Abstract
The longitudinal relationship between stunting and wasting in children is poorly characterized. Instances of wasting or poor weight gain may precede linear growth retardation. We analyzed longitudinal anthropometric data for 1599 children from 8 cohort studies to determine the effect of wasting [weight-for-length Z-score (WLZ) < −2] and variability in WLZ in the first 17 mo on length-for-age Z-score (LAZ) at 18–24 mo of age. In addition, we considered the effects of change in WLZ during the previous 6-mo period on length at 18 and 24 mo. Wasting at 6–11 or 12–17 mo was associated with decreased LAZ; however, children who experienced wasting only at 0–5 mo did not suffer any long-term growth deficits compared with children with no wasting during any period. Children with greater WLZ variability (≥0.5 SD) in the first 17 mo of life were shorter [LAZ = −0.51 SD (95% CI: −0.67, −0.36 SD)] at 18–24 mo of age than children with WLZ variability <0.5. Change in WLZ in the previous 6-mo period was directly associated with greater attained length at 18 mo [0.33 cm (95% CI: 0.11, 0.54 cm)] and 24 mo [0.72 cm (95% CI: 0.52, 0.92 cm)]. Children with wasting, highly variable WLZ, or negative changes in WLZ are at a higher risk for linear growth retardation, although instances of wasting may not be the primary cause of stunting in developing countries.
Introduction
Childhood undernutrition, in the form of stunting, wasting, or intrauterine growth restriction, is an important contributing factor to the high levels of childhood illness and death in developing countries. Undernutrition is an underlying cause of ~2.2 million child deaths and 21% of disability-adjusted life years lost, and >178 million children are stunted in developing countries (1). Therefore, decreasing child undernutrition is an important step in achieving the Millennium Development Goals (2). In addition, the long-term effects of childhood undernutrition include lower attained schooling, decreased economic potential, and chronic illness in adulthood (3–5).
Undernutrition results most commonly from limited quality or quantity of food, suboptimal feeding practices, and high rates of infectious diseases (6, 7). Wasting is usually considered to be a short-term (i.e., acute) response to inadequate intake or an infectious disease episode, whereas stunting is considered to be a longer-term response to a sustained poor dietary intake or repeated illnesses. If linear growth slows over the short term, catch-up growth may occur once the infection is resolved or if diet improves (8–10). However, in many resource-poor settings of low- and middle-income countries, dietary intake is consistently inadequate and infectious diseases are common, impeding the process of catch-up growth and potentially resulting in many stunted children.
Associations between stunting and wasting in children are not consistently found in analyses using cross-sectional data (11–14), likely because wasting is a short-term and potentially seasonal phenomenon resulting from a recent insult (infection or food insecurity), whereas stunting results from a longer term multifactorial process of undernutrition. In addition, wasting may precede linear growth retardation (15); therefore, cross-sectional data may not demonstrate a concurrent relationship. Using a large longitudinal dataset, we sought to explore the longitudinal relationship between stunting and wasting. Specifically, we determined if the history of wasting instances is related to stunting at 18–24 mo of age. By increasing our understanding of these relationships, we can develop better interventions that will improve child health and survival in developing countries.
Participants and Methods
Participants.
We used anthropometric data from 8 longitudinal studies obtained from a network of collaborators, 6 that were compiled previously for an analysis of growth and diarrhea (16–22) plus 2 newly added studies (23, 24) (Table 1). The studies were longitudinal cohorts that were conducted over a period of 2 decades and were performed in Africa, Asia, and Latin America.
TABLE 1.
