Abstract
Knowledge gaps persist about the efficacy of cancer prevention strategies based on dietary food components. Adaptations to nutrient supply are executed through tuning of multiple protein networks that include transcription factors, histones, modifying enzymes, translation factors, membrane and nuclear receptors, and secreted proteins. However, the simultaneous quantitative and qualitative measurement of all proteins that regulate cancer processes is not practical using traditional protein methodologies. Proteomics offers an attractive opportunity to fill this knowledge gap and unravel the effects of dietary components on protein networks that impinge on cancer. The articles presented in this supplement are from talks proffered in the “Nutrition Proteomics and Cancer Prevention” session at the American Institute for Cancer Research Annual Research Conference on Food, Nutrition, Physical Activity and Cancer held in Washington, DC on October 21 and 22, 2010. Recent advances in MS technologies suggest that studies in nutrition and cancer prevention may benefit from the adoption of proteomic tools to elucidate the impact on biological processes that govern the transition from normal to malignant phenotype; to identify protein changes that determine both positive and negative responses to food components; to assess how protein networks mediate dose-, time-, and tissue-dependent responses to food components; and, finally, for predicting responders and nonresponders. However, both the limited accessibility to proteomic technologies and research funding appear to be hampering the routine adoption of proteomic tools in nutrition and cancer prevention research.
Introduction
The adoption of “omic” technologies defined as the collection and analysis of large-scale measurements related to the organization and regulation of biological systems sparked new enthusiasm for the prevention of chronic diseases, including cancer (1). One of the central tenants of the Human Genome Project was to provide a blueprint to categorize cancers and develop biomarkers of cancer susceptibility based on genetic information (2). However, variations in epidemiologic trials of nutrients for cancer prevention and susceptibility informed that gene-environment interactions have the potential for influencing a person’s risk for cancer (3) or response to dietary intervention (4). For example, in BRCA-1 mutation carriers, the risk of breast tumors is either reduced by higher intake of fruits and vegetables (5) or increased by polymorphisms in the methyl-tetrahydro-folate reductase gene (6). The example of BRCA-1 illustrates that in the postgenomic era, there is a need for new investigative tools to explain how diet modifies the risk of cancer (4, 7).
The field of proteomics is concerned with the systematic study of all proteins in cell compartments, tissues, and biofluids. Of the ~20,000 protein-coding human genes discovered through the genome project, ~8000 (38%) reportedly lack experimental evidence at the protein level (8). Up to one million different protein molecules have been estimated to originate from the combined effects of alternative splicing, protein modifications, and pathological and physiological conditions. Therefore, it seems reasonable that proteomic tools should be adopted to make a direct assessment of all the proteins that influence biological processes associated with cancer (9).
Historically, large differences in chemical properties of proteins and the wide dynamic range of protein concentrations have made profiling proteins challenging (10). However, recent improvements in technologies allow the identification and quantitation of proteins, analysis of protein-protein interactions, and characterization of posttranslational modifications (11). Therefore, assessing the dynamic changes of protein profiles brought about by dietary components may offer new opportunities for the development of proteomic signatures for each bioactive food component or diet and predictive models of cancer risk.
Articles presented in this supplement are from talks proffered in the “Nutrition Proteomics and Cancer Prevention” session at the American Institute for Cancer Research Annual Research Conference on Food, Nutrition, Physical Activity and Cancer held in Washington, DC on October 21 and 22, 2010. This session originated from the concept that future progress in the implementation of nutritional strategies for cancer prevention requires knowledge of how dietary components influence protein targets that govern the transition from normal to malignant phenotype. Proteomic studies presented by Zhen Xiao et al. (12) highlighted that isothiocyanates (ITC)5 commonly found in cruciferous vegetables may target the microtubule network. They proposed that the antiproliferative effects of ITC may be related to covalent modifications of cysteine residues of tubulin leading to loss of tubulin polymerization, a process that is necessary for maintaining cell structure. The article presented by Baukje de Roos (13) addressed the benefits of using proteomic approaches to assess the influence of dietary fatty acids on mechanisms involved in carcinogenesis and discovery of new protein biomarkers of cancer risk. Angela Betancourt et al. (14) used proteomic technologies to discover proteins that modulated the response to the hormonally active chemical bisphenol A and the soy component genistein. Unequivocally, these studies offer compelling evidence that the future of nutritional proteomics in cancer prevention remains bright. However, efforts are needed for its incorporation into diagnostic tools for predicting benefits from dietary changes.
