Abstract
Alterations in appetite hormones favoring increased postprandial satiety have been implicated in both the glycemic control and potential weight-loss benefits of a low-glycemic diet. Racial differences exist in dietary glycemic load and appetite hormone concentrations. This study examined the impact of glycemic load on appetite hormones in 20 black women [10 normal weight, BMI = 22.8 ± 1.42 (mean ± SD); 10 obese, BMI = 35.1 ± 2.77] and 20 white women (10 normal weight, BMI = 22.9 ± 1.45; 10 obese, BMI = 34.3 ± 2.77). Each woman completed two 4.5-d weight-maintenance, mixed-macronutrient, high-glycemic vs. low-glycemic load diets that concluded with a test meal of identical composition. Blood samples collected before and serially for 3 h after each test meal were assayed for plasma ghrelin and serum insulin and glucose concentrations. Compared with the high-glycemic load meal, the low-glycemic load meal was associated with lower insulinAUC (P = 0.02), glucoseAUC (P = 0.01), and urge to eat ratings (P = 0.05) but with higher ghrelinAUC (P = 0.008). These results suggest the satiating effect of a low-glycemic load meal is not directly linked to enhanced postprandial suppression of ghrelin. Notably, these effects were significant among white but not black women, suggesting that black women may be less sensitive than white women to the glucoregulatory effects of a low-glycemic load. These findings add to a growing literature demonstrating racial differences in postprandial appetite hormone responses. If reproducible, these findings have implications for individualized diet prescription for the purposes of glucose or weight control in women.
Introduction
Consumption of a diet that is low in glycemic load is recognized as a useful tool for glycemic control in diabetes and has been linked with positive cardiovascular health outcomes (1–3) and indicators such as improved blood lipids and insulin sensitivity (4, 5). These benefits are not universal, however. Individual differences in response to a low-glycemic load can reflect underlying variability in response to a single food with a specific glycemic index (6). In addition, the response to a low-glycemic load can vary according to an individual’s level of glucose impairment (7). Racial/ethnic differences also may be important to consider because of underlying differences in disease risk (8, 9) and differences in chronic exposure to a high dietary glycemic load (9–11).
Some studies suggest a role for low-glycemic load in weight loss in overweight or obese individuals (12). The weight loss potential of a low-glycemic load diet is ascribed to the cumulative effects of low-glycemic load meals on postprandial satiety and subsequent food intake (13–15). The mechanisms linking low-glycemic load with increased satiety are thought to include alterations in appetite-regulating hormones. For example, glycemic load has been shown to affect ghrelin (16, 17), glucagon-like peptide-1, and cholecystokinin (16–18) but not peptide-YY (19).
Ghrelin is a gut-derived hormone that plays a major role in meal initiation (20) and nutrient sensing (21, 22). Food intake suppresses the plasma ghrelin concentration and this suppression is blunted in obese compared with normal-weight (23, 24) and black compared with white individuals (25, 26). In addition, ghrelin is sensitive to macronutrient manipulation, tending to be suppressed to a greater extent by carbohydrate than protein or fat in the early postprandial period (0–2 h) and by protein more than carbohydrate or fat in the later postprandial period (2–6 h) (27–29). The ghrelin response to whole food challenges that differ in glycemic load has been examined in a case study (16) and cross-sectionally (30). The current study is the first, to our knowledge, to examine the impact of glycemic load on postprandial ghrelin using a within-subject design.
The purpose of this study was to evaluate the effect of glycemic load on fasting and postprandial concentrations of ghrelin, insulin, and glucose in obese and normal-weight black and white women. We expected to find blunted ghrelin suppression and greater postprandial insulin and glucose responses in obese compared with normal-weight women. We hypothesized that postprandial ghrelin suppression would be greater and postprandial insulin and glucose responses would be lesser following a low-glycemic compared with a high-glycemic load meal. In addition, we hypothesized that these glycemic load effects would be more pronounced in white compared with black women.
Materials and Methods
Participants.