General description of studies included in the combined dataset including anthropometry data collected in children <24 mo of age
| Dates | Setting (reference) | Design | Purpose |
| 1985–1987 | Lima, Peru (urban) (19) | Observational | Effects of diarrhea on growth |
| 1989–1991 | Lima, Peru (urban) (20) | Observational | Effects of diarrhea on growth |
| 1995–1998 | Lima, Peru (urban) (21) | Observational | Effects of diarrhea on growth |
| 1989–2000 | Goncalves, Brazil (urban) (17) | Observational | Effects of diarrhea on growth |
| 1987–1990 | Bandim, Guinea-Bissau (urban) (22) | Observational | Identify risk factors for diarrhea in Africa |
| 1996–1998 | Bandim, Guinea-Bissau (urban) (18) | Randomized trial | Effects of dietary management of diarrhea on growth |
| 1993–1996 | Mirzapur, Bangladesh (rural) (24) | Observational | Identify risk factors for diarrhea |
| 2002–2006 | Vellore, India (urban) (23) | Observational | Comparative study of rotavirus epidemiology |
Inclusion criteria.
We retained children with at least one complete set of length-for-age Z-scores (LAZ) and weight-for-length Z-scores (WLZ) when they were 0–5, 6–11, 12–17, and 18–23 mo of age. We did not include anthropometric measurements during the first 7 d of life in our analysis, because we wanted to avoid including the natural dip in weight-for-length that sometimes occurs in the first days of life. Among the children who met the criteria for analysis, we further limited the children to those who had length measurements at 6, 12, 18, and 24 mo (±30 d) for an analysis of the effects of changes in WLZ on LAZ at 18 and 24 mo.
Definitions.
We defined stunting as LAZ < −2 and wasting as WLZ < −2. We used the WHO Multicentre Growth Reference Study (MGRS) programs to obtain Z-scores for this analysis (25). Length values that were highly inconsistent (>2.5 cm) with both the previous and following length measurements were not included, because a loss in length of 2.5 cm from one measurement to the next is likely the result of measurement or documentation error. We calculated the individual child’s variability in WLZ in the first 17 mo using the standard deviation formula
 |
and categorized that variability either as <0.5 or ≥0.5 WLZ SD. High WLZ variability may indicate periodic dietary insufficiency or illness, likely due to seasonality in agriculture or infectious diseases.
Biostatistical methods.
Correlation coefficients were calculated for the concurrent comparison of WLZ and LAZ. The proportion stunted at 18–24 mo was calculated by dividing the number of stunted children by the number of children retained in the dataset, using the last measurement for each child (between 18 and 24 mo). The proportion ever wasted at 0–17 mo was calculated as the number of children with any wasting prior to 18 mo of age divided by the number of children retained in the dataset. The odds of stunting at 12 mo were calculated using logistic regression and robust variance, accounting for clustering within study. Included in that model were 3 covariates, an indicator for total number of stunted measurements at the 3 time points (3, 6, and 9 mo). To determine if recent or frequent wasting was associated with deficits in LAZ at 18–24 mo, we considered 8 categories of wasting instances: never wasted, wasted only at 0–5 mo, wasted only at 6–11 mo, wasted only at 12–17 mo, wasted at both 0–5 and 6–11 mo, wasted at both 6–11 and 12–17 mo, wasted at both 0–5 and 12–17 mo, and wasted at all 3 age groups. We used a mixed effects model with study-specific random effects and robust variance, controlling for gender, to model the effect of wasting in the different age groups on LAZ at 18–24 mo, with the never-wasted group as the reference. Mixed effects models are particularly suited to handle longitudinal data with unbalanced measurements over time, as is the case in our multi-cohort data. A similar model was used to assess the effect of WLZ variability on LAZ at 18–24 mo.
We also determined the effect of change in WLZ in the previous interval (6–12 mo for the 18-mo length measurement and 12–18 mo for the 24-mo length measurement) on LAZ at 18 and 24 mo of age using a linear model with study-specific random intercepts and robust variance. For this model, we limited the analysis to the subset of 830 children with anthropometric measurements at 6, 12, 18, and 24 mo of age. For the model with the outcome of LAZ at 18, the covariates included were difference in WLZ from 6 to 12 mo, gender, and LAZ at 12 mo. The model for LAZ at 24 mo was similar, with WLZ difference from 12 to 18 mo, gender, and LAZ at 18 mo. Analyses were subsequently stratified by stunting status at 6 mo of age.