Protein Networks as Targets for Bioactive Food Components
Protein networks
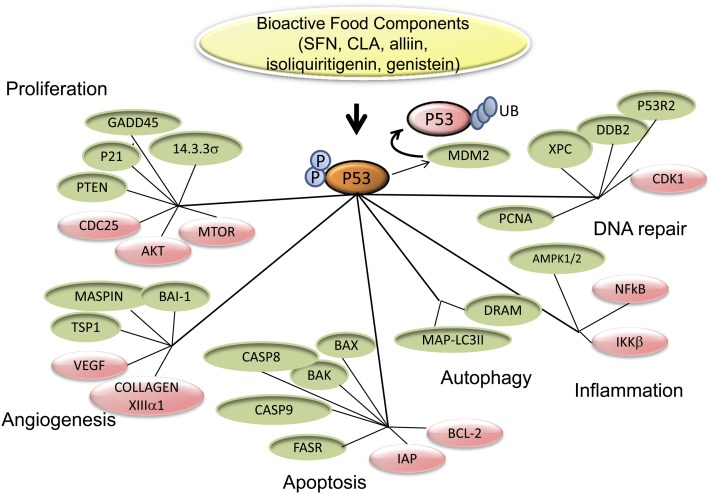
Implicit in the adoption of proteomic tools is the concept that the composition and functionality of protein networks determine disease risk (15). The value of targeting protein networks rather than individual proteins or protein modifications stems from the fact that protein inter-relationships regulate biological processes such as proliferation, apoptosis, autophagy, DNA repair, inflammation, and angiogenesis. For example, various food components that possess anticarcinogenic properties have been shown to activate the tumor suppressor protein, P53 (16–21), a highly connected nodal protein that regulates a vast number of signaling pathways (22) (Fig. 1). One important consequence of P53 activation is the halting of transition through G1/S phase by the stimulated expression of the tumor suppressor protein P21, which then interferes with the formation of cyclin-dependent kinase complexes necessary for cell cycle progression (23). In addition, P53 has been reported to block G2/M phase transition by inducing expression of 14–3-3σ, which anchors CYCLIN B1-cyclin-dependent kinase 1 in the cytoplasm, and of GADD45, which dissociates CDC2 from CYCLIN B1 and P21. Moreover, the P53 protein has been shown to repress the CYCLIN B1 and CDC2 genes, further reinforcing its effects on cell cycle arrest (24, 25). In addition to halting cell cycle progression, P53 induces apoptosis through inhibition of the antiapoptotic protein BCL-2, thus releasing BCL-2’s inhibition on the proapoptotic BAX and BAK. The latter proteins stimulate the release of cytochrome-c from mitochondria, the repression of inhibitors of apoptosis proteins, and hampering of AIP’s repression on caspase-9, leading to apoptosis (26). Also, P53 has been implicated in the regulation of proteins that participate in DNA repair (XPC, DDB2, P53R2), autophagy (DRAM, MAP-LC3II), inflammation (IKKβ/NFκB), and angiogenesis (MASPIN, TSP1, BAI1, VEGF, COLLAGEN VIIIα1) (22). Proteins regulated by P53 may in turn alter the expression levels, posttranslational modifications, DNA binding, protein-protein interactions, and localization of other proteins comprised in subnetworks further amplifying the duration and amplitude of the signal initiated by food components. Moreover, the P53 protein itself is extensively regulated through positive (transcription factor E2F) and negative (murine double minute-2) regulators, post-translational modifications that affect its levels, subcellular localization, DNA binding, and transactivation potential (27). The example of the P53 network provides an excellent proof-of-principle that proteomic tools are necessary to perform the measurement of quantitative and qualitative influences of food components on complex protein networks and subnetworks, the study of which is largely impractical using traditional protein methodologies.