Women who self-identified as non-Hispanic black (hereafter, black) and non-Hispanic white (hereafter, white) and who were age ≥18 y were eligible to participate. The study exclusion criteria were: lactating, pregnant, or planned pregnancy in the next 6 wk; self-reported history of diabetes or other metabolic disorder; current use of medications that influenced appetite or that had substantial weight gain or loss side effects; BMI not within the range for “normal” (18.5–24.9 kg/m2) or “obese” (Class I or II, 30–39.9 kg/m2); unable or unwilling to eat animal-derived foods; or current exercise routine involving vigorous activity ≥3 times/wk. Using printed advertisements and by word-of-mouth, 40 women (20 black, 20 white; 10 normal weight and 10 obese per racial group) were recruited from the local community and completed the study. The participants received monetary compensation for completing each outpatient diet and test meal and a bonus for completing all study-related activities in full. White participants were recruited to match black participants on the basis of BMI (±2 kg/m2) and age (±2 y). The Institutional Review Board of the University of North Carolina at Chapel Hill approved the protocol for this study and each participant provided written consent.
Design and procedures.
Each participant completed a screening visit during which height and weight were obtained and a fasting blood sample was drawn to determine prestudy glucose and insulin concentrations. Thereafter, each participant was evaluated under 2 conditions, which were separated by a minimum 1-wk washout period. The condition order was counterbalanced across participants. Each of the 2 conditions began with a preliminary 4-d outpatient dietary preload. To reduce the likelihood of drop-out, the study dietitian interviewed each participant for food allergies and aversions and adjusted the outpatient meal plans accordingly. The conditions were matched for macronutrient composition (55% carbohydrate, 30% fat, and 15% protein) but differed in glycemic load (high vs. low). Each condition concluded with an overnight fast in the Clinical and Translational Research Center followed by a morning test breakfast meal, which was either high or low in glycemic load to reflect the composition of the preceding 4-d outpatient diet (Supplemental Table 1).
Participants were allotted 20 min to complete each test meal, and time zero of the postprandial period occurred at the conclusion of that 20-min period. Before the meal (−20 min) and at postprandial min 30, 60, 90, 105, 120, 135, 150, and 180, blood samples were obtained via an indwelling i.v. catheter. Blood samples were immediately processed and stored at −80°C for later assay. Samples collected at min 30 were assayed for insulin and glucose but not ghrelin, because the ghrelin nadir value was expected at a later time point. In addition, using visual analogue scales (31) before and at 0, 30, 60, 120, and 180 min after each meal, participants rated various aspects of subjective appetite (hunger; fullness; urge to eat; and desire to eat sweet, salty, fatty, or savory foods) as well as meal characteristics (visual appeal, smell, taste, aftertaste, palatability).
Composition of outpatient diets and test meals.
The specific details of the study meals and outpatient protocol are described elsewhere (19). Briefly, glycemic load values were derived using The Food Processor SQL (version 10.2.0.0, ESHA Research) and outpatient diets were provided in 500-kcal increments commensurate with each individual’s estimated energy requirement adjusted for body weight. For example, the 2500-kcal diet was composed of 344 g carbohydrate, 83 g fat, and 94 g protein, with a target glycemic load of 200 (high) or 100 (low). These target loads were chosen for 2 reasons: they were achievable with the foods available to the dietitian, and they closely represented the highest and lowest quintiles of glycemic load observed in a representative sample of adult women in the US (1). Any food or beverage not consumed was returned and weighed to estimate the actual amount consumed. The test meals were developed using ProNutra software (version 3.2.1.0, Viocare Technologies) and standardized to 625 kcal (86 g carbohydrate, 21 g fat, and 23 g protein).
Bioassays.
Blood samples were assayed using commercially available RIA (Linco) to measure plasma total ghrelin [assay sensitivity = 93 pg/mL (27.9 pmol/L)] and serum insulin [assay sensitivity = 2.0 μIU/mL (13.9 pmol/L)]. Serum glucose was determined by an Ortho Clinical Diagnostics Vitros 950 analyzer [University of North Carolina Hospitals; assay sensitivity = 20 mg/dL (1.11 mmol/L)].
Statistical analysis.