Results
Descriptive statistics
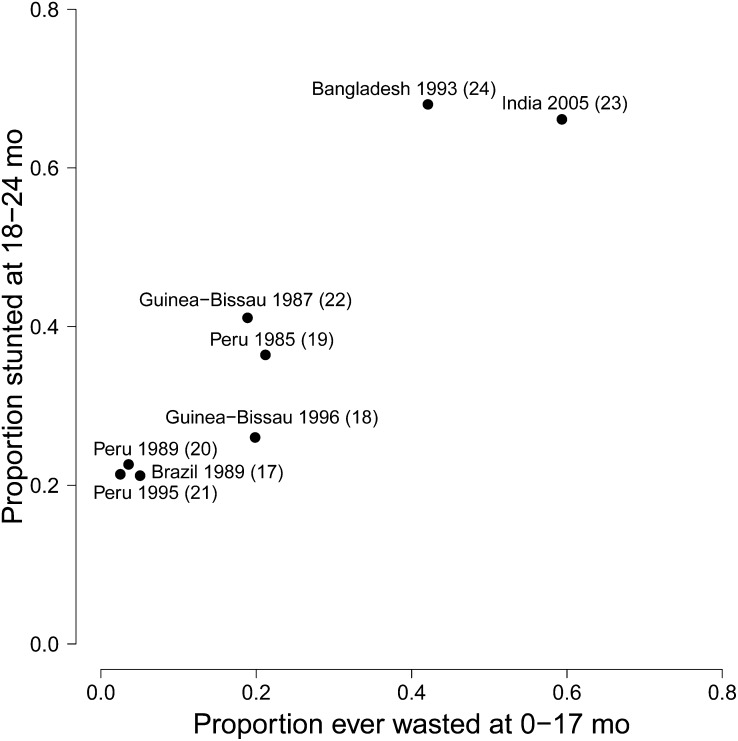
We included 1604 children with 27,117 measurements in our primary analysis (Table 2). Children were measured at least once every 3 mo and some studies measured children monthly. The percent of children with any wasting prior to 18 mo of age ranged from 3 to 59% across studies, and the percent that were stunted at 18–24 mo of age ranged from 21 to 68%. Considering the individual-level data as cross-sectional, the correlation between LAZ and WLZ increased with age (Fig. 1). Prevalence of stunting at 18–24 mo was correlated with history of wasting at the country level = 0.94; P < 0.001) ( .
TABLE 2.
Description of children included in the categorical wasting analysis in the combined dataset including anthropometry data collected in children <24 mo of age
| Setting (reference) | Participants | Weight and length in 4 periods1 | Girls | Mean age at first visit | Mean visits <18 mo | Any wasting 0–17 mo | Stunted at 18–24 mo |
| n | n | n (%) | mo | n | n (%) | n (%) | |
| Peru 1985 (19) | 673 | 118 | 54 (46) | 1.8 | 16 | 25 (21) | 43 (36) |
| Peru 1989 (20) | 217 | 84 | 43 (51) | 1.7 | 14 | 3 (4) | 19 (23) |
| Peru 1995 (21) | 224 | 159 | 70 (44) | 1.3 | 16 | 4 (3) | 34 (21) |
| Brazil 1989 (17) | 119 | 99 | 53 (54) | 3.2 | 5 | 5 (5) | 21 (21) |
| Guinea-Bissau 1987 (22) | 1,143 | 90 | 45 (50) | 3.2 | 5 | 17 (19) | 37 (41) |
| Guinea-Bissau 1996 (18) | 1,027 | 438 | 213 (49) | 2.9 | 7 | 87 (20) | 114 (26) |
| Bangladesh 1993 (24) | 288 | 247 | 106 (43) | 1.2 | 16 | 104 (42) | 168 (68) |
| India 2005 (23) | 373 | 369 | 185 (50) | 1.1 | 17 | 219 (59) | 244 (66) |
The 4 periods are 0–5, 6–11, 12–17, and 18–23 mo.
