Abstract
The Breastfeeding, Antiretrovirals, and Nutrition Study evaluated the effect of daily consumption of lipid-based nutrient supplements (LNS) by 2121 lactating, HIV-infected mothers on the growth of their exclusively breast-fed, HIV-uninfected infants from 0 to 24 wk. The study had a 2 × 3 factorial design. Malawian mothers with CD4+ ≥250 cells/mm3, hemoglobin ≥70 g/L, and BMI ≥17 kg/m2 were randomized within 36 h of delivery to receive either no LNS or 140 g/d of LNS to meet lactation energy and protein needs, and mother-infant pairs were assigned to maternal antiretroviral drugs (ARV), infant ARV, or no ARV. Sex-stratified, longitudinal, random effects models were used to estimate the effect of the 6 study arms on infant weight, length, and BMI. Logistic regression models were used to calculate the odds of growth faltering [decline in weight-for-age Z-score (WAZ) or length-for-age Z-score (LAZ) >0.67] using the control arm as the reference. Although some differences between study arms emerged with increasing infant age in boys, there were no consistent effects of the maternal supplement across the 3 growth outcomes in longitudinal models. At the ages where differences were observed, the effects on weight and BMI were quite small (≤200 g and ≤0.4 kg/m2) and unlikely to be of clinical importance. Overall, 21 and 34% of infants faltered in WAZ and LAZ, respectively. Maternal supplementation did not reduce the odds of infant weight or length faltering from 0 to 24 wk in any arm. These results indicate that blanket supplementation of HIV-infected lactating women may have little impact on infant growth.
Introduction
The improved availability of antiretroviral therapy for prevention of mother-to-child transmission has increased the number of HIV-exposed, uninfected infants (1, 2). Maintaining the health and growth of HIV-exposed infants is an important public health aim, linked to at least 2 of the United Nations Millennium Development Goals (3).
HIV-exposed infants living in resource-poor settings face many challenges compared with their unexposed peers. If they are fully weaned early or not breastfed, then they are at risk of growth faltering and early death due to increased incidence of infectious diseases and inadequate dietary intake (4–6). If they are breastfed in the absence of antiretroviral drugs (ARV)13, infants of HIV-infected mothers are at risk of contracting HIV through breast milk, especially when complementary foods are added to the infant diet during the first 6 mo of life (7–9). To address these issues in settings where the national authorities have decided to support breastfeeding and ARV use, the WHO currently recommends that HIV-infected mothers exclusively breastfeed their infants for 6 mo, introduce complementary foods thereafter, and continue breastfeeding until 12 mo (10). Breastfeeding should stop only when a safe, nutritionally adequate diet without breast milk is available.
Although exclusive breastfeeding is essential for the growth and health of HIV-exposed infants in low-resource settings, lactating HIV-infected women may become nutritionally depleted and suffer excess weight loss because of the increased metabolic demands of both breastfeeding and HIV infection (11–14). It is unclear how seriously these demands affect their own health. Two studies found that weight loss in HIV-infected mothers during lactation led to higher mortality among these women (15, 16), whereas 2 other studies found no association of breastfeeding with mortality (17, 18).
In addition to possible impacts on their own health, unmet nutritional needs of HIV-infected mothers may also affect their children through poor-quality breast milk and reduced capacity for child care [e.g., through the effect of anemia on maternal responsiveness (19, 20)]. Lactating HIV-infected mothers in resource-poor settings have lower levels of several micronutrients than uninfected mothers (21). The energy and micronutrient content of breast milk is dependent on maternal dietary intake and stores, and some nutrients in breast milk can be increased with supplementation (22, 23). The effects of maternal supplementation during pregnancy and lactation on infant growth are mixed (23–29), but it appears that improvements in maternal weight status and infant growth are more likely to occur in thinner mothers (23, 24). We expected to find that HIV-infected mothers in a country with regular food insecurity would fall into this category.
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) study was a randomized-controlled intervention trial designed to test the effects of daily consumption of lipid-based nutrient supplements (LNS) by lactating HIV-infected mothers on their weight status and the provision of ARV to mothers or infants during the period of exclusive breastfeeding on MTCT. Mothers in the study who received LNS lost less weight during 6 mo of exclusive breastfeeding than mothers who did not receive LNS regardless of ARV assignment (30). Here, we assess the effect of BAN study interventions on infant weight, length, and BMI during the period of exclusive breastfeeding, from 0 to 24 wk.
Participants and Methods
Study population.
The methods used in the BAN study have been described in detail elsewhere (31). Briefly, HIV-1–infected, pregnant women were recruited at antenatal clinics in Lilongwe, Malawi. Eligibility criteria included: ≥14 y if married or ≥18 y if not married, CD4+ count ≥250 cells/mm3 (CD4+ count ≥200 cells/mm3 before July 2006), hemoglobin ≥70 g/L, normal liver function tests, no previous ARV use, and no serious complications of pregnancy (such as hemorrhage or eclampsia). After delivery, mother-infant pairs who met the following secondary eligibility criteria were randomized: birth weight ≥2 kg, enrollment within 36 h of delivery, maternal acceptance of 7-d perinatal ARV regimen for herself and her infant, and no maternal condition that would preclude the use of a study drug (such as hypotension or severe infection). Data were collected on randomized mother-infant pairs from April 2004 to January 2010. Infants who were found to be HIV-positive by PCR methods within the first 2 wk of life were un-enrolled from the study, together with their mothers, and referred for ARV treatment.