Prior to analysis, AUC were calculated for ghrelin, insulin, and glucose with the trapezoidal method. In addition, insulin sensitivity was calculated using the Quantitative Insulin Sensitivity Check Index (32) on the basis of fasting insulin and glucose concentrations that were obtained at study entry. Mixed-model analyses were performed to assess the effects of race (black, white), obesity (yes, no), and glycemic load (high, low) on the ghrelinAUC, insulinAUC, glucoseAUC, and insulin sensitivity. To account for the correlation between the repeated observations within participants, a compound symmetric correlation matrix was assumed and was found to fit the observed correlation in the data. All main effects and 2-way and 3-way interaction terms were tested. Secondary analyses featured similar types of analyses that focused on assessing race, obesity, glycemic load, and sampling time main and interactive effects on ghrelin, insulin, glucose, subjective appetite, and meal characteristic levels. In both sets of analyses, significant interactive effects were followed by a post hoc comparison of least squares means for interpretation. The participants were encouraged but not forced to eat the test meals in entirety, resulting in small-magnitude individual differences in the glycemic load and macronutrient percentages actually consumed during each of the test meals. Thus, to evaluate the influence of individual differences in these factors, models were subsequently tested while controlling for actual test meal glycemic load, total energy, and percentages of protein, carbohydrate, and fat. All analyses were carried out using SAS software (v. 9.2, SAS Institute) and P < 0.05 was considered significant. Data in the text are unadjusted mean ± SD.
Results
Baseline measures.
Characteristics of the sample at study entry are presented in Table 1. Age (P = 0.01), BMI (P < 0.001), fasting glucose (P = 0.01), and fasting insulin (P = 0.009) were higher and insulin sensitivity (P < 0.001) was lower in obese women (n = 20) compared with normal-weight women (n = 20). The race effect and the race × obesity interaction effect were not significant for age, fasting glucose and insulin, and insulin sensitivity.