FIGURE 1.
Cross-sectional relationships between LAZ and WLZ by age group in combined dataset including anthropometry data collected in children <24 mo of age from 8 cohort studies. LAZ, length-for-age Z-score; WLZ, weight-for-length Z-score.
FIGURE 2.
Relationship between proportion stunted at 18–24 mo and history of wasting at 0–17 mo in combined dataset including anthropometry data collected in children <24 mo of age from 8 cohort studies.
Wasting and stunting as acute or chronic conditions.
Wasting is commonly thought to be a short-term condition, whereas stunting is thought to be a chronic condition. To confirm these assumptions, we calculated the percent of measurements that were determined to be wasted or stunted for each child. Persistent stunting was far more common than was persistent wasting. Twenty-five percent of children were found to be stunted in >50% of their measurements, whereas only 2% of children were found to be wasted in >50% of their measurements. Among the 1241 children with measurements at 3, 6, 9, and 12 mo of age, 10% of children were stunted and 1% were wasted at all 4 time points. Similar relationships were obtained when considering children with LAZ and WLZ < −1 (31% of children had LAZ < −1 and 7% of children had WLZ < −1 at all 4 time points). The likelihood of stunting at 12 mo of age increased as the number of prior measurements with stunting increased. Children with one stunted measurement prior to 12 mo of age were more likely to be stunted at 12 mo [OR = 2.6 (95% CI: 2.1, 3.1)] compared with children who had no stunting in the first 11 mo. The odds increased with additional stunted measurements at 3, 6, and/or 9 mo [2 stunted measurements: OR = 3.9 (95% CI: 3.0, 4.8); 3 stunted measurements: OR = 5.5 (95% CI: 4.9, 6.1)]. Nearly 80% (334/421, 79.3%) of children with stunting at 3, 6, or 9 mo were stunted at 12 mo, demonstrating limited recovery from stunting. Among the 177 children (14%) with wasting at 3, 6, or 9 mo, 70% (123) were not wasted at 12 mo of age, demonstrating recovery from wasting. Wasting appears to be more acute and reversible than stunting.
Natural history of malnutrition in early childhood
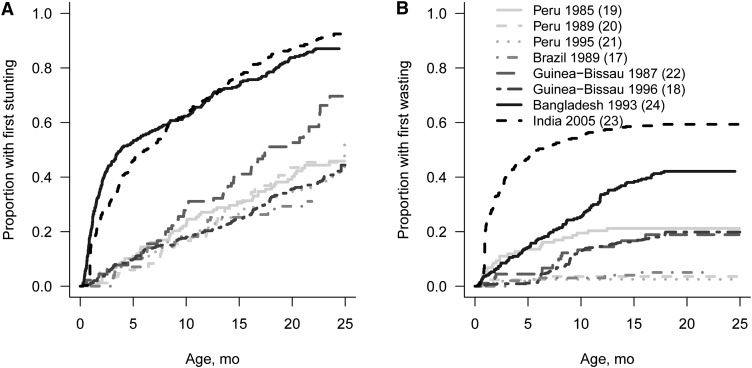
The first instances of stunting and wasting occurred at different times across studies. The percent of children who experienced their first stunting measurement continued to increase steadily throughout the first 2 y of life, but most of the initial wasting occurred primarily in the first 6–12 mo of life in most countries (Fig. 3). The prevalence of wasting was highest in the first 1–3 mo of age (~15%) and decreased thereafter (Fig. 4). Wasting prevalence in Asia and Latin America decreased over time, whereas in Guinea-Bissau, wasting prevalence appeared to increase until 9 mo of age, at which time a plateau was observed.
FIGURE 3.
Cumulative incidence of first stunting (A) and wasting (B) measurements in combined dataset including anthropometry data collected in children <24 mo of age from 8 cohort studies.