FIGURE 1.
Protein networks as targets for bioactive food components. A simplified scheme of how food components reported to induce p53, selected as a prototype interconnecting protein node, may influence proteins that belong to neighboring networks controlling biological processes. Stimulatory effects of p53 are shown in green and repressive effects are shown in red. Proteomic tools are needed to learn about the topology and dynamic behavior of protein networks that impinge on cancer risk and assess quantitative and qualitative influences of food components.
Post-translational modifications
Factors that contribute to increasing the complexity of protein networks are post-translational modifications such as phosphorylation, acetylation, methylation, glycosylation, myristoylation, nitrosylation, sumoylation, palmitoylation, and ubiquitination. Proteins can also be modified through oxidation, nitration, or binding to lipid moieties (28). Post-translational modifications influence protein structure, stability, and localization. Phosphorylation of the ERK, JNK, and P38 kinases are necessary for their translocation to the nucleus and activation of transcription factors (29). The activation of phosphatidylinositol 3-kinases by point mutations, receptors, small GTPASE rat sarcoma (RAS), and AKT induce system-wide protein responses leading to cell transformation (30). Hence, proteomics offers attractive opportunities for the qualitative and quantitative analysis of how food components influence post-translational modifications associated with growth stimulation (31) or metabolic stress (32).
The human kinome comprises over 500 protein kinases, which transiently phosphorylate predominantly serine and threonine residues, although a subgroup (~90 tyrosine kinases) phosphorylates tyrosine residues on receptors, including EGFR, IR, and FGFR as well as nonreceptor proteins (i.e., tyrosine kinase SRC and others). About one-half of the protein tyrosine kinases are linked to human cancers through constitutive activation (33). ERK alone can phosphorylate >80 substrates in the cytoplasm and the nucleus (34). Interestingly, quantitative phosphoproteomic studies illustrated that a cluster of tyrosine kinases mediated the invasive effects of SRC (35), which is overexpressed in ~80% of human colorectal cancers (36). Therefore, a proteomic overview of the kinome in colonic cells may help identify dysregulated protein networks and assist in the generation of working hypotheses for targeting of SRC and other tyrosine kinases with food components. Other studies that used proteomic approaches suggested that the anticarcinogenic properties of genistein may be related to inhibition of the tyrosine kinase activity of SRC, EGFR, PDGFR, and IR (37) as well as activation of phosphatases, which reverse phosphorylation. Proteomic studies of peripheral blood mononuclear cells from postmenopausal women revealed that supplementation with soy isoflavones increased the levels of protein tyrosine phosphatases (38).
An example of a post-translational modification that influences subcellular distribution and protein-protein interactions is palmitoylation, which enhances hydrophobic anchoring of proteins to the fatty acid chain into the lipid bilayer. Palmitoylation regulates trafficking and function of many transmembrane proteins, including receptors, SRC family kinases, and RAS proteins. Proteomic analyses have the potential to assess how dietary modulators of palmitoylation influence redistribution of certain proteins to various cellular compartments and regulate processes associated with carcinogenesis (39). For example, palmitoylation of the estrogen receptor-α is necessary for its association with the plasma membrane and interactions with caveolin-1 and for the nongenomic activation of ERK- and AKT-regulated pathways (40). Therefore, proteomics may be useful to investigate how food components influence cellular relocation of proteins involved in growth stimulation. Studies with mouse colonocytes reported that supplementation with DHA disrupted RAS signaling by displacing H-RAS from caveolae while excluding EGFR from lipid rafts (41). Given the large number of cancers with abnormal RAS signaling, the adoption of proteomic approaches may accelerate the development of preventive strategies based on supplementation with DHA and other food components.