Ethical approval of the study protocol was obtained from the Malawi National Health Science Research Committee and the Institutional Review Boards at the University of North Carolina at Chapel Hill and the U.S. CDC.
Study interventions.
The study had a 2 × 3 factorial design. Mothers were randomly assigned to receive LNS or no LNS, and mother-infant pairs were randomly assigned to maternal ARV, infant ARV, or no ARV. Following delivery and eligibility screening, mother-infant pairs were randomized, using a permuted block method, to 1 of 6 study arms: maternal LNS/maternal ARV (mLNS-mARV), maternal LNS/infant ARV (mLNS-iARV), maternal LNS (mLNS), maternal ARV (mARV), infant ARV (iARV), or control (C) (31). Mother and infant interventions began within 36 h of delivery and continued to 28 wk postpartum or until breastfeeding cessation, if earlier. Participants were followed until 48 wk. The nutrition intervention was given to one-half of the mothers in the form of 140 g/d of LNS, which contained 3120 kJ of energy and 100% of the recommended dietary allowance of micronutrients for lactating women (Table 1). The daily quantity of LNS was selected to offset the energy and protein costs of lactation (32) and was intended to be consumed in addition to regular foods. LNS were produced by Nutriset and contained peanut butter, vegetable oil, dried skimmed milk powder, dry whey, sugar, and micronutrients. The maternal ARV intervention consisted of a 3-drug, highly-active regimen, and the infant regimen included daily oral nevirapine. Details about the ARV regimens are described elsewhere (8). In addition, all mothers and infants were given an intrapartum dose of nevirapine and postpartum ARV for 7 d.
TABLE 1.
Composition of the daily ration of LNS given to BAN study mothers during lactation1
| Component | Provides |
| unit/140 g | |
| Energy, kJ | 3120 |
| Protein, g | 20.8 |
| Iron, mg | 15 |
| Zinc, mg | 19 |
| Phosphorus, g | 1.2 |
| Selenium, μg | 75 |
| Thiamin, mg | 1.6 |
| Riboflavin, mg | 1.8 |
| Niacin, mg equivalent | 20 |
| Pyridoxine, mg | 2.2 |
| Cyanocobalamine, μg | 2.6 |
| Ascorbic acid, mg | 100 |
| α-Tocopherol, mg | 12 |
| Folic acid, μg | 300 |
| Iodine, μg | 200 |
| Potassium, g | 1.1 |
| Magnesium, mg | 124 |
| Copper, mg | 0.3 |
| Calcium, mg | 294 |
LNS contained ground peanuts, dried skimmed milk, vegetable fat, sugar, and a multivitamin-mineral premix. BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
All BAN-enrolled women, including those receiving LNS, were given 2 kg/wk of maize flour for family consumption. From February to August 2005, there was a drought during which the World Food Programme (WFP) provided food aid to HIV-infected women in Lilongwe. The WFP ration, which included a similar quantity of corn/soy flour as the BAN maize supplement plus 1 L of vitamin A-fortified corn oil per month, was provided in lieu of the maize supplied by the study to ~260 BAN mothers during at least part of their study participation. The mothers who received the WFP ration were evenly distributed across study arms.
All women were intensively counseled to exclusively breastfeed their infants until 24 wk of age and to wean rapidly by 28 wk (33). Although it is possible that there are delayed effects of maternal supplementation on infant growth, only data up to 24 wk are included in the present analysis, because the infants began receiving LNS for their own consumption at that time.
Mothers who had >5% weight loss between study visits or BMI <17 kg/m2 but who were not assigned to one of the maternal supplement arms were given the same type and dose of LNS as mothers in the supplement arms until their weight stabilized. Of the 25 mothers who received LNS due to excess weight loss, 13 were in the mARV arm, 8 were in the iARV arm, and 4 were in the C arm. On average, they consumed LNS for 6 (range 2–16) wk. On March 26, 2008, the data safety monitoring board stopped enrollment in the no-ARV (mLNS and C) arms, because HIV transmission through breast milk occurred more frequently in these arms. Mothers in the mLNS and C arms were offered the choice of remaining in the same arm or switching to either the maternal or infant ARV interventions (34). Thirty-four mothers in our analysis sample chose to switch arms: 30 to the maternal ARV and 4 to the infant ARV arms. We conducted sensitivity analyses and found that removing those infants whose mothers were given LNS to stabilize their weight and those who switched from mLNS or C to one of the ARV arms did not change our results. Consequently, these infants were retained in their originally assigned study arms for the analysis.
Anthropometric measurements and other study procedures.
All study visits and data collection took place at the BAN study clinic at Bwaila Hospital in Lilongwe, Malawi. Weight and recumbent length were obtained from infants at the age of 0, 1, 2, 4, 6, 8, 12, 18, 21, and 24 wk using Tanita digital infant scales (0.1 kg increment) and wooden length boards made to UNICEF specifications (0.1 cm increment) (35). Scales were calibrated daily using a standard weight. Nurses and nutrition assistants were trained to take anthropometric measurements and their measurements were standardized (35).
