Abstract
Over the last several years, national programs to lower the content of industrially produced (IP) C18:1 and C18:2 trans fatty acids in foods have been implemented, but whether this has resulted in lower blood trans fatty acid levels is unknown. Likewise, an increased perception of the health benefits of fish oils rich in EPA and DHA may have resulted in an increase in consumption and blood levels of these fatty acids. To explore these issues, we analyzed the changes in RBC fatty acid composition between the 7th (1998–2001) and 8th (2005–2007) examination cycles in a random sample of the Framingham Offspring cohort. This was a retrospective cohort study of 291 participants from whom blood was drawn at both examinations and for whom complete covariate data were available. Overall, the proportion of trans fatty acids in RBC changed by −23% (95% CI: −26 to −21%). RBC EPA+DHA proportions increased by 41% (95% CI: 31 to 52%) in 38 individuals who were taking fish oil supplements at examination 8, but in 253 participants not taking fish oil, the proportion of RBC EPA+DHA did not change. In conclusion, in a random subsample of Framingham Offspring participants with serial observations over 6.7 y, the proportion of trans fatty acids in RBC decreased. Those of EPA+DHA increased in people taking fish oil supplements. These changes could potentially translate into a lower risk for cardiovascular disease.
Introduction
The 2 types of dietary fatty acids that are likely to affect their blood levels most are the trans and long-chain (n-3) fatty acids, in great part because neither is synthesized to a large extent in vivo. Trans versions of C18:1 and C18:2 derive largely from industrially produced (IP)10, partially hydrogenated vegetable oils (1). A small proportion (~20%) of the total trans fatty acid intake comes from ruminant-produced (RP) fats (with trans C16:1 as a marker) (2). The marine (n-3) fatty acids [EPA, docosapentaeonoic acid, and DHA] are derived almost exclusively from fish and fish oils (3). Both of these classes of fatty acids have been associated with risk for cardiovascular disease: beneficially with the (n-3) class (4) and adversely with industrially produced trans-fatty acid (IP-trans) (5). The risk posed by ruminant-produced trans-fatty acid (RP-trans) remains unclear (6). Consequently, changes in blood/tissue levels of these fatty acids may be expected to alter cardiovascular risk, either directly or via changes in surrogate risk markers.
Beginning in the late 1990s, efforts to remove IP-trans fatty acids from the food supply were underway in the US (1) and other countries (7), most notably Denmark, where they were effectively banned in the early 2000s (8). The effects that these efforts have had on IP-trans fat intakes in the US are unknown due to the inability of major nutrient databases to keep abreast of the constant changes in food composition. However, if blood levels of IP-trans fatty acids decreased over the last decade, then it would be reasonable to conclude that the national efforts to remove IP-trans fats from the food supply were at least somewhat successful, as suggested by a recent report from the NHANES survey (9).
Just as efforts to decrease trans fatty acids in the diet were growing, recommendations to increase the intake of the long-chain (n-3) fatty acids were being made by the AHA (3) and other governmental agencies around the world (10). As with the trans fatty acids, the most reliable indicator that such advice was being heeded would be changes in circulating levels of the marine (n-3) fatty acids in the population. The RBC membrane has been used by several investigators to quantify relatively long-term dietary exposure to trans and marine (n-3) dietary fatty acids (11–13) and has been shown to correlate with outcomes (e.g., sudden cardiac death) better than dietary questionnaire-derived estimates of (n-3) fatty acid intake (13). The purpose of this study was to track serial changes in RBC fatty acid content in a well-characterized cohort of American adults between the late 1990s and mid-2000s to determine the extent to which fatty acid intakes may have changed.
Methods
Study sample.
Children (and their spouses) of the original Framingham Heart Study cohort, recruited in 1971, constitute the Framingham Offspring Study (14). For this study, 299 participants were randomly selected who had RBC available at both examination cycles 7 (1998–2001) and 8 (2005–2007). The study was approved by the institutional review board of Boston University Medical Center.
Laboratory methods.