State of Development of Proteomic Technologies and Challenges
An in-depth discussion of the evolution and state of proteomic technologies is beyond the scope of this manuscript. Therefore, we refer to excellent reviews of the scientific principles of various proteomic platforms (42–44). The following paragraphs offer some considerations about the dynamic range and versatility of MS-based proteomics and examples of applications in nutrition and cancer prevention research. Table 1 provides a list of preclinical and clinical investigations that have used various MS platforms to assess the impact of various food components and dietary mixtures on biological processes that impinge on cancer.
TABLE 1.
MS platforms and proteomic studies of food components in cancer prevention research1
| Food component | Model | Proteomic platform | Combined effects of food components on biological processes | Reference |
| Cruciferous | ||||
| BITC, PEITC, SFN | Lung, A549 | 2D-nano RPLC-MALDI-TOF/TOF | Disruption of cytoskeleton organization | 48 |
| BITC, PEITC, SFN | Breast, Ras-MCF10A | LC-ESI-Q-TOF | Induction of apoptosis | 69 |
| SFN | Breast, MCF10, MCF12A | SILAC, LC-QTOF | Upregulation of hydroxysteroid metabolism | 59 |
| SFN | Prostate, LNCaP | 2D-MALDI-TOF/TOF | Induction of apoptosis | 47 |
| SFN | Colon, Caco-2 | 2D-MALDI-TOF | Decrease of neurotransmitter receptors | 46 |
| SFN | Liver, huh-7 | 2D-MALDI-TOF | Induction of apoptosis | 70 |
| PEITC | Liver, HepG2 | 2D-MALDI-TOF/TOF | Proapoptotic, antiinflammatory | 71 |
| PEITC | Plasma, TRAMP mice | 2D-MALDI-TOF/TOF | Induction of autophagy | 72 |
| PEITC | Cervix, Hela | ESI-LTQ | Inhibition of proinflammatory cytokine (MIF) | 73 |
| Indol-3-carbinol | Lung, A/J mice | iTRAQ-SCX-LC-TOF/TOF | Detoxification, antiproliferative | 60 |
| Brussels Sprouts | PBMC, human | 2D-LC-nanospray-MS | Growth arrest, proapoptotic, antioxidant | 74 |
| Brassica Sp. | Serum, human | MALDI-TOF | Inhibition of receptor tyrosine kinase | 75 |
| Brassica Sp. | Serum, human | MALDI-TOF | Reduced lipolysis in GSTM1+ subjects | 52 |
| Vegetable diets | ||||
| Mixture | Colon, C57BL6 mice | 2D-MALDI-TOF | Maintenance of intracolonic pH, | 76 |
| Antioxidant | Brain, canine | 2D-MALDI-TOF | Reduced protein oxidation | 77 |
| Isoflavones | ||||
| Genistein | Breast, Sprague-Dawley rat | 2D-MALDI-TOF | Increased differentiation | 78 |
| Genistein | Breast, Sprague Dawley rat | 2-D-MALDI-TOF/TOF,LC-ESI-MS/MS | Reduced EGFR signaling | 49 |
| Genistein | Stomach, SGC-7901 | SILAC-SCX-LC-trap/Orbitrap | Impaired signaling, cell growth, invasion | 37 |
| Genistein | Leukemia, HL-60 | 2D-MALDI-TOF/TOF | Proapoptotic | 79 |
| Genistein | Stomach, SGC-7901 | SILAC, SCX-LC-LTQ-Orbitrap | Induction of G2/M arrest and apoptosis | 53 |
| Genistein | Endothelial, EA.hy 926 | 2D-MALDI-TOF | Increased detoxification-GST | 80 |
| Soy isoflavones | PBMC, human | 2D-MALDI-TOF | Antiinflammatory | 38 |
| Red clover isoflavones | Liver, Sprague Dawley rat | 2D-MALDI-TOF | Reduced levels of 3a-hydroxysteroid-dehydrogenase | 81 |