Infant HIV status was tested using Amplicor 1.5 DNA PCR assay at 0, 2, 12, and 28 wk to identify infants who were infected postnatally through breast milk. Positive HIV tests were confirmed with a new specimen on the following visit. Dried blood spots, which were collected at every study visit except 21 wk, were tested using Gen-Probe Aptima HIV-1 Qualitative Assay to determine more precisely the timing of infection in infants with positive PCR tests. A total of 58 infants in our analysis sample became HIV infected between 2 and 24 wk.
Information on socio-economic characteristics was obtained from mothers during screening. At 1, 4, 8, 12, and 21 wk, mothers who received LNS reported how many full and half packets of the 2-packet daily dose they consumed the day prior to the study visit. They also stated how many of the packets they received on the last visit they had remaining. Mothers assigned to LNS received it for 28 wk or until they reported breastfeeding cessation, if this occurred first. Infants’ exclusive breastfeeding status was assessed by maternal report at 4, 8, 12, 18, 21, and 24 wk. Eighteen infants were fully weaned and 128 consumed a mixed diet by 21 wk; most of these events occurred between 18 and 21 wk. An additional 71 infants became fully weaned and 126 became mixed fed between 21 and 24 wk.
The BAN study initiated cotrimoxazole preventive therapy (CPT) for infants 6–36 wk of age starting in June 2006, based on WHO recommendations (36). Within our analysis sample, ~1500 infants received CPT.
Statistical analysis.
The background characteristics of the 6 study arms were compared to assess similarity using t tests for continuous, normally distributed variables and chi-squared tests for categorical variables. Nonparametric tests were conducted for skewed continuous variables. Statistical analyses were carried out using Stata 11.2.
Longitudinal random effects models were used to analyze the effects of study interventions on 3 outcomes: infant weight, length, and BMI. The outcomes were modeled as having an exchangeable correlation structure, which reflects our assumption that a participant’s measurement at one visit is correlated with measurements at their other visits. All analyses were stratified by sex due to significant differences in growth patterns of boys and girls for all measures of growth. Based on a 2 × 3 factorial design (i.e., 2 levels for LNS supplementation and 3 levels for ARV treatment), the initial analyses indicated a significant interaction between the ARV and supplement interventions. Thus, in all subsequent analyses, the 2 × 3 factorial design was collapsed into one factor consisting of the 6 study arms. Because inclusion of maternal BMI, education, and CD4+ count and infant CPT did not influence study arm coefficients, they were excluded from the final longitudinal models, which controlled for the corresponding birth measurement (e.g., birth weight in the weight models) and treatment × age and treatment × age2 interactions, where appropriate. Likelihood ratio tests were used to assess the joint significance of treatment × age and treatment × age2 interactions.
In a separate analysis, growth faltering was defined as >0.67-unit decline in weight-for-age Z-score (WAZ) or length-for-age Z-score (LAZ) from 0 to 24 wk. A 0.67-SD decrease in Z-score represents the width of a percentile band on growth charts and is a clinically significant event (37, 38). The odds of faltering were estimated using logistic regression, controlling for initial Z-scores.
Randomized infants were included in the longitudinal analysis sample if they were singletons, did not become HIV infected or die within the first 2 wk after birth, and had anthropometric measurements on at least 2 of the 10 time points. Measurements for infants who became HIV infected from 2 to 24 wk were included up to the point of infection (determined by analysis of regularly collected dried blood spots). Sensitivity analyses were conducted to examine the effect of removing all measurements of infants who became HIV infected between 2 and 24 wk of age and measurements of infants after they were no longer exclusively breastfed. We found that separate or simultaneous exclusion of these groups of infants did not influence the results, so they were retained in the models to maintain the intent of the clinical trial and limit selection bias. Infants in the longitudinal analysis sample were included in the faltering analysis if they had data at 0 and 24 wk. A small number (<1%) of implausible post-birth weights and lengths were replaced by values interpolated from prior and subsequent measures, assuming mean growth rates observed in longitudinal regression models of plausible values stratified by sex.
Sample size and analysis sample.
The method used to calculate sample size for the main BAN study outcomes has been described elsewhere (31). The present analysis had 80% power to detect a difference in mean weights of 200 g (~0.2 SD difference) between study arms at 24 wk, when the sample was the smallest.
Of the 3572 HIV-1–infected pregnant women who underwent study screening, 2791 delivered infants and 2369 mother-infant pairs were randomized to the 6 study arms (Supplemental Fig. 1). Following the exclusions described above, 2121 infants remained in the longitudinal analysis sample for weight. The WAZ faltering analysis included 1661 infants who had data at 0 and 24 wk. Sample sizes for longitudinal analyses of length (n = 2105) and BMI (n = 2103) as well as the analysis of LAZ faltering (n = 1650) were slightly smaller than for weight and WAZ due to missing length values. Among infants in the longitudinal analysis sample for weight, 19% were not included in the faltering analysis due to loss to follow-up and 1% due to death. These proportions did not differ by study arm (loss to follow-up, P = 0.24; death, P = 0.69). Three percent of the infants were not included in the faltering analysis, because they became HIV infected between 2 and 24 wk of age. The number of HIV-infected infants was larger in the no ARV arms (mLNS and C), consistent with a previous report (8).