FIGURE 4.
Prevalence of wasting (percentage of measurements with WLZ < −2) by month of age in combined dataset including anthropometry data collected in children <24 mo of age from 8 cohort studies and by region, with cubic smoothing splines added to emphasize trend. Latin America includes Peru (3 studies) and Brazil, Asia includes Bangladesh and India, and Africa includes Guinea-Bissau (2 studies). WLZ, weight-for-length Z-score.
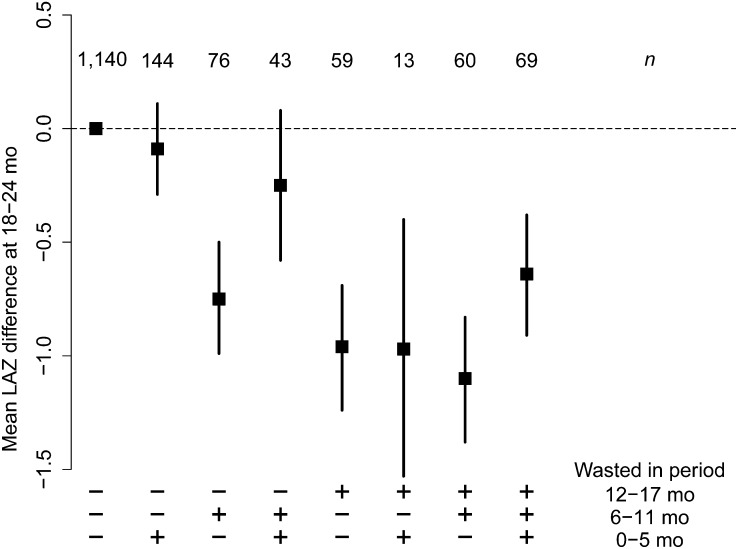
Longitudinal instances of wasting on LAZ
We identified the mean effect of all of the different permutations of wasting in the three 6-mo periods on LAZ (Fig. 5) compared with children without wasting. Although the numbers are small in some categories, they demonstrate no long-term effect of wasting in the first 6 mo of life on LAZ at 18–24 mo if no further wasting was identified, whereas more recent wasting was associated with lower LAZ at 18–24 mo. Similar results were observed when the analysis was done by geographic region (Supplemental Fig. 1) and including only children who were not stunted at baseline (Supplemental Fig. 2).
FIGURE 5.
Effect of wasting in different age groups (+ indicates wasting was experienced during that period, – indicates no wasting during that period) on mean LAZ at 18–24 mo in combined dataset including anthropometry data collected in children <24 mo of age. Number of participants (n) contributing data to each category is at the top of the figure. LAZ, length-for-age Z-score.
WLZ variability on LAZ
Children with greater WLZ variability in the first 17 mo of life were shorter than those with lower WLZ variability [LAZ = −0.51 (95% CI: −0.67, −0.36)]. The relationship appeared relatively homogenous across regions and among children who were stunted/not stunted at baseline (Supplemental Fig. 3). Mean WLZ was also included in the model and was directly associated with attained length at 18–24 mo [LAZ = 0.35 (95% CI: 0.29, 0.40)].
Association between LAZ and change in WLZ in previous period
We also considered the effect of a change in WLZ during the previous 6-mo period on LAZ at 18 and 24 mo. A 1-unit increase in WLZ from 6 to 12 mo of age was associated with increased LAZ at 18 mo of age [0.13 LAZ (95% CI: 0.09, 0.17)] after controlling for gender and LAZ at 12 mo of age. A 1-unit increase in WLZ from 12 to 18 mo of age was not significantly associated with a mean difference in LAZ at 24 mo [0.03 LAZ (95% CI: −0.01, 0.08)]. Similar results were found in 18-mo-old children who were stunted at 6 mo of age [0.13 LAZ (95% CI: 0.05, 0.21)] and in those who were not [0.14 LAZ (95% CI: 0.09, 0.19)]. For the 24-mo-old children, there was no significant effect of a 1-unit increase of WLZ from 12 to 18 mo on LAZ at 24 mo for the children who were stunted at 6 mo of age [−0.03 (95% CI: −0.12, 0.06)], but there was a significant effect in children who were not [0.06 LAZ (95% CI: 0.01, 0.10)].