| Flavonoids | ||||
| Quercetin | Colon, HT29 | 2D-MALDI-TOF | Proapoptotic, disruption of cytoskeleton | 82 |
| Quercetin | Colon, F344 rat | MALDI-FT-ICR MALDI-TOF/TOF | Reduced glycolysis, increased fatty acid oxidation, proapoptotic | 83 |
| Quercetin | Colon, SW480 | 2D-MALDI-TOF/TOF | Antiangiogenic | 84 |
| Quercetin | Liver, HepG2 | SILAC-NanoHPLC-ESI-Q-TOF | Disruption of cytoskeleton, antiangiogenic | 85 |
| Quercetin | Neuroblastoma, SJ-N-KP | HPLC-ESI-Q-TOF | Antiproliferative, proapoptotic | 86 |
| Quercetin | Ovary, 2774 | 2D-SELDI-TOF | Proapoptotic | 87 |
| Quercetin | Prostate, PC-3 | 2D-NanoHPLC-ion trap | Proapoptotic, reduced glycolysis | 88 |
| Flavone | Colon, HT-29 | 2D-MALDI-TOF | Antiproliferative, proapoptotic | 89 |
| Fatty acids and cyclopentenone PG | ||||
| Fish oil | Serum, human | 2D-MALDI-TOF, NanoLC-Q-Trap | Inhibition of acute-phase response, antiinflammatory | 90 |
| Fish oil | Liver, APOE3*Leiden Mice | 2D-MALDI-TOF | Inhibition of hypoxia, enhanced fatty acid oxidation | 91 |
| t10,c12-CLA | Liver, E3*Leiden, mice | 2D-MALDI-TOF | Increased lipid storage | 91 |
| Liver, APOE−/−, mice | 2D-LC-ESI-MALDI/TOF | Inhibition of TCA cycle, stimulation of gluconeogenesis | 92 | |
| c9,t11-CLA | Liver, APOE−/−, mice | 2D-LC-ESI-MALDI/TOF | Antiproliferative, antiinflammatory | 92 |
| Butyrate | Colon, HCT-116 | iTRAQ/ICAT-2D-LC-MALDI-MS | Antiproliferative, proapoptotic, inhibition of glycolysis, increased oxidative phosphorylation | 93 |
| Butyrate | Colon, HT-29 | 2D-MALDI-TOF | Proapoptotic | 94 |
| Butyrate | Colon, HT-29 | 2D-MALDI-TOF/TOF | Antiproliferative, proapoptotic, disruption of glycolytic pathways | 95 |
| PGA1 | Fibroblast, NIH-3T3 | NanoHPLC-ESI-Q/TOF | Disruption of cytoskeleton, proapoptotic | 96 |
| Grape compounds | ||||
| Grape seed extracts | Brain, Sprague Dawley rat | 2D-MALDI/-TOF 2D-RPLC-ESI-D-TOF | Neuroprotective | 97 |
| Resveratrol | Lymphoma, Jeko-1 | 2D-Nano RP-HPLC-ESI–ion trap, Q-TOF | Proapoptotic | 45 |
| Resveratrol | Colon, HCT-116(Bax+/−) | 2D-MALDI-TOF/TOF | Induced proapoptotic signaling | 98 |
| Resveratrol | Prostate, LNCaP | 2D-MALDI-MS | Reduced glycolysis | 99 |
| Garlic | ||||
| Diallyl-trisulfide | Stomach, BGC823 | 2D-MALDi/TOF, 2D-LC-ion trap | Stimulation of apoptosis | 100 |
| Diallyl-trisulfide | Bone, Saos-2 | 2D-MALDI-TOF | Impaired signaling, disruption of cytoskeleton, proapoptotic | 101 |
| Tea compounds | ||||
| Green tea extracts | Lung, A549 | 2D-HPLC-ESI-Q-TOF | Inhibition of cell motility | 102 |
| EGCG | Neuroblastoma, SH-SY5Y | 2D-RPLC-ion trap | Neuroprotective | 103 |
| Theobroma cacao | ||||
| Procyanidin | Breast, MDA-MB-231,-436,-468; SKBR3 | Antibody protein array | Antiproliferative, proapoptotic | 104 |
| Selenium | ||||
| Selenomethyl-selenocysteine | Plasma, Wistar rat | 2D-MALDI-TOF | Stimulation of acute-phase response | 105 |
| Vitamins | ||||
| Retinoic acid | Breast, MCF-7 | 2D-MALDI-TOF | Cell cycle arrest, antiproliferative, proapoptotic | 106 |