Results
Baseline characteristics of the mother-infant pairs included in the longitudinal analysis were generally well balanced among the 6 study arms (Table 2), although the proportions with education beyond primary school and low BMI (<18.5 kg/m2) were lower in the C and iARV arms, respectively.
TABLE 2.
Background characteristics of 2121 mother-infant pairs in the BAN study, by study arm1
| Characteristic | mLNS-mARV | mARV | mLNS-iARV | iARV | mLNS | C | P value |
| n | 377 | 374 | 391 | 381 | 299 | 299 | |
| Mothers | |||||||
| Age, y | 25 (23–30) | 26 (22–29) | 26 (22–29) | 25 (22–29) | 26 (22–30) | 26 (22–29) | 0.97 |
| Education beyond primary school, % | 35.0 | 37.5 | 39.2 | 33.7 | 36.1 | 27.8 | 0.043 |
| Married, % | 94.4 | 89.3 | 94.1 | 91.9 | 91.0 | 93.0 | 0.08 |
| First birth, % | 10.6 | 12.5 | 13.0 | 12.9 | 11.2 | 10.4 | 0.81 |
| BMI <18.5,2 % | 4.1 | 2.1 | 2.2 | 0.6 | 4.7 | 1.5 | 0.007 |
| BMI >25,2 % | 21.8 | 24.3 | 20.3 | 17.9 | 22.2 | 25.0 | 0.26 |
| CD4+ count,3 cells/mm3 | 439 (328–582) | 437 (329–565) | 448 (324–611) | 436 (337–559) | 437 (326–578) | 444 (337–574) | 0.84 |
| Infants | |||||||
| Male sex, % | 49.6 | 49.7 | 50.6 | 49.3 | 50.2 | 53.2 | 0.95 |
| Birth weight, kg | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 0.80 |
| Low birth weight,4 % | 5.8 | 8.8 | 6.1 | 6.8 | 6.7 | 5.7 | 0.57 |
| Birth length, cm | 48.3 ± 2.0 | 48.2 ± 2.0 | 48.1 ± 1.9 | 48.3 ± 1.9 | 48.3 ± 2.1 | 48.0 ± 1.9 | 0.46 |
| Born during hungry season, % | 30.2 | 30.2 | 30.7 | 29.7 | 36.5 | 35.1 | 0.27 |
| CPT,5 % | 80.1 | 79.2 | 78.3 | 79.2 | 72.7 | 73.1 | 0.16 |
Values are mean ± SD, median (IQR), or percent. BAN, Breastfeeding, Antiretrovirals, and Nutrition; C, control; CPT, cotrimoxazole preventive therapy; iARV, infant antiretroviral drug; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement/infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement/maternal antiretroviral drug.
BMI at 2 wk postpartum.
CD4+ during pregnancy.
Low birth weight defined as 2.0–2.5 kg, as infants were required to weigh ≥2.0 kg to be eligible for the study.
Proportion receiving CPT at 24 wk.
Based on adherence data collected at 5 study visits, 93.4% of mothers reported consuming the full amount of LNS on the previous day. The proportions were similar across LNS study arms (mLNS, mLNS-mARV, and mLNS-iARV) and by infant sex. The proportion of mothers who reported exclusive breastfeeding at 21 wk was 96.5% and did not differ by study arm or sex.
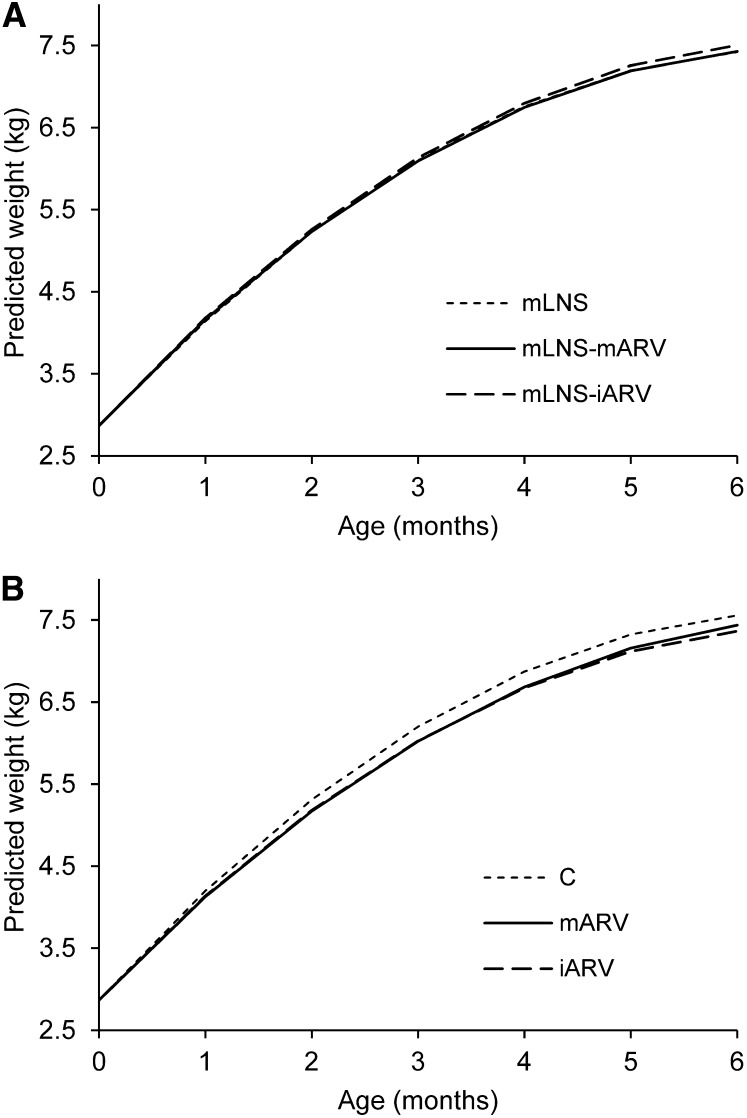
Mean infant weight, length, and BMI at each study visit are reported in Supplemental Table 1. The longitudinal models indicate that the average effect of the interventions on growth was minimal, but there were some differences in effects over time (Supplemental Table 2). Among infants whose mothers received LNS, there were no differences in weight, length, or BMI by ARV assignment (mLNS, mLNS-mARV, and mLNS-iARV) (Fig. 1A). However, among infants whose mothers did not receive LNS, boys in the C arm weighed more than boys in either the iARV or mARV arms (Fig. 1B). Weights predicted from the longitudinal model indicate that differences were largest between the C and iARV arms, ranging from 130 g at 2 mo to 200 g at 5 mo of age. Based on the predicted means, boys in the C arm also had higher BMI than boys in the iARV arm from 3 to 4 mo of age; the maximum difference was 0.4 kg/m2 at 4 mo. In girls, there were differences between study arms over time in weight and BMI, but not length. However, the differences were smaller for girls than for boys and not clinically relevant.