Methods for the analysis of non-HDL and HDL cholesterol and creatinine were previously described (14). The fatty acid content of unwashed, packed RBC was measured in cells isolated from blood drawn after a 10- to 12-h fast and frozen at −80°C immediately after collection. Methanol containing 14% boron trifluoride (Sigma-Aldrich) and hexane (EMD Chemicals) were sequentially added (250 μL each) to a 25-μL aliquot of RBC. The vial was briefly vortexed and then placed in an aluminum bead hot bath at 100°C for 10 min. After cooling, 250 μL of HPLC grade water was added, the tubes were recapped, vortexed, and centrifuged for 3 min at 1500 × g to separate layers. A 50-μL aliquot of the hexane (upper) layer was transferred to a GC vial. FAME analysis was carried out using a GC2010 Gas Chromatograph (Shimadzu) equipped with a SP2560, 100-m fused silica capillary column (0.25-mm i.d., 0.2-μm film thickness; Supelco). Run conditions were: carrier gas, hydrogen; linear velocity, 22 cm/s; injector temperature, 230oC; oven program, initial temperature of 140oC hold for 5 min, ramp temperature at a rate of 4oC/min to 240oC and hold for 15 min (total run time, 45 min); and detector temperature, 240oC. FAME were identified by comparison with a standard mixture of fatty acids characteristic of RBC (GLC 727, NuCheck Prep), which was also used to determine individual FAME response factors. Fatty acid composition was expressed as a percentage of total identified FAME. The chromatographic conditions used in this study were sufficient to isolate the C16:1 trans isomers [marker for RP trans (15)] and the C18:2 Δ 9t-12c, 9t-12t, and 9c-12t isomers. However, each individual C18:1 trans molecular species (i.e., C18:1 Δ6 thru Δ13) could not be segregated but appeared as 2 blended peaks that eluted just before oleic acid. The areas of these 2 peaks were summed and referred to a C18:1 trans. The sum of all C18:2 and C18:1 trans species is here termed IP-trans (16), although these 2 classes were also separately analyzed due to their potential differential associations with cardiovascular disease (11). The CV were 3% for EPA + DHA and 7% for total trans fats.
Diet assessment.
(n-3) and trans fatty acid intakes at examinations 7 and 8 were estimated by FFQ (17). The validity of this instrument for fatty acid intake has been documented by comparison with adipose tissue fatty acid composition (18, 19). A single nutrient database that was released in 2002 was used to calculate the fatty acid composition of diets at both examinations, because 2 separate databases reflecting food composition during 1998–2001 and 2005–2007 did not exist. The composition of foods containing (n-3) fatty acids was largely stable from 1998 to 2007, so using a single nutrient database provided relatively accurate estimates of intake at both examinations (although only the sum of EPA+DHA was available, not the individual fatty acids nor docosapentaenoic acid). However, due to the continual reformulation of cooking fats and oils during the study period in anticipation of new federal labeling regulations, which were to take effect in January 2006, the trans fat data are more likely to reflect food composition at examination 7 than at examination 8. Therefore, estimates of the change in trans fat intakes between these examinations is likely to be biased and was not attempted; the baseline (1999) intake estimates should be reasonably accurate and are thus reported. Fish oil supplement use was self-reported under Medications as “fish oil” or “omega-3” but not “flaxseed oil” and was available at both examinations.
Statistical methods.
Samples from 299 participants who had RBC available at examinations 7 (1998–2001) and 8 (2005–2007) were randomly selected to study temporal changes in fatty acid composition. One participant was excluded due to gastric by-pass surgery between examinations and 7 participants were missing covariates, leaving 291 participants for analyses. Differences between examinations 7 and 8 in fatty acids and patient characteristics were tested using paired t test for continuous and McNemar’s test for categorical variables.
Changes between examinations 7 and 8 in RBC content of C18:1t + C18:2t (IP-trans species) and EPA+DHA [omega-3 index (21)] were analyzed as the dependent variables in adjusted linear models that included age, sex, and the baseline RBC fatty acid level at examination 7. PROC GLMSELECT was used with stepwise variable selection and Schwarz Bayesian Criterion (20) to select the best model (21) from 14 candidate variables (fish oil use at examination 8, changes in the following variables: non-HDL and HDL cholesterol, TG, statin use, dietary fatty acid intake, serum creatinine, diastolic and systolic blood pressure, BMI, glasses of wine per week, initiated hypertension treatment, newly diagnosed diabetes, and smoking cessation). Residual diagnostics were conducted to verify model assumptions.
The critical level α was set to 0.05 for the a priori hypotheses and Bonferroni adjustment was used for univariate differences in participant characteristics (0.05/15 = 0.0033) and individual fatty acids (0.05/22 = 0.0023). Analyses were performed using SAS software (version 9.2; SAS Institute).
Results
Potential explanatory baseline variables (examination 7) and their differences at examination 8 were identified (Table 1). At baseline, 97% of the trans fatty acid intake was from IP-trans. The intake of RP-trans did not change (P = 0.86), but, as discussed earlier, the change in IP-trans intake could not be reliably calculated. The dietary EPA+DHA intake increased by ~20% between the examinations (P = 0.002).
TABLE 1.