| Retinoic acid | Neuroblastoma, SH-SY5Y | 2D-MALDI-TOF/TOF, iTRAQ-LC-NanoESI- Q-TOF | Alteration of mRNA splicing and translation | 107 |
| L-Ascorbic acid | Leukemia, NB4 | 2D-MALDI-MS | Alteration of contractile system | 108 |
| Folate | Plasma, human | 2D-NanoLC-Q-Trap | Activation of immune function | 109 |
| Folate deficiency | Colon, NCM460 | 2D-NanoLC-Q-Trap | Increased DNA damage, metastasis reduced phase II enzymes | 110 |
BITC, benzyl isothiocyanate; EGCG, (-)-epigallocatechin gallate; ESI, electrospray ionization; FT-ICR, Fourier transform-ion cyclotron resonance; ICAT, isotope-coded affinity tag; iTRAQ, isobaric tag for relative and absolute quantification; LTQ, linear trap quadrupole; MALDI, matrix-assisted laser desorption/ionization; PEITC, phenetyl isothiocyanate; RPLC, reverse-phase-LC; SELDI, surface-enhanced laser desorption/ionization; SFN, sulforaphane; SILAC, stable isotope-based labeling; SXC, strong cation exchange; TOF, time-of-flight; 2D, 2-dimensional gel electrophoresis; Q, quadrupole.
Top-down and bottom-up proteomics
Of the several proteomic methods that have been developed, all involve protein digestion, fractionation, and MS analysis of peptide ions, from which it is possible to derive the amino acid sequence and post-translational modifications and calculate the amount of selected peptides. In top-down proteomics, separation and analysis are performed directly on intact proteins, followed by digestion and MS analysis. The top-down approach starts with the intact protein and it draws inferences about amino acid composition, post-translational modifications, and protein functionality. Conversely, in bottom-up proteomics, protein samples first undergo proteolytic digestion followed by separation of peptides and analytical measurement by MS. The concept behind the bottom-up approach is to use information about amino acid and post-translational modifications to reconstruct the protein of interest and gain knowledge about its functionality. The latter method is more sensitive, but it has the drawback of not capturing all information about small proteins (<30 kD), because they generate fewer peptides (42).
Protein separation techniques
Two-dimensional (2D) SDS-PAGE has been widely used to detect differentially expressed proteins based on mass and charge. 2D electrophoresis utilizes isoelectric focusing prior to gel separation, which can be followed by gel excision of proteins of interest, digestion, and MS analysis. 2D gel electrophoresis separation followed by in-gel trypsin digestion and MS analysis have been used to identify protein targets of various food components, including grape resveratrol in lymphoma cells (45), cruciferous ITC and indole compounds in colon (46) and prostate (47, 48) cancer cells, and the soy isoflavone genistein in developing normal mammary tissue (49) (Table 1). However, 2D gels have a bias against membrane proteins, large proteins, and low-abundance proteins. Also, proteins with an extreme isoelectric point (10 < isoelectric point < 3) are not effectively resolved (50).
