing PA 2. Fluorescence images of PA 2, control PA 1, and H2O2 negative control are shown, with red indicating dead cells and green indicating live cells. Quantification of viability was performed by cell counting (n = 5–8 for each group). Significance for PA 2 and CORM-3 is shown relative to control and PA 1. All images are set to the same scale.
An advantage of using the PA framework to deliver CO is the ability for PAs to form robust gels upon charge screening.23 Based on our previous work on NO release from PA nanofiber gels, we expected an increase in the half-life of CO release upon gelation.38 To test this hypothesis, we formed gels of PA 2 with the aid of a strongly gelling PA (C16V2A2E2; Fig. S7). CaCl2 was added to a solution of the two PAs, and a robust gel formed immediately. An SEM image of the gel is shown in Fig. 3. The fibrous aggregates seen in the SEM are bundles of nanofibers that entangle to form a highly hydrated three-dimensional network structure (~1 wt.% PA). This morphology is consistent with many other PA gels studied in our laboratory. To form gels for CO-release experiments, aqueous CaCl2 was added to a freshly dissolved PA solution at the bottom of a cuvette. The total amount of PA 2 in the gel was the same as that used for the soluble nanofiber kinetic studies. After waiting a few seconds for gelation, dMb was layered on top. Conversion of dMb to COMb was measured over time, and the half-life of CO release from these PA nanofiber gels was found to increase to 17.8 ± 1.4 min (Fig. 2B). This represents more than an 8-fold increase compared to the half-lives of both CORM-3 and PA 2 in solution. We speculate that the decreased rate of CO release is a result of reduced water accessibility upon nanofiber bundling during gelation. One potential advantage of this PA gel would be its ability to localize CO-release by retaining the CO-releasing species within the tissue of interest. This strategy could limit off-target effects of CO therapy and reduce the total dose of CO compared with systemically delivered therapeutics such as CORM-3.
Fig. 3.
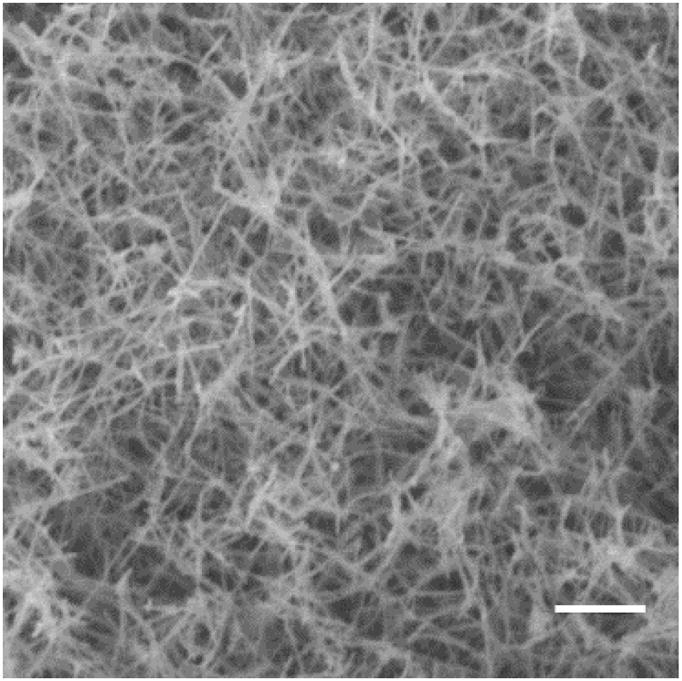
SEM image of PA 2 mixed with diluent PA C16V2A2E2 and gelled by addition of CaCl2. Scalebar = 0.5 μm.
We have reported here the synthesis, release kinetics, and biological activity of a CO-releasing peptide material. This material is based on a Ru-carbonyl-containing amphiphile and can deliver CO either in its soluble state or as a biodegradable gel. Gelation was found to dramatically prolong CO release, addressing the problem of rapid depletion of the gas in most other structures. Additionally, the cardioprotective capacity of the PA was established in vitro on oxidatively stressed cardiomyocytes. Most significantly, this first example of a CO-releasing gel material offers the possibility of localizing the therapy by injection into a tissue of interest. As such, CO-releasing materials could improve on the therapeutic efficacy already demonstrated by CORM-3 and other small-molecules. We believe that CO-releasing materials are an important new direction for medicine, especially as more becomes known about the wide-ranging therapeutic effects of this potent signalling gas.
Supplementary Material
Fig. 4.

(A) Absorbance spectra showing the conversion of dMb to COMb for CO release from a gel containig PA 2. (B) Kinetics plot derived from these spectra, showing an 8-fold decrease in release rate compared with soluble PA 2.
Acknowledgments
The authors acknowledge Christina Newcomb and Jessica Lehrman for experimental assistance. We are grateful to the following Northwestern University Core facilities: IBNAM Peptide Synthesis Core, Biological Imaging Facility (BIF), Cell Imaging Facility, and Keck Biophysics Facility. We thank Dr Steven Weigand and the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) Synchrotron Research Center at the Advanced Photon Source (APS) at Argonne National Lab for assistance with SAXS measurements. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. JBM was supported by an NIH postdoctoral fellowship (Grant No. 1F32AR061955-01). This project was supported by NIH grants 1P01HL108795-01 and 5R01EB003806-07.