Changes in variables potentially affecting proportions of RBC fatty acids in a random sample of the Framingham Offspring cohort between 1996 and 20071
| Variable | Examination 7 | Examination 8 – Examination 7 |
||
| n initiating/n discontinuing | P value2 | |||
| Statin use, n (%) | 58 (20) | 70/15 | <0.0001 | |
| Serum non-HDL-C, mmol/L | 3.81 ± 0.93 | −0.52 ± 1.01 | <0.0001 | |
| Serum TG, mmol/L | 1.48 ± 0.81 | −0.17 ± 0.71 | <0.0001 | |
| Serum HDL-C, mmol/L | 1.45 ± 0.47 | 0.05 ± 0.26 | 0.0034 | |
| Hypertension treatment, n (%) | 97 (33) | 60/4 | <0.0001 | |
| Diastolic, mm Hg | 75 ± 9.5 | −2.6 ± 10 | <0.0001 | |
| Systolic, mm Hg | 127 ± 18 | 2.5 ± 17 | 0.013 | |
| Diabetes, n (%) | 31 (11) | 16/0 | <0.0001 | |
| Serum creatinine, μmol/L | 76 ± 16 | 3.5 ± 14.1 | 0.0001 | |
| Current smoker, n (%) | 26 (9) | 1/7 | 0.034 | |
| BMI, kg/m2 | 28.1 ± 5.1 | 0.2 ± 2.1 | 0.13 | |
| Wine, glass/wk | 2.2 ± 3.5 | −0.2 ± 2.7 | 0.22 | |
| Fish oil supplementation, n (%) | 9 (3) | 33/4 | <0.0001 | |
| Dietary intake,3 g/d | ||||
| 16:1 trans | 0.09 ± 0.05 | 0.004 ± 0.05 | 0.86 | |
| 18:1 trans | 2.00 ± 1.13 | —5 | ||
| 18:2 trans | 0.49 ± 0.26 | — | ||
| EPA+DHA | 0.30 ± 0.24 | 0.06 ± 0.29 | 0.0019 | |
Values are mean ± SD, = 291 unless otherwise indicated. Age at examination 7 was 62 ± 9 y and the group was 41% male. HDL-C, HDL cholesterol.
Bonferroni significance is 0.05/15 tests or <0.0033.
Dietary intake had ~9 and 15% missing data for individual exams and paired differences, respectively.
<0.01 g/d.
Nutrition database was released in 2002 and dietary intakes of C18 fats are valid for only examination 7.
due to subsequent reformulation of foods and cooking oils.
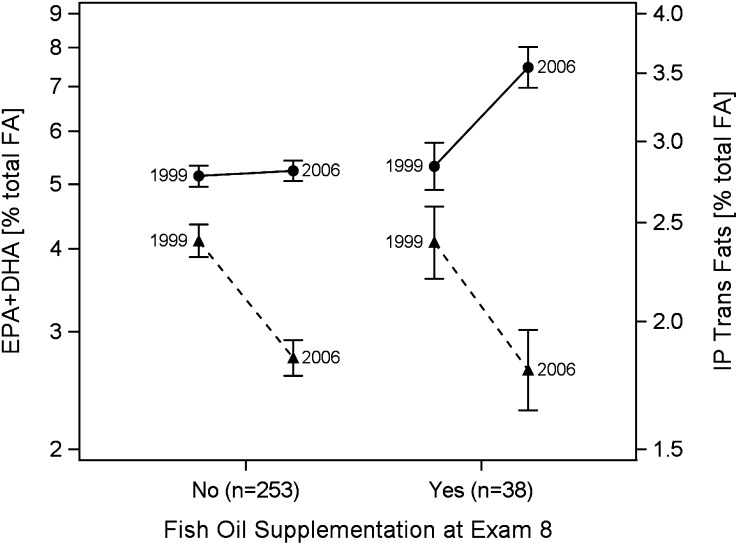
At examination 7, IP-trans fatty acids constituted 93% of the total RBC trans fatty acids (Table 2). At examination 8, total trans fatty acid content was reduced by 21%, with the IP-subset accounting for 98% of the total change. At examination 7, EPA+DHA accounted for 67% of the total marine (n-3) fatty acids in RBC membranes and 71% of the change in total marine (n-3) fatty acids was from increases in EPA and DHA. The changes in proportions of (n-3) fatty acid between examinations 7 and 8 were driven predominantly by the 38 participants who reported taking fish oil at examination 8. In these participants, the omega-3 index increased by 41% (Fig. 1), whereas it did not significantly change in the 253 that were not taking fish oil. The 23% decrease in the proportion of IP-trans in RBC was unaffected by fish oil supplementation status (Fig. 1).
TABLE 2.