TABLE 1.
Characteristics of the sample at study entry1
| Black normal weight | White normal weight | Black obese | White obese | P value2 | |
| Age, y | 26.5 ± 6.08 | 29.4 ± 7.93 | 35.6 ± 7.62 | 34.1 ± 10.5 | 0.01 |
| BMI, kg/m2 | 22.8 ± 1.42 | 22.9 ± 1.45 | 35.1 ± 2.78 | 34.3 ± 2.77 | <0.001 |
| Fasting serum glucose, mmol/L | 4.64 ± 0.49 | 4.71 ± 0.41 | 5.36 ± 0.60 | 5.02 ± 0.89 | 0.01 |
| Fasting serum insulin, pmol/L | 59.2 ± 36.8 | 49.0 ± 28.3 | 175 ± 189 | 132 ± 121 | 0.009 |
| Insulin sensitivity (Quantitative Insulin Sensitivity Check Index) | 0.36 ± 0.04 | 0.38 ± 0.05 | 0.31 ± 0.04 | 0.32 ± 0.03 | <0.001 |
Values are unadjusted means ± SD, = 10.
Obesity main effect; the main effect of race and the race × obesity interaction were nonsignificant.
Outpatient diet and test meal actual glycemic load intake.
The glycemic loads of the high and low outpatient diets were 213 ± 31.2 and 108 ± 25.2, respectively. During the test meals, the glycemic loads were 59.1 ± 5.94 and 31.1 ± 3.61 for the high and low meals, respectively. There were no significant differences between black and white women or between obese and normal-weight participants in test meal glycemic load (data not shown).
Obesity effects.
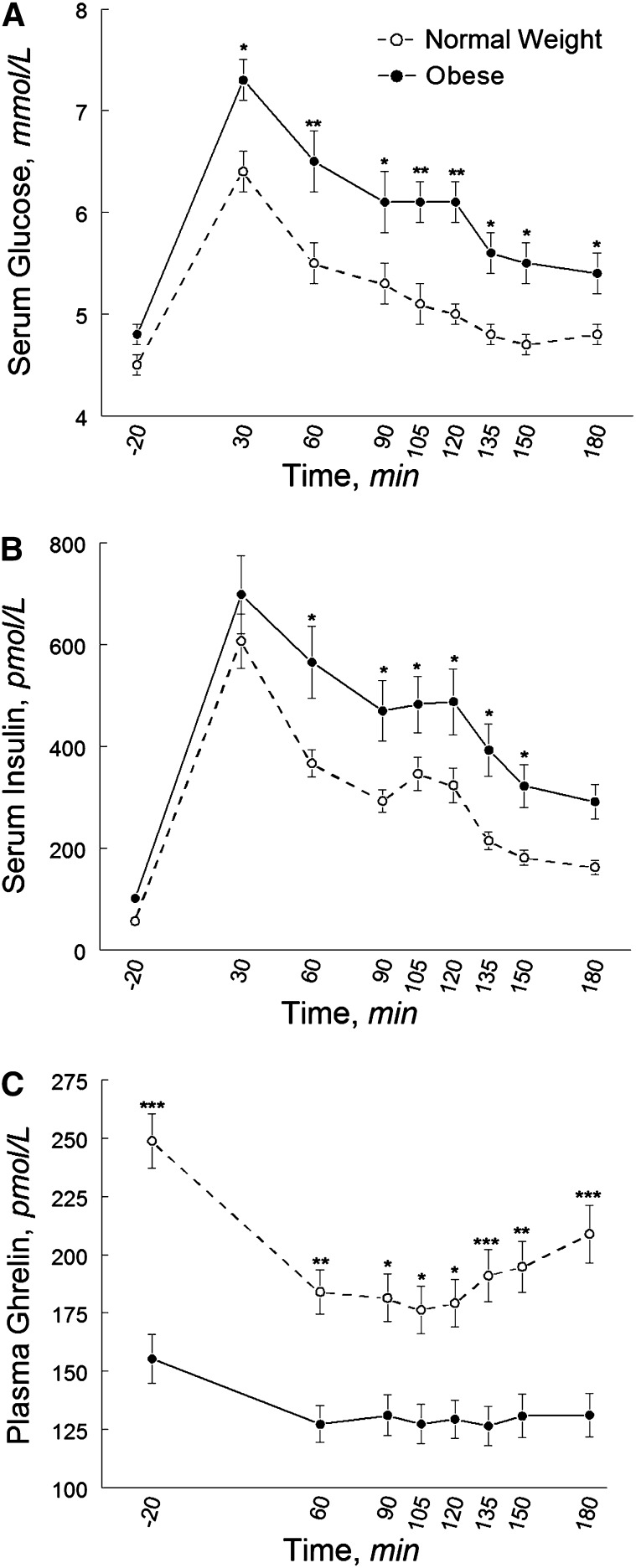
The glucoseAUC (P = 0.009) and ghrelinAUC (P = 0.002) differed between obese and normal-weight women; the insulinAUC also tended to differ by obesity status (P = 0.06). Compared with normal-weight women, obese women had a greater peak glucose response, higher postprandial glucose and insulin concentrations, a lower fasting ghrelin concentration, and a blunted ghrelin response (i.e., less suppression) (Fig. 1). Ghrelin was suppressed to 24.3% below the fasting concentration in the normal-weight group and 15.3% in the obese group. The diet × obesity and race × obesity interactions were not significant for the glucoseAUC, insulinAUC, and ghrelinAUC.
FIGURE 1.