FIGURE 1.
Boys’ predicted weight through age 6 mo in the mLNS, mLNS-mARV, and mLNS-iARV arms of the longitudinal model (A) and in the C, mARV, and iARV arms of the longitudinal model (B). C, control; iARV, infant antiretroviral drug; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement/infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement/maternal antiretroviral drug.
From 0 to 24 wk, mean WAZ increased in all arms, whereas mean LAZ remained static in girls and declined modestly in boys, particularly in the C and mLNS arms (not shown). Approximately 21% of infants faltered in WAZ from 0 to 24 wk and LAZ faltering occurred in 34% of infants. Overall, there was no difference in the proportion of boys (21%) and girls (20%) whose WAZ faltered (P = 0.51), but a larger proportion of boys (39%) than girls (30%) faltered in LAZ (P < 0.001). The proportion of infants faltering in weight and length from 0 to 24 wk did not vary by study arm for either sex (Table 3). Controlling for the corresponding Z-score at birth, the odds of WAZ or LAZ faltering from 0 to 24 wk did not differ in any study arm compared with the C arm (Table 3). In both sexes, the supplement did not influence WAZ or LAZ faltering within any ARV arm and ARV was not associated with faltering within the supplement arms.
TABLE 3.
Proportion of BAN infants whose WAZ and LAZ faltered by study arm and odds of growth faltering from 0–24 wk compared to the control arm1
| Boys |
Girls |
|||||||
| % Who faltered2 | OR3 | 95% CI | P value | % Who faltered | OR | 95% CI | P value | |
| WAZ faltering4 | n = 855 | n = 806 | ||||||
| C (ref) | 19.5 | — | — | — | 20.2 | — | — | — |
| mLNS | 24.8 | 1.25 | (0.64, 2.42) | 0.51 | 23.6 | 1.30 | (0.65, 2.60) | 0.46 |
| mARV | 23.7 | 1.25 | (0.68, 2.31) | 0.47 | 19.9 | 0.85 | (0.44, 1.67) | 0.64 |
| mLNS-mARV | 19.2 | 0.97 | (0.52, 1.82) | 0.93 | 20.4 | 1.00 | (0.52, 1.93) | 0.99 |
| iARV | 22.1 | 1.09 | (0.59, 2.02) | 0.79 | 20.5 | 0.99 | (0.52, 1.88) | 0.97 |
| mLNS-iARV | 19.9 | 1.16 | (0.62, 2.16) | 0.64 | 17.2 | 0.75 | (0.39, 1.45) | 0.39 |
| LAZ faltering5 | n = 846 | n = 804 | ||||||
| C (ref) | 30.3 | — | — | — | 28.7 | — | — | — |
| mLNS | 44.8 | 1.68 | (0.94, 3.03) | 0.08 | 20.8 | 0.59 | (0.30, 1.14) | 0.11 |
| mARV | 40.9 | 1.71 | (0.99, 2.95) | 0.06 | 26.7 | 0.76 | (0.42, 1.39) | 0.37 |
| mLNS-mARV | 41.0 | 1.64 | (0.95, 2.83) | 0.08 | 30.1 | 0.92 | (0.51, 1.63) | 0.76 |
| iARV | 38.2 | 1.44 | (0.83, 2.50) | 0.19 | 32.1 | 1.01 | (0.58, 1.78) | 0.97 |
| mLNS-iARV | 36.9 | 1.48 | (0.86, 2.56) | 0.16 | 36.3 | 1.35 | (0.77, 2.35) | 0.28 |
Faltering is >0.67 decline in Z-score. BAN, Breastfeeding, Antiretrovirals, and Nutrition; C, control; iARV, infant antiretroviral drug; LAZ, length-for-age Z-score; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement/infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement/maternal antiretroviral drug; WAZ, weight-for-age Z-score.