Changes in proportions of RBC fatty acids in a random sample of the Framingham Offspring cohort between 1999 and 20061
| Fatty acid | Examination 7 | Examination 8 – Examination 7 paired change |
|
| % total fatty acids | P value2 | ||
| 18:1 trans | 2.15 ± 0.63 | −0.47 ± 0.53 | <0.0001 |
| 18:2 trans | 0.35 ± 0.09 | −0.09 ± 0.10 | <0.0001 |
| 16:1 trans | 0.18 ± 0.04 | −0.01 ± 0.05 | 0.0019 |
| 20:5(n-3) | 0.60 ± 0.28 | 0.17 ± 0.43 | <0.0001 |
| 22:5(n-3) | 2.59 ± 0.34 | 0.15 ± 0.48 | <0.0001 |
| 22:6(n-3) | 4.76 ± 1.31 | 0.20 ± 1.15 | 0.0030 |
| 14:0 | 0.34 ± 0.09 | −0.04 ± 0.11 | <0.0001 |
| 16:0 | 21.5 ± 1.27 | −0.13 ± 1.22 | 0.067 |
| 18:0 | 17.7 ± 0.92 | 0.18 ± 1.04 | 0.0030 |
| 24:0 | 0.44 ± 0.12 | −0.01 ± 0.19 | 0.43 |
| 16:1 | 0.38 ± 0.18 | 0.01 ± 0.20 | 0.21 |
| 18:1 | 13.8 ± 1.02 | 0.18 ± 0.88 | 0.0006 |
| 20:1 | 0.23 ± 0.04 | 0.04 ± 0.10 | <0.0001 |
| 24:1 | 0.43 ± 0.13 | 0.01 ± 0.20 | 0.31 |
| 18:3(n-3) | 0.20 ± 0.09 | −0.02 ± 0.13 | 0.013 |
| 18:2(n-6) | 11.0 ± 1.46 | 0.28 ± 1.90 | 0.012 |
| 18:3(n-6) | 0.06 ± 0.03 | 0.04 ± 0.09 | <0.0001 |
| 20:2(n-6) | 0.29 ± 0.04 | −0.01 ± 0.04 | 0.015 |
| 20:3(n-6) | 1.65 ± 0.36 | −0.06 ± 0.22 | <0.0001 |
| 20:4(n-6) | 16.8 ± 1.31 | −0.23 ± 1.38 | 0.0056 |
| 22:4(n-6) | 3.83 ± 0.77 | −0.17 ± 0.71 | <0.0001 |
| 22:5(n-6) | 0.70 ± 0.17 | −0.05 ± 0.17 | <0.0001 |
Values are mean ± SD, = 291.
Bonferroni significance is 0.05/22 tests or <0.0023.
FIGURE 1.
Changes between 1999 and 2006 in proportions of IP-trans (C18:1t+C18:2t, dashed lines) and EPA+DHA (solid lines) in RBC from a random sample of the Framingham Offspring cohort by use of fish oil supplements at examination 8. Values are geometric mean (95% CI). For IP-trans, the −23% (−26 to −21%; P < 0.0001) was independent of fish oil use. For EPA+DHA, levels increased by 41% (95%CI: 31 to 52%; P < 0.0001) in those supplementing at examination 8 but did not change (P = 0.46) in those not taking fish oil. IP-trans, industrially produced trans-fatty acid.
With respect to which fatty acids may have replaced those that changed between examinations, the 0.57% decrease in total trans fatty acids appears to have been predominately offset by small increases in stearic and oleic acids (Table 2). The 0.52% increase in the marine (n-3) fatty acids appears to have been compensated for by similar decreases in the 20- and 22-carbon, highly unsaturated (n-6) fatty acids.
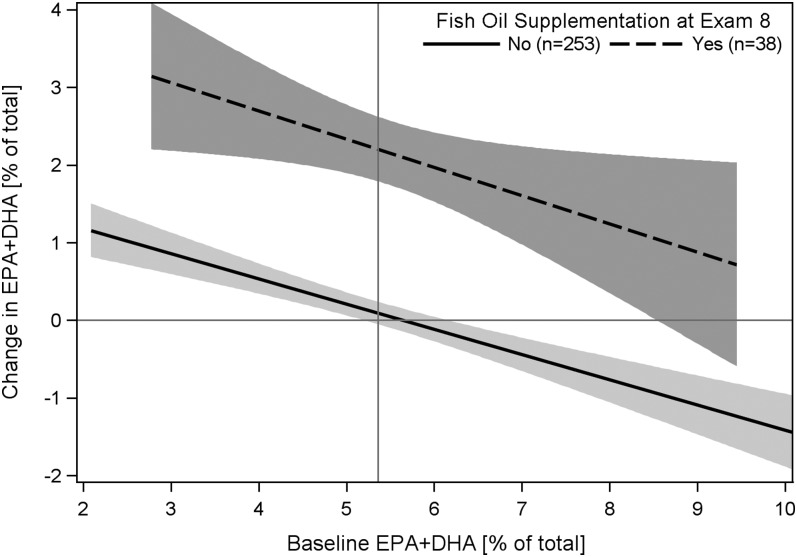
We further explored factors associated with changes in the IP-trans and EPA+DHA using multivariable linear regression and allowed the variables listed in Table 1 to enter the models. The changes in the omega-3 index were directly related to fish oil supplementation, dietary EPA+DHA intake, changes in wine consumption, and starting statins and inversely related to baseline EPA+DHA level (Table 3). In the adjusted model, the omega-3 index increased by ~2 percentage points for participants who used fish oil supplements at examination 8, regardless of their RBC baseline level. The negative slope estimate for the change in the index compared with baseline values illustrates regression to the mean regardless of supplementation status (Fig. 2). Changes in the proportions of IP-trans were directly related to baseline age and male gender and to changes in non-HDL cholesterol (non-HDL-C) and were inversely related to changes in HDL-C and baseline IP-trans levels (again, regression to the mean) (Table 3). The multivariable models explained 33 and 44% of the variability in changes of IP-trans and EPA+DHA, respectively. As noted above, the increases in the omega-3 index were due mainly to the participants who were taking fish oil supplements at examination 8; however, these participants also significantly increased their dietary EPA+DHA by 241 ± 275 mg/d (means ± SD). There was no significant change in dietary EPA+DHA intake in the participants that were not supplementing at examination 8 (30 ± 287 mg/d).