Pre- and postprandial circulating concentrations of glucose (A), insulin (B), and total ghrelin (C) in normal-weight and obese black and white women after high- and low-glycemic load test meals. Data are unadjusted mean ± SE, n = 40. Different from obese at that time, *P < 0.05; **P < 0.001; ***P < 0.001.
Glycemic load effects.
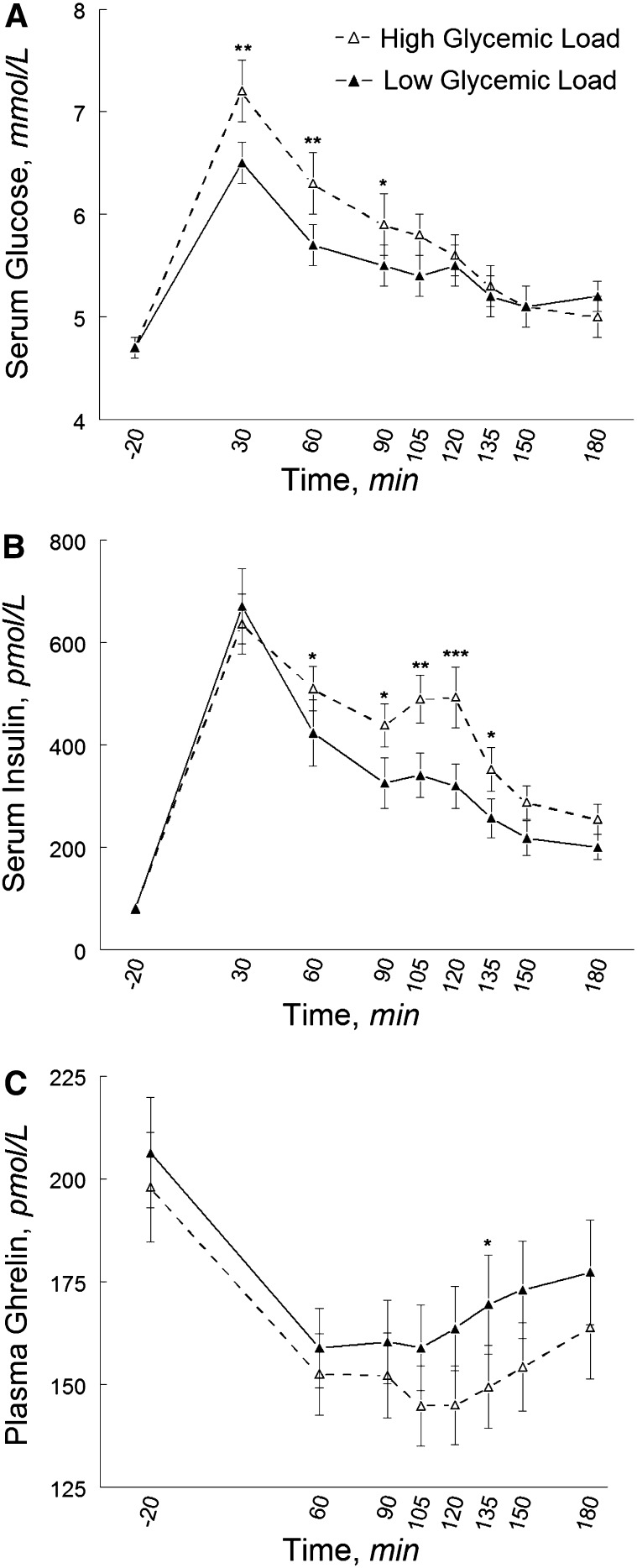
Fasting concentrations of glucose, insulin, and ghrelin did not differ by glycemic load; however, glycemic load affected the glucoseAUC (P = 0.01), insulinAUC (P = 0.02), and ghrelinAUC (P = 0.0008). These effects were essentially unchanged when accounting for the fact that not every participant consumed every morsel of food provided during each of the 2 test meals (i.e., when we accounted for individual differences in actual consumption). For example, the effect of the high- compared with the low-glycemic load meal on the ghrelinAUC remained significant when we accounted for total energy (P = 0.0008) as well as the percentage of fat (P = 0.0009), carbohydrate (P = 0.0007), and protein (P = 0.0009), and also glycemic load (P = 0.0007). The peak glucose response was greater and postprandial glucose and insulin concentrations were higher after the high- compared with the low-glycemic load meal; however, contrary to our hypothesis, the overall ghrelin response (i.e., ghrelin suppression) was greater after the high- than the low-glycemic load meal (Fig. 2). The mean ghrelin suppression below fasting concentration was 22% after the high-glycemic load meal and 18% after the low-glycemic load meal. The mean maximal ghrelin suppression was 25% after the high-glycemic load meal and 23% after the low-glycemic load meal. For both meals, the maximal ghrelin suppression was observed at min 105; this level of suppression was maintained through min 120 following the high-glycemic load meal but not following the low-glycemic load meal.
FIGURE 2.
Pre- and postprandial circulating concentrations of glucose (A), insulin (B), and total ghrelin (C) in normal-weight and obese black and white women after high- and low-glycemic load test meals. Data are unadjusted mean ± SE, n = 40. Different from low-glycemic load at that time, *P < 0.05; **P < 0.001; ***P < 0.001
Race effects.