Discussion
In this study, children with wasting only in the first 6 mo of life had similar LAZ at 18–24 mo to those with no wasting in any period, whereas more recent wasting appeared to be associated with stunting and a lower LAZ compared with children with no wasting. Greater variability in WLZ during the first 17 mo of life was associated with lower LAZ compared with children with lower WLZ variability at 18–24 mo. Finally, positive changes in WLZ during the previous 6-mo period (6–12, 12–18) were found to be associated with greater length at 18 and 24 mo.
In the past, investigations describing the relationship between stunting and wasting have primarily been cross-sectional and attempts to describe the relationships in a longitudinal fashion have faced sample size limitations, because wasting is relatively rare and generally short term. By combining data from 8 different studies, we were able to overcome some of the sample size limitations and explore the longitudinal experience of stunting and wasting in children <2 y of age. However, even in the multi-country cohort study, sample size was limited, because many of the studies began collecting data on older children and we wanted to consider the impact of wasting in early childhood.
Victora (11) found a correlation between concurrent stunting and wasting prevalence in Asia and the Eastern Mediterranean, but low correlation in Africa or Latin America; however, he found comparable degrees of stunting across the regions, leading him to conclude that wasting and stunting prevalence may reflect underlying dietary insufficiency in different ways. This notion was confirmed later by Frongillo and Hanson (26), who determined that national characteristics explained many of the differences among the regions. Stunting occurs as a response to a number of different acute and chronic factors, including micro- and macro-nutrient deficiencies, serial infectious diseases, inadequate feeding practices, and exposure to pathogens in the environment, among others. Wasting, on the other hand, is thought to be a short-term response to food shortage or infectious disease that may or may not lead to stunting, depending on whether the child is able to recover his or her trajectory via catch-up growth. Areas with a lower prevalence of wasting may continue to have a relatively high stunting prevalence because of ongoing nutritional deficiencies.
First, we explored the wasting and stunting experiences of these children to determine if these were acute or chronic states. Far more children were stunted than wasted and children with stunting in early life were unlikely to become nonstunted later, whereas most of those children who experienced wasting recovered WLZ by the next anthropometric assessment. Wasting, in general, appeared to be an infrequent and temporary state, whereas stunting was more likely to be chronic and irreversible.
Children who were wasted only in the first 6 mo of life were not observed to have linear growth deficits at the end of follow-up compared with children who were never wasted. Presumably, catch-up growth in length was adequate for those children who had their only wasting during the first 6 mo of life, whereas time was insufficient for catch-up linear growth in those children with more recent wasting. Alternatively, perhaps catch-up growth in length does not occur as readily in older age groups and if we were to follow these children into their third or fourth year of life, we would find persistent linear deficits. Children with wasting in the first 2 time periods (0–5 and 6–11 mo) did not differ from those children with no wasting in LAZ at 18–24 mo, and this could be because of catch-up growth in these 43 children during the 12- to 17-mo time period. Wasting in all 3 time periods appeared to have less of a detrimental effect on LAZ than wasting in the 12- to 17-mo time period. We found that the results were similar when the models were run on the subgroups of children who were stunted and nonstunted at baseline.