TABLE 3.
Variables associated with changes in proportions of RBC fatty acids in a random sample of the Framingham Offspring cohort between 1999 and 2006
| Estimate | SE | P value | Lower | Upper | |
| 95% CI | |||||
| 18:1 trans + 18:2 trans (n = 291) | |||||
| Sex (male)1 | 0.13 | 0.06 | 0.029 | 0.01 | 0.25 |
| Age (10 y)1 | 0.10 | 0.03 | 0.0007 | 0.04 | 0.16 |
| Baseline RBC 18:1t + 18:2t1 | −0.46 | 0.04 | <0.0001 | −0.54 | −0.38 |
| Change in non-HDL-C (1 SD = 1 mmol/L) | 0.09 | 0.03 | 0.0032 | 0.03 | 0.15 |
| Change in HDL-C (1 SD = 0.26 mmol/L) | −0.07 | 0.03 | 0.0085 | −0.13 | −0.02 |
| EPA + DHA (n = 246)2 | |||||
| Sex (male)1 | 0.15 | 0.14 | 0.30 | −0.13 | 0.42 |
| Age (10 y)1 | 0.01 | 0.08 | 0.94 | −0.14 | 0.16 |
| Baseline RBC EPA + DHA1 | −0.31 | 0.05 | <0.0001 | −0.40 | −0.22 |
| Fish oil supplementation at examination 8 | 2.04 | 0.20 | <0.0001 | 1.64 | 2.45 |
| Change in EPA+DHA dietary intake (1 SD = 294 mg) | 0.22 | 0.07 | 0.0024 | 0.08 | 0.36 |
| Started statins | 0.48 | 0.16 | 0.0031 | 0.16 | 0.80 |
| Change in drinking wine (+1 glass/wk) | 0.06 | 0.03 | 0.016 | 0.01 | 0.11 |
Forced into Multivariable Linear Regression Models.
The sample size was reduced from 291 to 246 due to missing dietary information.
FIGURE 2.
Changes from 1999 to 2006 in proportions of EPA+DHA in RBC relative to proportions at baseline in a random sample of the Framingham Offspring cohort by use of fish oil supplements at examination 8. Linear regression with 95% CI bands shows that EPA+DHA increased by 2.04 (1.64, 2.45; P < 0.0001) percentage points for participants who used fish oil supplements at examination 8, regardless of their baseline level. The slope estimate for the baseline value of −0.31 (−0.40, −0.22; P < 0.0001) shows that proportions of EPA+DHA tended to increase in those with low levels at baseline and tended to decrease in those with high baseline levels (i.e. regression to the mean).
Discussion
In this study, we sought to determine to what extent and in what manner RBC membrane fatty acid composition might have changed over a median 6.7-y period in a random subsample of Framingham Offspring Study participants. We observed a 21% decrease in the proportion of RBC trans fatty acids, virtually all (98%) from reduced levels of those trans species that are produced by the commercial hydrogenation of vegetable oils. Based on emerging evidence from the 1990s that trans fats increased risk for cardiovascular disease (5), the Institute of Medicine of the National Academy of Sciences recommended in 2002 that dietary trans fat intake be reduced as much as possible (22). In 2003, the FDA announced, and the press widely reported, that beginning in January of 2006, nutrition labels on packaged foods must list the amount of trans fats per serving. Anticipating such a regulation, fat and oil producers had begun to reformulate their products to be able to list “0” on the label’s “trans fat per serving” line. In mid-2006, the AHA launched a national campaign to educate the public about the health hazards of trans fats, and in December of 2006, the New York City Board of Health voted to remove artificial trans fats in public restaurants beginning in July of 2007. These official actions were taking place during the time when the Offspring examination 8 blood samples were drawn and likely heightened the public awareness of the dangers of consuming trans-fat rich foods. But, as noted earlier, the food industry had for many years been reformulating their products in order to be able to comply with the impending regulations. Hence, our findings of a 21% reduction in RBC levels of IP-trans fatty acids [along with similar findings from NHANES (9)] suggest that these cumulative efforts were at least somewhat successful. The lack of meaningful change in the RP-trans species (−0.01 percentage points, a 6% decrease) similarly suggests that the reductions in total trans were due to reformulations of foods with partially hydrogenated oils, not reductions in the intake of dairy products or meat.