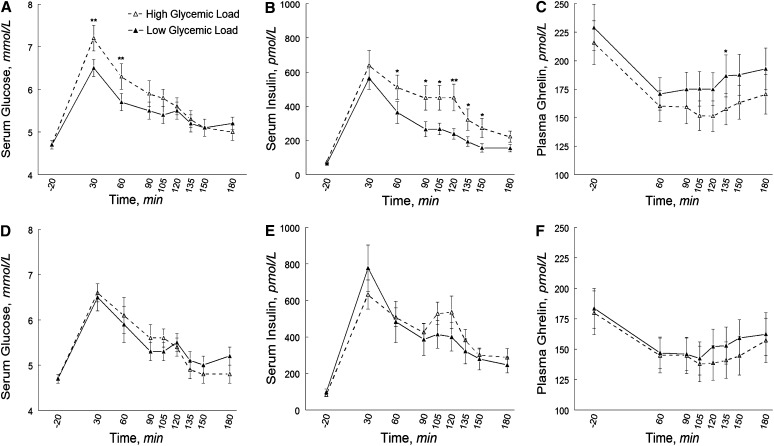
Fasting ghrelin tended to be lower in black (182 ± 12.0 pmol/L) than in white (223 ± 13.8 pmol/L) women (P = 0.06), but race did not affect fasting insulin and glucose or the glucoseAUC, insulinAUC, and ghrelinAUC. However, the glycemic load effect on the glucoseAUC differed by race (P-interaction = 0.02), with similar trends observed for the insulinAUC (P-interaction = 0.07) and ghrelinAUC (P-interaction = 0.08) (Supplemental Table 2). These effects were essentially unchanged when models were controlled for individual differences in actual consumption during test meals. Compared with the low-glycemic load meal, the high-glycemic load meal was associated with greater glucoseAUC (P = 0.001) and insulinAUC (P = 0.005) in white women; in black women, glycemic load did not affect the glucoseAUC or insulinAUC (Fig. 3). Similarly, the high-glycemic load meal was associated with a lower ghrelinAUC (i.e., greater ghrelin suppression) in white women (P = 0.0005) but not in black women, although individual ghrelin responses to the glycemic load meals varied within each group (Supplemental Fig. 1).
FIGURE 3.
Pre- and postprandial concentrations of circulating glucose (A,D), insulin (B,E), and total ghrelin (C,F) in white (A–C) and black (D–F) normal-weight and obese women after high- and low-glycemic test meals. Data are unadjusted mean ± SE, n = 20. Different from low-glycemic load at that time, *P < 0.05; **P < 0.001; ***P < 0.001.
Subjective appetite ratings and meal characteristic effects.
Compared with the low-glycemic load meal, the high-glycemic load meal had higher visual appeal (P = 0.05), taste (P = 0.01), and palatability (P = 0.04) (data not shown). The urge to eat AUC was higher after the high-glycemic load meal (data not shown in graph; P = 0.05); by visual inspection of the data, this effect appeared to be greater in magnitude in white than in black women, although the diet × race interaction was not significant (P = 0.16) (Supplemental Fig. 2). All other results for subjective appetite and cravings were not significant.
Discussion
Compared with the high-glycemic load meal, the low-glycemic load meal resulted in lower postprandial glucose and insulin responses and a lesser postprandial rating of urge to eat. These results extend those recently reported by Krog-Mikkelsen et al. (30), in which postprandial glucose and insulin were lower and ratings of fullness were higher in a group of women who consumed a low-glycemic diet for 10 wk compared with a group of women who consumed a high-glycemic diet for 10 wk. Contrary to our hypothesis, however, the low-glycemic load meal was associated with a lesser rather than a greater postprandial ghrelin suppression. These findings challenge the notion that the satiating effect of a low-glycemic load meal is directly linked to enhanced postprandial ghrelin suppression resulting in diminished appetitive signaling. Instead, the extent and pattern of postprandial ghrelin suppression appeared to be more closely linked to the insulin response, which was enhanced after high glycemic intake. However, it is important to consider that the duration of the postprandial window in the current study was 3 h. Thus, our data do not address whether the duration (as opposed to degree or magnitude) of ghrelin suppression below the baseline fasting concentration or the extent of ghrelin rebound above the baseline fasting concentration differed as a function of glycemic load in a later postprandial window. Foster-Schubert et al. (27) showed that ghrelin concentrations remained suppressed below the fasting baseline for 6 h after both protein and fat intake but rebounded and actually exceeded baseline concentrations 4 h after carbohydrate intake. Therefore, we cannot rule out the possibility that ghrelin is suppressed below the fasting baseline for a longer duration following a low- compared with a high-glycemic load meal and that this dynamic is responsible for the prolonged satiating effect ascribed to low glycemic intake and the potential weight maintenance benefits of a low-glycemic diet. Studies that compare high- and low-glycemic load effects on ghrelin and subjective appetite after breakfast, lunch, and dinner spaced ≥5 h apart may be instrumental in addressing this question. Further studies are also warranted to better understand the functional significance of a 4% difference in ghrelin suppression, which was the mean difference between the high- and low-glycemic index meals. Clearly, some individuals were more responsive to the dietary manipulation than others and it is worthwhile to consider the applicability of this individual difference to dietary prescription for health benefits.