Footnotes
Electronic Supplementary Information (ESI) available: Full experimental details, characterization data, and cell viability studies. See DOI: 10.1039/b000000x/
Notes and references
- 1.Tenhunen R, Marver HS, Schmid R. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–897. doi: 10.1152/ajplung.00366.2009. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Choi AM. Novartis Found Symp. 2007;280:165–175. discussion 175–181. [PubMed] [Google Scholar]
- 4.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 5.Motterlini R, Otterbein LE. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 6.Bauer I, Pannen BHJ. Crit Care. 2009;13:10. [Google Scholar]
- 7.Motterlini R, Mann BE, Foresti R. Expert Opin Investig Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 8.Haas KL, Franz KJ. Chem Rev. 2009;109:4921–4960. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Circ Res. 2002;90:E17–24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 10.Sawle P, Hammad J, Fairlamb IJS, Moulton B, O’Brien CT, Lynam JM, Duhme-Klair AK, Foresti R, Motterlini R. J Pharmacol Exp Ther. 2006;318:403–410. doi: 10.1124/jpet.106.101758. [DOI] [PubMed] [Google Scholar]
- 11.Kunz PC, Huber W, Rojas A, Schatzschneider U, Spingler B. Eur J Inorg Chem. 2009:5358–5366. [Google Scholar]
- 12.Rimmer RD, Richter H, Ford PC. Inorg Chem. 2010;49:1180–1185. doi: 10.1021/ic902147n. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez MA, Fry NL, Burt R, Davda R, Hobbs A, Mascharak PK. Inorg Chem. 2011;50:3127–3134. doi: 10.1021/ic2000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretschmer R, Gessner G, Gorls H, Heinemann SH, Westerhausen M. J Inorg Biochem. 2011;105:6–9. doi: 10.1016/j.jinorgbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Circ Res. 2003;93:e2–8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 16.Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Curr Pharm Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- 17.Vadori M, Seveso M, Besenzon F, Bosio E, Tognato E, Fante F, Boldrin M, Gavasso S, Ravarotto L, Mann BE, Simioni P, Ancona E, Motterlini R, Cozzi E. Xenotransplantation. 2009;16:99–114. doi: 10.1111/j.1399-3089.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa U, van der Vlies AJ, Simeoni E, Wandrey C, Hubbell JA. J Am Chem Soc. 2010;132:18273–18280. doi: 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]
- 19.Yu YC, Berndt P, Tirrell M, Fields GB. J Am Chem Soc. 1996;118:12515–12520. [Google Scholar]
- 20.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 21.Collier JH, Rudra JS, Gasiorowski JZ, Jung JP. Chem Soc Rev. 2010;39:3413–3424. doi: 10.1039/b914337h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang HZ, Guler MO, Stupp SI. Soft Matter. 2007;3:454–462. doi: 10.1039/b614426h. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield MA, Hoffman JR, de la Cruz MO, Stupp SI. Langmuir. 2010;26:3641–3647. doi: 10.1021/la9030969. [DOI] [PubMed] [Google Scholar]
- 24.Matson JB, Stupp SI. Chem Commun. 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudra JS, Tian YF, Jung JP, Collier JH. Proc Natl Acad Sci U S A. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelain F, Unsworth LD, Zhang SG. J Control Release. 2010;145:231–239. doi: 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. Biomaterials. 2010;31:9527–9534. doi: 10.1016/j.biomaterials.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matson JB, Stupp SI. Chem Commun. 2011;47:7962–7964. doi: 10.1039/c1cc12570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webber MJ, Newcomb CJ, Bitton R, Stupp SI. Soft Matter. 2011;7:9665–9672. doi: 10.1039/c1sm05610g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 31.Holmes TC, de Lacalle S, Su X, Liu GS, Rich A, Zhang SG. Proc Natl Acad Sci U S A. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standley SM, Toft DJ, Cheng H, Soukasene S, Chen J, Raja SM, Band V, Band H, Cryns VL, Stupp SI. Cancer Res. 2010;70:3020–3026. doi: 10.1158/0008-5472.CAN-09-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aulisa L, Forraz N, McGuckin C, Hartgerink JD. Acta Biomater. 2009;5:842–853. doi: 10.1016/j.actbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Webber MJ, Kessler JA, Stupp SI. J Intern Med. 2010;267:71–88. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah RN, Shah NA, Lim MMD, Hsieh C, Nuber G, Stupp SI. Proc Natl Acad Sci U S A. 2010;107:3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajangam K, Behanna HA, Hui MJ, Han XQ, Hulvat JF, Lomasney JW, Stupp SI. Nano Lett. 2006;6:2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 37.Webber MJ, Han XQ, Murthy SNP, Rajangam K, Stupp SI, Lomasney JW. J Tissue Eng Regen M. 2010;4:600–610. doi: 10.1002/term.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Hrabie JA, Murar J, Martinez J, Popowich DA, Jiang Q, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MP. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson CS, Schmitt S, Dou QP, Kodanko JJ. Inorg Chem. 2011;50:5336–5338. doi: 10.1021/ic200676s. [DOI] [PubMed] [Google Scholar]
- 40.Pfeiffer H, Rojas A, Niesel J, Schatzschneider U. Dalton Trans. 2009:4292–4298. doi: 10.1039/b819091g. [DOI] [PubMed] [Google Scholar]
- 41.Matson JB, Newcomb CJ, Bitton R, Stupp SI. Soft Matter. 2012;8:3586–3595. doi: 10.1039/C2SM07420F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su CY, Chong KY, Chen J, Ryter S, Khardori R, Lai CC. J Mol Cell Cardiol. 1999;31:845–855. doi: 10.1006/jmcc.1998.0923. [DOI] [PubMed] [Google Scholar]
- 43.Singla DK, McDonald DE. Am J Physiol Heart Circ. 2007;293:H1590–H1595. doi: 10.1152/ajpheart.00431.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