Baseline RBC levels of trans fats were similar in this study to those reported in the Nurses’ Health Study in samples collected between 1989 and 1990 (23) and in the King County Primary Cardiac Arrest study in samples collected between 1988 and 1999 (11). The only other study that has tracked trans fat levels in biological samples during this period of heightened concern about (and regulation of) trans fats was from Canada. Similar to the decrease observed here, levels of trans fatty acids in human breast milk decreased by 35% between 1998 and 2005 (24). Likewise, there was a decrease in adipose tissue trans fat levels documented by Clifton et al. (25) in Australia after a reformulation of vegetable oils in the mid 1990s.
It has been estimated that reducing U.S. trans fat intakes from ~2% of energy to 1% could reduce the proportion of preventable coronary heart disease events by >10% (5). The mechanisms responsible could be via effects on serum lipids, inflammatory markers, adipose tissue metabolism, and endothelial function, as outlined by Mozaffarian et al. (5). Non-HDL-C (directly) and HDL-C (inversely) were selected as predictor variables into the IP trans model (Table 3). However, the converse was not true, i.e., these changes in lipoprotein cholesterol were better explained by other known factors (e.g., changes in statin use, TG, BMI, and the prevalence of diabetes) than by changes in IP-trans levels (Supplemental Table 1).
Proportions of marine (n-3) fatty acids in RBC did not change between examinations in those participants who did not start taking fish oil supplements, increased substantially in those who did, and decreased in the 4 who stopped, confirming the reliability and modifiability of this marker. The percent of the U.S. population reportedly taking fish oil supplements increased from ~2% in the late 1990s to 14% in 2006 (26), which is generally consistent with our observation that the proportion of Framingham participants who reported taking supplements increased from 3 to 12% between 1999 and 2006. The 40% increase in the omega-3 index in those who started taking fish oil supplements was due to a combination of supplementation and concomitantly increasing dietary intake of EPA+DHA. It is not surprising that those who chose to begin fish oil supplementation also chose to add more fish to their diets. Initiating statin use was associated with an increase in the omega-3 index independent of changes in supplement use or fish consumption. Rise et al. (27) reported that in vitro incubation with statins increased levels of EPA and DHA in a human acute leukemia cell line, suggesting increased desaturase activity, but this was not observed in hepatocytes. In humans, statin treatment did not affect EPA or DHA levels in plasma cholesteryl esters, but it did increase arachidonic acid levels (28). The effect of statins on RBC marine fatty acid levels has not been reported. Another factor independently associated with an increase in the omega-3 index was increased wine intake. This confirms the association observed between wine intake and both plasma (29) and RBC (30) EPA+DHA levels in 2 European cohorts. Whether unmeasured confounding factors or some biological mechanism is responsible for this association is not clear.
We observed no effect of aging per se on the omega-3 index. Previous cross-sectional studies reported significant direct relations between these fatty acids and age, even when controlling for the intake of oily fish and fish oil supplements (31–38). Although a biological explanation for this phenomenon is possible (e.g., continued tissue accretion even with small intakes), it may also be explained by “the attrition of the susceptible,” i.e., because a lower (n-3) fatty acid content has been associated with increased risk for death in both randomized trials (39–41) and prospective cohort studies (42–44), older cohorts would ordinarily be enriched with individuals having higher concentrations. Hence, aging per se does not raise blood (n-3) fatty acid concentrations but higher concentrations may promote longevity (43, 45).
With the increase in RBC (n-3) fatty acids, we found an associated decrease in long-chain (n-6) fatty acids consistent with the inhibition of Δ-5 desaturase by EPA (46), which results in a presumed substitution of an (n-3) for an (n-6) fatty acid in the sn-2 position of membrane phospholipids.
The omega-3 index is highly correlated with cardiac EPA+DHA levels (47, 48) and have been proposed as a potential new risk factor for coronary heart disease mortality (49). Indeed, RBC fatty acid patterns were recently reported to be superior to the standard risk factors in discriminating acute coronary syndrome patients from controls (50). The 2.2-percentage point increase in the omega-3 index in those who supplemented might be expected to reduce risk for sudden cardiac death by ~50% based on prospective data from the Physicians’ Health Study (44). The potential effects of a change in RBC trans fat levels (particularly C18:2 trans) on risk for sudden cardiac death may be estimated from the King County Study to be of a similar magnitude (11). Hence, the RBC fatty acid changes observed in the Framingham study, both trans and (n-3), may have substantial clinical relevance. Because proportions of both (n-3) and trans fatty acids in RBC are influenced by dietary intake, the observations made here indicate that the fatty acids consumed changed in Framingham Offspring participants between 1999 and 2006. For the (n-3) fatty acids, these changes were intentional (initiation of fish oil supplementation, choosing to eat more fish); however, for trans fatty acids, the changes were most likely unintentional and resulted from shifts in the fatty acid composition of the conventional food supply.