Stunting is far more common than prevalence of earlier wasting instances can explain. It is likely that the cause of stunting in each country is due to a mixture of exposures, some having more to do with quality of diet or lack of specific micronutrients, others having to do with environmental exposures or access to treatment of infectious diseases, and only some of these potential causes would involve wasting. To look at these factors across cultures and better determine the role of wasting in stunting, one would require a large, longitudinal, international study with standardized protocols and comprehensive follow-up. Wasting is a short-term condition that is incompletely ascertained through infrequent anthropometric measurements; therefore, we consider the findings from this study to be underestimates of the actual relationships. In addition, the definition of wasting is somewhat insensitive due to the binary nature of the WLZ <−2 cutoff. A study with larger sample sizes and comprehensive follow up will allow for a more thorough investigation of the relationship between changes in weight and length in early childhood.
A novel way of looking at undernutrition is to consider the variability in WLZ as a risk factor for stunting. Children who vary considerably in their WLZ are presumably subject to food insecurity and seasonal infections. Thus, swings in WLZ may result in linear growth faltering. Mean LAZ was lower among children who had greater variability in WLZ, suggesting that perturbations in the weight acquisition process can have a lasting impact on linear growth.
Walker et al. (15) described an association between change in weight-for-length during the previous 6-mo interval and attained length in the following interval in stunted children. There are several differences between our study and the Walker et al. (15) study. First, Walker et al. (15) had a smaller sample size compared with our study and only included children who were stunted at baseline, whereas we had both stunted and nonstunted children at baseline in our dataset. Second, children in the Walker et al. (15) study were 9–24 mo at baseline and our analysis included children who were ~6 mo at baseline. Third, the analysis performed by Walker et al. (15) combined the children of different ages and used time in study to categorize them, whereas we compared the impact of previous change in WLZ on LAZ in children of the same age. Even with these differences in study design, our results concur with the findings of Walker et al. (15) that the change in weight-for-length during the previous 6-mo period may be associated with length in early childhood.
Childhood undernutrition is a risk factor for child illness and death, and attained growth has been associated with economic productivity and health status in adulthood (3, 5). Prevention of undernutrition is therefore highly important. This study indicates that acute malnutrition in the form of wasting is associated with the process of stunting, and prevention of wasting could potentially increase attained stature in children. Increasing the nutritional status of children involves a multidisciplinary approach that includes improved dietary quality and quantity, and better disease control strategies.
Supplementary Material
Acknowledgments
S.A.R. and W.C. were responsible for the development of the concept and methods; S.A.R. conducted the analysis and wrote the first draft of the manuscript. Each of the 8 studies included in this analysis are represented by one author who participated in the original collection of data. All authors edited the manuscript and assisted in interpretation of the results. All authors read and approved the final manuscript.
Footnotes
Supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, NIH (to W. Checkley).
Supplemental Figures 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Other members of the Childhood Malnutrition and Infection Network include: Dr. Sean R Moore (University of Cincinnati, USA), Dr. Aldo AM Lima (Universidade Federal do Ceará in Fortaleza, Brazil), Dr. Relana C Pinkerton (University of Virginia, USA), Dr. Peter Aaby (Statens Serum Institut, Denmark and the Bandim Health Project, Bissau, Guinea-Bissau), Lilia Z Cabrera (A.B. PRISMA, Peru), Dr. Caryn Bern (Centers for Disease Control, USA), Dr. Charles R Sterling (University of Arizona, USA), Dr. Leonardo D Epstein (Universidad Adolfo Ibanez, Chile), Dr. Lawrence Moulton (Johns Hopkins University, USA), Dr. Michael Perch (Statens Serum Institut, Copenhagen), Dr. Thea K Fischer (Statens Serum Institut, Copenhagen), Dr. Halvor Sommerfelt (Center of International Health, University of Bergen, Norway), Dr. Hans Steinsland (Center of International Health, University of Bergen, Norway), Hector Verastegui (Instituto de Investigación Nutricional, Perú).