An alternative method to 2D separation is affinity chromatography, which can be used to capture proteins of interest. For example, in human plasma, ~99% of the protein mass is due to ~22 proteins, and their removal through a chromatographic approach (e.g., matrix immobilized antibody) is advantageous prior to MS to enhance the detection of less abundant proteins (51). The preliminary precipitation of albumin and Ig through affinity chromatography prior to MS analysis has been used successfully in clinical studies that examined the effects of the glutathione-S-transferase-M1 phenotype on the serum peptidome following supplementation with cruciferous vegetables (52). Other studies that investigated the anticarcinogenic properties of ITC utilized affinity chromatography with streptavidin-Sepharose beads to purify cysteine-containing protein targets of ITC (50). In the latter study, MS analysis of bound proteins identified macrophage migration inhibitory factor, a proinflammatory cytokine, as a primary binding target for ITC.
To by-pass challenges related to protein separation, the entire proteome from a biological sample (e.g., cell, tissue, biofluid) can be first digested, typically by trypsin. Then, the resulting peptides can be separated using various techniques, including ion exchange chromatography, isoelectric focusing, ion-pairing reversed-phase HPLC, and phosphopeptide chromatography. To investigate the effects of genistein on the phosphoproteomic profile of gastric cancer SGC-7901 cells, hydrophilic interaction chromatography methods with the metal oxide TiO2 were utilized for the enrichment of phosphopeptides prior to light chromatography (LC)-tandem MS (MS/MS) analysis (53). These studies led to the identification of novel phopshoprotein targets for genistein, including receptors, signal adaptors, protein kinases, protein phosphatase regulatory subunits, and transcription regulators. Principles and applications of global and site-specific quantitative phosphoproteomics are reviewed elsewhere (54).
Ionization of peptides
For MS analysis, peptides first need to be ionized. Two main ionization techniques are commonly used and include matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI). In MALDI, the peptide mixture is cocrystallized with a matrix that upon excitement with a UV laser leads to the ionization of peptides through gain of a proton. The ionized peptide molecule is usually referred to as [M+H]+ (55). ESI utilizes a solvent system to dissolve the peptide mixture, which is then electro-sprayed into a vacuum chamber. Then, through solvent evaporation or extraction methods, the peptides are ionized. With ESI, most peptide ions gain more than one proton charge ([M+nH]n+) (56). Regardless of the technique used for ionization, peptide ions are analyzed based on their m/z (42–44). Several examples of studies that utilized MALDI or ESI methods in nutrition and cancer prevention research are reported in Table 1.
MS
In MS, 3 types of information are necessary for each peptide and include mass, peptide ion intensity, and list of peptide ion fragments (44). An MS method for peptide ion mass determination is time-of-flight (TOF), in which travel distance of peptide ions is calculated based on the square root of m/z, i.e., peptides with a high m/z travel slower compared with those with a lower m/z. Then, the m/z values of unknown peptide ions are calculated against TOF of internal peptide ion standards. A second MS method utilizes quadrupole (Q) chambers that measure “spiraling” trajectories of peptide ions of preselected m/z values. Because of their sensitivity, triple Q have been used for quantitative measurement of single or multiple fragment ions. In single/multiple reaction monitoring, the first Q chamber is used to select the peptide ion of interest; in the second Q, the peptide ion is fragmented; and in the third Q, one or a few peptide ions are collected. Therefore, multiple Q are used as mass filters that allow the passage of ions of selected m/z ratios (57). A third approach for MS determination is based on ion traps, which eject peptide ions of different m/z values onto the MS detector. In general, ion traps are useful, because they accumulate ions of interest but have limited resolution (500–2000) compared with TOF analyzers (>10,000). A fourth group of MS known as Orbitraps and Fourier transform-ion cyclotron resonance separate ions based on oscillation frequencies and have a mass resolution >60,000 (44).
Platforms for proteomic analysis need to combine an ionization technique with an MS platform. Widely used combinations are MALDI-TOF for 2D electrophoresis and ESI-ion trap/Orbitrap for LC-MS. MS platforms commonly used today include a chromatographic technique (e.g., nano HPLC) followed by ESI-MS/MS analysis. Because the on-line nanoHPLC-ESI combination operates in the liquid phase, it eliminates losses due to separation and collection steps. Conversely, when combining LC with MALDI, eluted peptides need to be mixed with the appropriate matrix for subsequent MALDI analysis. The latter solution is more time-consuming compared with the LC-based platforms (42).