Proportion of infants who faltered from 0 to 24 wk of age.
Estimated OR of faltering with C as the reference group (ref) based on logistic regression.
The models were adjusted for WAZ at birth.
The models were adjusted for LAZ at birth.
Discussion
The maternal nutritional supplementation component of the BAN study was conducted with the expectation that at least 10% of HIV-infected mothers would have low BMI (<18.5 kg/m2) (39) and many would have depleted energy or micronutrient stores (21, 40, 41). The LNS were intended to improve or maintain the mothers’ own nutritional status (42, 43), which could promote the growth of infants in these study arms. The effect of ARV on the growth of HIV-exposed infants was unknown and we did not have a priori hypotheses regarding their influence on the outcomes. We found no consistent effects of the maternal supplement across the 3 infant growth outcomes. ARV, particularly infant nevirapine, had a negative effect on the weight and BMI of boys as their age increased. As this effect was not seen in boys whose mothers were nutritionally supplemented, LNS may have buffered an adverse effect of ARV on growth. The differential effects of BAN study interventions on boys and girls may be due to sex differences in the growth process (44). During infancy, boys gain weight and length more rapidly than girls (45), which could make boys more susceptible to environmental insults, including the ingestion of ARV.
This research had some limitations. First, we cannot calculate the net increase in maternal energy and nutrient intake attributable to LNS in the absence of dietary intake data. Second, it is possible that our analysis of growth faltering from 0 to 24 wk underestimates the actual prevalence of faltering in this population due to the fairly sizeable number of participants who were lost to follow-up by 24 wk. Infants who were lost to follow-up were evenly distributed among the study arms.
The potential effects of maternal LNS supplementation on infant growth may have been limited by the generally high BMI of the BAN mothers. Compared with women of reproductive age in Lilongwe (46), the BAN mothers at 8 wk postpartum were less likely to have BMI <18.5 kg/m2 (3.4 vs. 6.0%, respectively) and more likely to have BMI >25 kg/m2 (20.2 vs. 16.1%, respectively). Thus, although BAN mothers were HIV infected, they remained better nourished during lactation than the general population of women of reproductive age in the same city. Other research has shown that mothers and infants who were well nourished had little if any benefit from maternal supplementation during pregnancy or lactation (23, 24, 47). Mothers in the BAN study who received the LNS during lactation had less weight loss than those who were not supplemented (30), indicating that there were some mothers whose nutritional status allowed them to benefit from the LNS.
The results of previous studies examining the effects of maternal supplementation on infant growth have been mixed. Three studies conducted in Columbia (24), Guatemala (25, 26), and Tanzania (27) provided maternal supplements during pregnancy and lactation and found positive effects on infant growth at different time points ranging from 6 to 24 mo of age, whereas a study in Taiwan found no effect (28). It is notable that the study in Guatemala found benefits on infant growth only in supplemented mothers who were thinner (24). Two other studies in Guatemala (23) and South Africa (29) supplemented mothers during lactation only and reported positive effects for thinner mothers themselves but found no impact on infant growth, which is consistent with the results of the BAN study. The Guatemala study showed that thinner, supplemented mothers had higher breast milk energy content and output, indicating one of the biological mechanisms through which maternal supplementation could improve infant growth (23). The South African study is most comparable to the one presented here, because the supplement and study population were similar and high mean maternal BMI likely limited the effect of maternal supplementation in both cases (29). Taken together, these results suggest that maternal supplementation should be targeted at women with poor nutritional status, even in HIV-infected populations.
Because we found no large differences in infant growth between the study arms, it is useful to put our findings into context. Infants in previous studies of HIV-exposed, uninfected children usually had a short period of exclusive breastfeeding followed by mixed feeding, which contributed to similar patterns of growth faltering in HIV-exposed and unexposed children (48). Compared with 2 other groups of Malawian infants in Lungwena with short periods of exclusive breastfeeding and <20% prevalence of HIV among mothers, on average, the BAN infants were approximately the same weight but ~1–3 cm longer at 6 mo of age (49, 50). Possible explanations for the somewhat better linear growth of BAN infants are residence in an urban area compared with the rural Lungwena cohorts and the extensive counseling and support for exclusive breastfeeding provided by the BAN study nurses (33), which was not available in Lungwena.
In conclusion, the growth of infants whose mothers received LNS did not differ from infants whose mothers did not receive LNS during the period of exclusive breastfeeding. Boys who were not exposed to ARV had slightly better weight and BMI with increasing age than those with ARV exposure. These differences were small and unlikely to have clinical importance. Although the study interventions had little effect on growth compared with the controls, the BAN HIV-exposed infants had somewhat better linear growth than other Malawian infants. This suggests that good counseling on exclusive breastfeeding may be beneficial to the growth of HIV-exposed infants.