Limitations
This study was conducted with a small (~10%) but random subset of the Framingham Offspring population. Accordingly, we did not discuss changes in traditional risk factors, which may not be representative of the full cohort. As originally constituted, this cohort was ethnically homogeneous and thus our findings may not be applicable to non-white groups. In addition, the participants may be unlike other cohorts of similar age and race owing to their potentially heightened attentiveness to healthy lifestyles as a result of having participated in extensive examinations every 4–8 y as part of this well-known study. Indeed, the mean intake of marine (n-3) fatty acids in this cohort was ~300 mg/d, which is higher than that in several other cohorts [165 mg/d (51), 188 mg/d (52), 214 mg/d (53), 226 mg/d (54), and 231 mg/d (55)], but lower than that in the Cardiovascular Health Study [452 mg/d (56)]. We could not explore in more detail which fatty acids may have replaced the trans and marine (n-3) fatty acids, because the sample size is small (~300) for multivariate analysis considering a 22-dimensional fatty acid profile and the changes were small on the order of 0.5% percentage point.
In conclusion, we observed that RBC trans fat levels decreased in a random sample of the Framingham cohort in parallel with national efforts to reduce the trans fatty acid content of the American diet. The omega-3 index was unchanged in participants who did not change their intake of marine (n-3) fatty acids but rose substantially with increased intakes from diet and supplements. Taken together, these 2 changes in fatty acids would suggest a potential for reduced risk for coronary heart disease in this community-based sample.
Supplementary Material
Acknowledgments
The authors thank Andrew Christianson and Joseph Ashmore for performing the RBC fatty acid analyses. W.S.H., S.J.R., R.S.V., and M.G.L. designed research (project conception, development of overall research plan, and study oversight); W.S.H. conducted research (oversaw the laboratory analyses); J.V.P. (with oversight from M.G.L.) analyzed data or performed statistical analysis; W.S.H. and J.V.P. wrote the paper with major editorial input from S.J.R., M.G.L., and R.S.V.; and W.S.H. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by the National Heart Lung and Blood Institute (NHLBI; R01 HL089590) and by contract N01-HC-25195, the Framingham Heart Study (NHLBI) and Boston University School of Medicine.
Author disclosures: W.S.H. is a scientific advisor to companies with interests in fatty acids, including Monsanto, Aker Biomarine, Neptune, Omthera, Amarin, and GlaxoSmithKline, and was a speaker for the latter. In addition, he is the owner of OmegaQuant, LLC and an employee of Health Diagnostics Laboratory, Inc., both of which commercially offer blood fatty acid testing. J. V. Pottala, R. S. Vasan, M. G. Larson, and S. J. Robins, no conflicts of interest.
Supplemental Table 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Present affiliation Health Diagnostic Laboratory, Inc., Richmond, VA 23219.
Abbreviations used: HDL-C, HDL cholesterol; IP, industrially produced; IP-trans, industrially produced trans-fatty acid; omega-3 index, RBC EPA+DHA; RP, ruminant-produced; RP-trans, ruminant-produced trans-fatty acid.
Literature Cited
- 1.Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: report of the Trans Fat Conference Planning Group. Circulation. 2007;115:2231–46 [DOI] [PubMed] [Google Scholar]
- 2.Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999;99:166–74 [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 4.Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33:436–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13 [DOI] [PubMed] [Google Scholar]
- 6.Tardy AL, Morio B, Chardigny JM, Malpuech-Brugere C. Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases. Nutr Res Rev. Epub 2011; Feb 15 [DOI] [PubMed] [Google Scholar]
- 7.L'Abbe MR, Stender S, Skeaff M. Ghafoorunissa, Tavella M. Approaches to removing trans fats from the food supply in industrialized and developing countries. Eur J Clin Nutr. 2009;63:S50–6719190645 [Google Scholar]
- 8.Stender S, Dyerberg J, Astrup A. Consumer protection through a legislative ban on industrially produced trans fatty acids in foods in Denmark. Scand J Food Nutr. 2006;50:155–60 [Google Scholar]
- 9.Vesper HW, Kuiper HC, Mirel LB, Johnson CL, Pirkle JL. Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA. 2012;307:562–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris WS. International recommendations for consumption of long-chain omega-3 fatty acids. J Cardiovasc Med. 2007;8 Suppl 1:S50–2 [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701 [DOI] [PubMed] [Google Scholar]
- 12.Harris WS. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–7 [DOI] [PubMed] [Google Scholar]
- 13.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–7 [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90 [DOI] [PubMed] [Google Scholar]
- 15.Stender S, Astrup A, Dyerberg J. Ruminant and industrially produced trans fatty acids: health aspects. Food Nutr Res. 2008;52:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids. 2008;43:805–11 [DOI] [PubMed] [Google Scholar]
- 17.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr. 2009;139:1150–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30 [DOI] [PubMed] [Google Scholar]
- 19.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–5 [DOI] [PubMed] [Google Scholar]
- 20.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–4 [Google Scholar]
- 21.Yang Y. Can the strengths of AIC and BIC be shared? A conflict between model identification and regression estimation. Biometrika. 2005;92:937–50 [Google Scholar]
- 22.Food and Nutrition Board, Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, DC, National Academies Press; 2002 [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Ma J, Campos H, Hankinson SE, Manson JE, Stampfer MJ, Rexrode KM, Willett WC, Hu FB. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007;115:1858–65 [DOI] [PubMed] [Google Scholar]
- 24.Friesen R, Innis SM. Trans fatty acids in human milk in Canada declined with the introduction of trans fat food labeling. J Nutr. 2006;136:2558–61 [DOI] [PubMed] [Google Scholar]
- 25.Clifton PM, Keogh JB, Noakes M. Trans fatty acids in adipose tissue and the food supply are associated with myocardial infarction. J Nutr. 2004;134:874–9 [DOI] [PubMed] [Google Scholar]
- 26. Frost and Sullivan and Global Organization for EPA and DHA. Omega-3 ingredients markets. San Antonio: Frost amp Sullivan; 2009.