Notably, our findings suggest that the hormonal and subjective appetite effects of glycemic load were collectively greater in magnitude in white than in black women. In white women, insulin and glucose responses, urge to eat, and ghrelin suppression were significantly less after the low- compared to the high-glycemic load meal. However, within the subset of 20 black women, the effect of glycemic load was nonsignificant across all outcome measures. For white women, these findings underscore the connection between ghrelin and insulin as well as the role of insulin in the satiating effect of low-glycemic load meals. For black women, to the extent that these results are reproducible in larger samples of women, these findings challenge the generalizability of nutritional counseling and dietary intervention for glucose or appetite control based on glycemic load. Our data also add to a growing literature indicating racial differences in postprandial appetite hormone responses (19, 25, 26), including blunted ghrelin suppression in blacks compared with whites (25). In the present study, independent of obesity status, black women tended to have a lower fasting ghrelin concentration compared with white women. Generally, lower fasting ghrelin concentrations and blunted postprandial suppression are associated with insulin resistance and higher BMI; however, our groups of black and white women did not differ on these measures. Further studies are needed to determine whether the observed racial differences in ghrelin regulation are innate or rather due to long-term dietary (or other) exposures not accounted for thus far. It has been suggested, but remains to be shown, that racial differences in postprandial ghrelin could contribute to racial disparities in obesity. The current findings encourage further examination of ghrelin × diet/environment interactions that may uniquely contribute to the pathogenesis of obesity among African American women. In addition to its primary role in food intake, ghrelin acts directly on the heart and vasculature (33); therefore, it also may be worthwhile to consider ghrelin’s role in racial disparities in obesity-related comorbidities.
There are several limitations to our study. First, the sample size was relatively small; however, the black and white participants within obesity subgroups were closely matched on BMI, age, and insulin sensitivity. Nonetheless, future studies might improve on our study design by evaluating insulin sensitivity under dynamic conditions (e.g., oral glucose tolerance test or euglycemic clamp) rather than relying on fasting values to estimate insulin sensitivity. A larger sample size might also permit more detailed examination of the relations among hormonal and subjective appetite responses as well as the functional significance of the observed differences in peripheral hormone levels. Future studies also may benefit from recent advances in assay techniques, which offer enhanced specificity for detecting postprandial changes in ghrelin (34). A second limitation of our study is that it did not address the extent to which racial differences in ghrelin response reflect racial differences in body composition. Future studies that measure body fat percentage or distribution, rather than simply BMI, may help clarify these relations. Future studies may also benefit by including measures of psychological factors that may affect appetite hormone responses (35) and examining other appetite-regulating hormones such as glucagon-like peptide-1 and cholecystokinin. Furthermore, including other racial minorities, men and children, and individuals with diabetes in future studies will maximize the understanding of both the limitations and benefits of dietary glycemic load at a population level. Finally, we cannot rule out the possibility that the outpatient preload diets may have obscured preexisting racial differences in glycemic load and its effect on ghrelin, insulin, or glucose. Future studies could directly address this question by assessing postprandial responses both before and after dietary glycemic manipulation.
In summary, our findings demonstrate that postprandial insulin, glucose, ghrelin, and subjective appetite respond to glycemic load in an integrated manner in white but not black women. Future studies are needed to determine whether overweight and diabetic black women benefit from a longer term, low-glycemic load intervention to the same extent as their white counterparts and whether racial differences in the weight and glucoregulatory effects of a low-glycemic load intervention are mediated by ghrelin. At an even more fundamental level, it is provocative to consider whether the racial ethnic diversity of the population used to compile the glycemic values of foods was sufficient and whether this issue warrants further study.
Supplementary Material
Acknowledgments
The authors thank Porshia Underwood for her editorial assistance. K.B. designed the research with assistance from B.M.; K.B., B.M., and S.H. conducted the research; A.H. performed prestudy physical exams and was responsible for overall participant safety; and J.G. was responsible for data analysis. All authors contributed to the interpretation of the results. All authors read and approved the final manuscript.