Literature Cited
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 2.The Millennium Development Goals Report 2010. New York: United Nations Department of Economic and Social Affairs; 2010 [Google Scholar]
- 3.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371:411–6 [DOI] [PubMed] [Google Scholar]
- 5.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller W. The epidemiology of stunting. : Waterlow JC, editor Linear growth retardation in developing countries. New York: Nestec Ltd., Vevey/Raven Press, Ltd; 1988. p. 17–38 [Google Scholar]
- 7.Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. Disease control priorities in developing countries (2nd edition). New York: Oxford University Press; 2006. pp. 551–68 [Google Scholar]
- 8.Ashworth A. Growth rates in children recovering from protein-calorie malnutrition. Br J Nutr. 1969;23:835–45 [DOI] [PubMed] [Google Scholar]
- 9.Walker SP, Golden MHN. Growth in length of children recovering from severe malnutrition. Eur J Clin Nutr. 1988;42:395–404 [PubMed] [Google Scholar]
- 10.Waterlow JC. Observations on the natural history of stunting. : Waterlow JC, editor Linear growth retardation in developing countries. New York: Nestec Ltd., Vevey/Raven Press, Ltd; 1988. p. 1–16 [Google Scholar]
- 11.Victora CG. The association between wasting and stunting: an international perspective. J Nutr. 1992;122:1105–10 [DOI] [PubMed] [Google Scholar]
- 12.Keller W. Choice of indicators of nutritional status. : Schurch B, editor Evaluation of nutrition education in third world communities. Bern: Hans Huber; 1983. p. 101–13 [Google Scholar]
- 13.Martorell R. Child growth retardation: a discussion of its causes and its relationship to health. : Blaxter KL WJ, editor Nutritional adaptation in man. London: John Libbey; 1985. p. 13–30 [Google Scholar]
- 14.Gorstein J, Sullivan K, Yip R, de Onis M, Trowbridge F, Fajans P, Clugston G. Issues in the assessment of nutritional status using anthropometry. Bull World Health Organ. 1994;72:273–83 [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SP, Grantham-McGregor SM, Himes JH, Powell CA. Relationships between wasting and linear growth in stunted children. Acta Paediatr. 1996;85:666–9 [DOI] [PubMed] [Google Scholar]
- 16.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–64 [DOI] [PubMed] [Google Scholar]
- 18.Valentiner-Branth P, Steinsland H, Santos G, Perch M, Begtrup K, Bhan MK, Dias F, Aaby P, Sommerfelt H, Molbak K. Community-based controlled trial of dietary management of children with persistent diarrhea: sustained beneficial effect on ponderal and linear growth. Am J Clin Nutr. 2001;73:968–74 [DOI] [PubMed] [Google Scholar]
- 19.Lanata CF, Black RE, Maurtua D, Gil A, Gabilondo A, Yi A, Miranda E, Gilman RH, Leon-Barua R, Sack RB. Etiologic agents in acute vs persistent diarrhea in children under three years of age in peri-urban Lima, Peru. Acta Paediatr Suppl. 1992;381:32–8 [DOI] [PubMed] [Google Scholar]
- 20.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506 [DOI] [PubMed] [Google Scholar]
- 21.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–75 [DOI] [PubMed] [Google Scholar]
- 22.Mølbak K, Jensen H, Ingholt L, Aaby P. Risk factors for diarrheal disease incidence in early childhood: a community cohort study from Guinea-Bissau. Am J Epidemiol. 1997;146:273–82 [DOI] [PubMed] [Google Scholar]
- 23.Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le Fevre A, Iturriza-Gomara M, Gray JJ, Bose A, Estes MK, Brown DW, et al. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathela P, Zahid Hasan K, Roy E, Huq F, Kasem Siddique A, Bradley Sack R. Diarrheal illness in a cohort of children 0–2 years of age in rural Bangladesh. I. Incidence and risk factors. Acta Paediatr. 2006;95:430–7 [DOI] [PubMed] [Google Scholar]
- 25.WHO WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: WHO; 2006 [Google Scholar]
- 26.Frongillo EA, Jr, de Onis M, Hanson KM. Socioeconomic and demographic factors are associated with worldwide patterns of stunting and wasting of children. J Nutr. 1997;127:2302–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.