Supplementary Material
Acknowledgments
M.E.B., C.S.C., D.K., M.G.H., R.J.K., A.S., D.J.J., C.M.v.d.H., and L.S.A. designed and conducted the study; V.L.F. and L.S.A. carried out the analysis; and V.L.F. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by the Prevention Research Centers Special Interest Project of the CDC (SIP 13-01 U48-CCU409660-09 and SIP 26-04 U48-DP000059-01), The Bill and Melinda Gates Foundation (OPP53107), the National Institute of Allergy and Infectious Diseases, the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 Tw01039-06), Fogarty International Clinical Research Fellows Program at Vanderbilt (R24TW007988), the University of North Carolina Center for AIDS Research (P30-AI50410), the Carolina Population Center (NICHD 5 R24 HD050924), Abbott Laboratories, GlaxoSmithKline, the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This trial was registered at http://www.clinicaltrials.gov as NCT00164736.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; C, control; CPT, cotrimoxazole preventive therapy; iARV, infant antiretroviral drug; LAZ, length-for-age Z-score; LNS, lipid-based nutrient supplement; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement/infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement/maternal antiretroviral drug; WAZ, weight-for-age Z-score; WFP, World Food Programme.
Literature Cited
- 1.UNAIDS. WHO AIDS epidemic update: November 2009. Geneva: UNAIDS; 2009 [Google Scholar]
- 2.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–87 [DOI] [PubMed] [Google Scholar]
- 3.UN The Millenium Development Goals report 2010. New York: United Nations; 2010 [Google Scholar]
- 4.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, Thea DM, Kuhn L. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, Hu CC, Tsai WY, Thea DM, Aldrovandi GM. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, Kumwenda NI, Nkanaunena K, Mipando L, Mofenson LM. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13 [DOI] [PubMed] [Google Scholar]
- 7.Fawzi W, Msamanga G, Spiegelman D, Renjifo B, Bang H, Kapiga S, Coley J, Hertzmark E, Essex M, Hunter D. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–8 [DOI] [PubMed] [Google Scholar]
- 8.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, Downing R, Malamba S, Tappero J, Mermin J. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2010;53:28–35 [DOI] [PubMed] [Google Scholar]
- 10.WHO Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO Press; 2010 [PubMed] [Google Scholar]
- 11.WHO Nutrient requirements for people living with HIV/AIDS: report of a technical consultation. Geneva: WHO Press; 2003 [Google Scholar]
- 12.Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J Nutr. 1992;122:1643–55 [DOI] [PubMed] [Google Scholar]
- 13.Ladner J, Castetbon K, Leroy V, Nyiraziraje M, Chauliac M, Karita E, De Clercq A, Van de Perre P, Dabis F. Pregnancy, body weight and human immunodeficiency virus infection in African women: a prospective cohort study in Kigali (Rwanda), 1992–1994. Pregnancy and HIV Study Group (EGE). Int J Epidemiol. 1998;27:1072–7 [DOI] [PubMed] [Google Scholar]
- 14.Papathakis PC, Van Loan MD, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Body composition changes during lactation in HIV-infected and HIV-uninfected South African women. J Acquir Immune Defic Syndr. 2006;43:467–74 [DOI] [PubMed] [Google Scholar]
- 15.Nduati R, Richardson BA, John G, Mbori-Ngacha D, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Kreiss J. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet. 2001;357:1651–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyanagi A, Humphrey JH, Moulton LH, Ntozini R, Mutasa K, Iliff P. Ruff AJ, The Zvitambo Study Group. Predictive value of weight loss on mortality of HIV-positive mothers in a prolonged breastfeeding setting. AIDS Res Hum Retroviruses. 2011;27:1141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutsoudis A, Coovadia H, Pillay K, Kuhn L. Are HIV-infected women who breastfeed at increased risk of mortality? AIDS. 2001;15:653–5 [DOI] [PubMed] [Google Scholar]
- 18.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Vwalika C, Tsai WY, Aldrovandi GM, Thea DM. Prolonged breast-feeding and mortality up to two years post-partum among HIV-positive women in Zambia. AIDS. 2005;19:1677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahmanifar A, Kirksey A, Wachs TD, McCabe GP, Bishry Z, Galal OM, Harrison GG, Jerome NW. Diet during lactation associated with infant behavior and caregiver-infant interaction in a semirural Egyptian village. J Nutr. 1993;123:164–75 [DOI] [PubMed] [Google Scholar]
- 20.Perez EM, Hendricks MK, Beard JL, Murray-Kolb LE, Berg A, Tomlinson M, Irlam J, Isaacs W, Njengele T, Sive A, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850–5 [DOI] [PubMed] [Google Scholar]
- 21.Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr. 2007;85:182–92 [DOI] [PubMed] [Google Scholar]
- 22.Allen LH. Maternal micronutrient malnutrition: effects on breast milk and infant nutrition, and priorities for intervention. SCN News. 1994:21–4 [PubMed] [Google Scholar]
- 23.González-Cossío T, Habicht JP, Rasmussen KM, Delgado HL. Impact of food supplementation during lactation on infant breast-milk intake and on the proportion of infants exclusively breast-fed. J Nutr. 1998;128:1692–702 [DOI] [PubMed] [Google Scholar]