- 27.Risé P, Ghezzi S, Priori I, Galli C. Differential modulation by simvastatin of the metabolic pathways in the n-9, n-6 and n-3 fatty acid series, in human monocytic and hepatocytic cell lines. Biochem Pharmacol. 2005;69:1095–100 [DOI] [PubMed] [Google Scholar]
- 28.Jula A, Marniemi J, Ronnemaa T, Virtanen A, Huupponen R. Effects of diet and simvastatin on fatty acid composition in hypercholesterolemic men: a randomized controlled trial. Arterioscler Thromb Vasc Biol. 2005;25:1952–9 [DOI] [PubMed] [Google Scholar]
- 29.de Lorgeril M, Salen P, Martin JL, Boucher F, de Leiris J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J. 2008;155:175–81 [DOI] [PubMed] [Google Scholar]
- 30.di Giuseppe R, de Lorgeril M, Salen P, Laporte F, Di Castelnuovo A, Krogh V, Siani A, Arnout J, Cappuccio FP, van Dongen M, et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89:354–62 [DOI] [PubMed] [Google Scholar]
- 31.Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomark J. 2008;1:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7 [DOI] [PubMed] [Google Scholar]
- 33.Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–5 [PubMed] [Google Scholar]
- 34.Aarsetoey H, Pönitz V, Grundt H, Staines H, Harris WS, Nilsen DW. (n-3) Fatty acid content of red blood cells does not predict risk of future cardiovascular events following an acute coronary syndrome. J Nutr. 2009;139:507–13 [DOI] [PubMed] [Google Scholar]
- 35.Nogi A, Yang J, Li L, Yamasaki M, Watanabe M, Hashimoto M, Shiwaku K. Plasma n-3 polyunsaturated fatty acid and cardiovascular disease risk factors in Japanese, Korean and Mongolian workers. J Occup Health. 2007;49:205–16 [DOI] [PubMed] [Google Scholar]
- 36.Dewailly EE, Blanchet C, Gingras S, Lemieux S, Sauve L, Bergeron J, Holub BJ. Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am J Clin Nutr. 2001;74:603–11 [DOI] [PubMed] [Google Scholar]
- 37.Dewailly E, Blanchet C, Lemieux S, Sauve L, Gingras S, Ayotte P, Holub BJ. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73 [DOI] [PubMed] [Google Scholar]
- 38.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr. 2002;76:85–92 [DOI] [PubMed] [Google Scholar]
- 39.GISSI-HF Investigators, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30 [DOI] [PubMed] [Google Scholar]
- 40.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903 [DOI] [PubMed] [Google Scholar]
- 41.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–61 [DOI] [PubMed] [Google Scholar]
- 42.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10 [DOI] [PubMed] [Google Scholar]
- 43.Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: The Heart and Soul Study. Circ Cardiovasc Qual Outcomes. 2010;3:406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 45.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000;130:1925–31 [DOI] [PubMed] [Google Scholar]
- 47.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplant patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9 [DOI] [PubMed] [Google Scholar]
- 48.Metcalf RG, Cleland LG, Gibson RA, Roberts-Thomson KC, Edwards JR, Sanders P, Stuklis R, James MJ, Young GD. Relation between blood and atrial fatty acids in patients undergoing cardiac bypass surgery. Am J Clin Nutr. 2010;91:528–34 [DOI] [PubMed] [Google Scholar]
- 49.Harris WS. The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep. 2010;12:503–8 [DOI] [PubMed] [Google Scholar]
- 50.Shearer GC, Pottala JV, Spertus JA, Harris WS. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS ONE. 2009;4:e5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. USDA, Agriculture Research Service. Nutrient intakes from food: mean amounts consumed per individual, one day, 2005–2006. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0506/Table_1_NIF_05.pdf.
- 52.Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004;160:1005–10 [DOI] [PubMed] [Google Scholar]
- 53.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–12 [DOI] [PubMed] [Google Scholar]
- 54.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. Am J Clin Nutr. 1995;62:564–71 [DOI] [PubMed] [Google Scholar]
- 55.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–60 [DOI] [PubMed] [Google Scholar]
- 56.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.