Footnotes
Supplemental Tables 1 and 2 and Supplemental Figures 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Literature Cited
- 1.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–61 [DOI] [PubMed] [Google Scholar]
- 2.Kelly S, Frost G, Whittaker V, Summerbell C. Low glycaemic index diets for coronary heart disease. Cochrane Database Syst Rev. 2004;CD004467. [DOI] [PubMed] [Google Scholar]
- 3.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;CD006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosdøl A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr. 2007;86:988–94 [DOI] [PubMed] [Google Scholar]
- 5.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 6.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials. 2010;31:5–11 [DOI] [PubMed] [Google Scholar]
- 7.Howlett J, Ashwell M. Glycemic response and health: summary of a workshop. Am J Clin Nutr. 2008;87:S212–6 [DOI] [PubMed] [Google Scholar]
- 8.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1998;280:356–62 [DOI] [PubMed] [Google Scholar]
- 9.Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–90 [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Block G, Sternfeld B, Sowers M. Dietary glycemic load, glycemic index, and associated factors in a multiethnic cohort of midlife women. J Am Coll Nutr. 2009;28:636–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy DS, Hoelscher DM, Aragaki C, Stevens J, Steffen LM, Pankow JS, Boerwinkle E. Association of glycemic index and glycemic load with risk of incident coronary heart disease among whites and African Americans with and without type 2 diabetes: the Atherosclerosis Risk in Communities Study. Ann Epidemiol. 2010;20:610–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007;CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball SD, Keller KR, Moyer-Mileur LJ, Ding Y-W, Donaldson D, Jackson WD. Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics. 2003;111:488–94 [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Cruz A, Gutierrez-Gonzalez AN, Bacardi-Gascon M. Low glycemic index lunch on satiety in overweight and obese people with type 2 diabetes. Nutr Hosp. 2005;20:348–50 [PubMed] [Google Scholar]
- 15.Warren JM, Henry CJ, Simonite V. Low glycemic index breakfasts and reduced food intake in preadolescent children. Pediatrics. 2003;112:e414. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J, Ristow M, Otto B, Heldwein W, Tschop M, Pfeiffer AF, Mohliq M. Post-prandial decrease of human plasma ghrelin in the absence of insulin. J Endocrinol Invest. 2003;26:RC19–22 [DOI] [PubMed] [Google Scholar]
- 17.Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Smout AJPM, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007;293:E743–53 [DOI] [PubMed] [Google Scholar]
- 18.Chaikomin R, Doran S, Jones KL, Feinle-Bisset C, O'Donovan D, Rayner CK, Horowitz M. Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab. 2005;289:E504–7 [DOI] [PubMed] [Google Scholar]
- 19.Brownley KA, Heymen S, Hinderliter AL, MacIntosh B. Effect of glycemic load on peptide-YY levels in a biracial sample of obese and normal weight women. Obesity (Silver Spring). 2010;18:1297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9 [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WG, Chen X, Jiang H, Jiang ZY. Effects of ghrelin on glucose-sensing and gastric distension sensitive neurons in rat dorsal vagal complex. Regul Pept. 2008;146:169–75 [DOI] [PubMed] [Google Scholar]
- 23.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. [DOI] [PubMed] [Google Scholar]
- 24.le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068–71 [DOI] [PubMed] [Google Scholar]
- 25.Bacha F, Arslanian SA. Ghrelin and peptide YY in youth: are there race-related differences? J Clin Endocrinol Metab. 2006;91:3117–22 [DOI] [PubMed] [Google Scholar]
- 26.Brownley KA, Light KC, Grewen KM, Bragdon EE, Hinderliter AL, West SG. Postprandial ghrelin is elevated in black compared with white women. J Clin Endocrinol Metab. 2004;89:4457–63 [DOI] [PubMed] [Google Scholar]
- 27.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003;88:5510–4 [DOI] [PubMed] [Google Scholar]
- 29.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–9 [DOI] [PubMed] [Google Scholar]
- 30.Krog-Mikkelsen I, Sloth B, Dimitrov D, Tetens I, Bjorck I, Flint A, Holst JJ, Astrup A, Elmstahl H, Raben A. A low glycemic index diet does not affect postprandial energy metabolism but decreases postprandial insulinemia and increases fullness ratings in healthy women. J Nutr. 2011;141:1679–84 [DOI] [PubMed] [Google Scholar]
- 31.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48 [DOI] [PubMed] [Google Scholar]
- 32.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10 [DOI] [PubMed] [Google Scholar]
- 33.Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854–9 [DOI] [PubMed] [Google Scholar]
- 34.Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE, Thorner MO, Geysen HM. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab. 2010;95:2351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burton-Freeman BM, Keim NL. Glycemic index, cholecystokinin, satiety and disinhibition: is there an unappreciated paradox for overweight women? Int J Obes (Lond). 2008;32:1647–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.