- 24.Herrera MG, Mora JO, de Paredes B, Wagner M. Maternal weight/height and the effect of food supplementation during pregnancy and lactation. In: Aebi H, Whitehead R, editors. Maternal nutrition during pregnancy and lactation. Bern: Hans Huber Publishers; 1980 [Google Scholar]
- 25.Delgado HL, Valverde VE, Martorell R, Klein RE. Relationship of maternal and infant nutrition to infant growth. Early Hum Dev. 1982;6:273–86 [DOI] [PubMed] [Google Scholar]
- 26.Lechtig A, Klein RE. Maternal food supplementation and infant health: results of a study in rural areas of Guatemala. In: Aebi H, Whitehead R, editors. Maternal nutrition during pregnancy and lactation. Bern: Hans Huber Publishers; 1980 [Google Scholar]
- 27.Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr. 2005;81:880–8 [DOI] [PubMed] [Google Scholar]
- 28.Wohlleb JC, Pollitt E, Mueller WH, Bigelow R. The Bacon Chow study: maternal supplementation and infant growth. Early Hum Dev. 1983;9:79–91 [DOI] [PubMed] [Google Scholar]
- 29.Kindra G, Coutsoudis A, Esposito F. Effect of nutritional supplementation of breastfeeding HIV positive mothers on maternal and child health: findings from a randomized controlled clinical trial. BMC Public Health. 2011;11:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, Chigwenembe M, Ellington S, Hosseinipour MC, Kourtis AP, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr. 2012;95:759–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, Fiscus S, Hudgens M, Kazembe P, Bentley M, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FAO Human energy requirements: report of a joint FAO/WHO/UNU expert consultation. Rome: FAO; 2001 [Google Scholar]
- 33.Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, Piwoz EG, Zulu C, Chasela C, Soko A, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: The Ban Study in Lilongwe, Malawi. AIDS Educ Prev. 2009;21:141–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavula C, Long D, Mzembe E, Kayira D, Chasela C, Hudgens MG, Hosseinipour M, King CC, Ellington S, Chigwenembe M, et al. Stopping the control arm in response to the DSMB: mother's choice of HIV prophylaxis during breastfeeding in the BAN Study. Contemp Clin Trials. 2012;33:55–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cogill B. Anthropometric indicators measurement guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2003 [Google Scholar]
- 36.WHO Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach. Geneva: WHO; 2006 [Google Scholar]
- 37.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lampl M, Gotsch F, Kusanovic JP, Espinoza J, Goncalves L, Gomez R, Nien JK, Frongillo EA, Romero R. Downward percentile crossing as an indicator of an adverse prenatal environment. Ann Hum Biol. 2008;35:462–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uthman OA. Prevalence and pattern of HIV-related malnutrition among women in sub-Saharan Africa: a meta-analysis of demographic health surveys. BMC Public Health. 2008;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friis H, Gomo E, Koestel P, Ndhlovu P, Nyazema N, Krarup H, Michaelsen KF. HIV and other predictors of serum beta-carotene and retinol in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr. 2001;73:1058–65 [DOI] [PubMed] [Google Scholar]
- 41.Friis H, Gomo E, Koestel P, Ndhlovu P, Nyazema N, Krarup H, Michaelsen KF. HIV and other predictors of serum folate, serum ferritin, and hemoglobin in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr. 2001;73:1066–73 [DOI] [PubMed] [Google Scholar]
- 42.Ndekha MJ, van Oosterhout JJ, Zijlstra EE, Manary M, Saloojee H, Manary MJ. Supplementary feeding with either ready-to-use fortified spread or corn-soy blend in wasted adults starting antiretroviral therapy in Malawi: randomised, investigator blinded, controlled trial. BMJ. 2009;338:b1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Oosterhout JJ, Ndekha M, Moore E, Kumwenda JJ, Zijlstra EE, Manary M. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS Care. 2010;22:737–42 [DOI] [PubMed] [Google Scholar]
- 44.Lampl M, Thompson AL, Frongillo EA. Sex differences in the relationships among weight gain, subcutaneous skinfold tissue and saltatory length growth spurts in infancy. Pediatr Res. 2005;58:1238–42 [DOI] [PubMed] [Google Scholar]
- 45.Nelson SE, Rogers RR, Ziegler EE, Fomon SJ. Gain in weight and length during early infancy. Early Hum Dev. 1989;19:223–39 [DOI] [PubMed] [Google Scholar]
- 46.National Statistical Office Malawi. ORC Macro Malawi demographic and health survey 2004. Calverton (MD): NSO and ORC Macro; 2005 [Google Scholar]
- 47.Winkvist A, Habicht JP, Rasmussen KM. Linking maternal and infant benefits of a nutritional supplement during pregnancy and lactation. Am J Clin Nutr. 1998;68:656–61 [DOI] [PubMed] [Google Scholar]
- 48.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr Rev. 2009;67:343–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maleta K, Virtanen S, Espo M, Kulmala T, Ashorn P. Timing of growth faltering in rural Malawi. Arch Dis Child. 2003;88:574–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Arch Pediatr Adolesc Med. 2008;162:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.