After peptide mass determination, a second goal in MS is to determine the amino acid sequence of the peptides of interest. This is accomplished through fragmentation of the peptide and recording of the m/z values of the fragments in a tandem mass spectrum. This approach relies on the use of 2 distinct (tandem) MS analyzers or the sequential use of the same MS analyzer. Examples of tandem platforms include Q-TOF, triple Q, and TOF-TOF (44). Sequential platforms utilize ion traps or Fourier transform-ion cyclotron resonance analyzers (42). The fragmentation of peptide ions can be accomplished through collision with gas molecules such as He, N2, or Ar, which cause preferential cleavage of peptide bonds and weak modifications such as glycosylation and phosphorylation linkages (42). An alternative fragmentation technique utilizes electron transfer, in which positively charged peptide ions react either with an electron donor (e.g., fluoranthene) or electrons generated by heat, leading to the gain of an unpaired electron and peptide bond cleavage. Compared with collision methods, the electron transfer approach appears to be more accurate for the analysis of large peptides or peptides with post-translational modifications (54).
The selection of MS peaks for sequencing is commonly carried out using 3 strategies. In shotgun or discovery proteomics, a full scan of the peptide ions entering the MS is performed. Then, peptide ions are selected for fragmentation and determination of the amino acid sequence. This strategy has a bias for more intense protein signals. A second protein identification approach involves 2 separate MS analyses for quantification and sequencing. This method improves quantitative measurements in favor of less abundant proteins. A third approach is targeted proteomics, which focuses on determination of the spectrum of fragment ions from a preselected list of peptides (43, 57).
When determining the amino acid sequence of the peptides of interest, the fragmentation spectrum of a peptide is compared with theoretical fragmentation patterns of peptides contained in databases. Then, the fidelity of the predicted amino acid sequence is scored using different computational tools. An example of a database of consensus spectra is available through the PeptideAtlas project (58). A main issue in proteomic experiments is that of discriminating true- from false-positive matches. In de novo sequencing, the fragment ion spectrum is used to determine the peptide sequence (44).
Quantitative assessment and prediction of protein networks
Several LC-MS/MS–based methods have been developed for quantitative proteomics and include label-free methods (42), and stable isotope methods such as metabolic stable isotope-based labeling (59), enzymatic isotope-coded affinity tag (42), and chemical isobaric tag for relative and absolute quantification (60) labeling. In label-free methods, the MS is used directly for quantitation based on signal intensity of peptides or spectral counting. In MS methods that use stable isotope labeling, quantitation is based on the mass increase of the label. For example, linear ion trap-Orbitrap and quantitative stable isotope-based labeling analyses were adopted in shotgun proteomics to identify in gastric cancer SGC-7901 cells the phosphoproteins and their regulatory sites in signaling pathways targeted by genistein (53). These investigations helped to identify proteins that mediated genistein-induced G2/M phase arrest and apoptosis. Specifically, phosphorylation of BCLAF1 at Ser-512 was identified as the regulatory event involved in the repression of Bcl-2 expression in response to genistein (Table 1). These proteomic studies suggested that specific phosphosites rather than the whole protein should be examined to learn about the impact of food components on the regulation of protein networks. Similarly, proteomic studies that adopted a Q-TOF approach (45) revealed that the grape compound resveratrol induced apoptosis in lymphoma cells through upregulation of Ser-3 phosphorylated cofilin, which functions in mitochondria as a checkpoint for programmed cell death (61).
Results of shotgun proteomic studies suggest this is the method of choice when no prior knowledge is available and for measurements of relative and absolute protein abundance (62). One of the limitations of the shotgun approach is that repeated analyses of the same samples may generate different, partially overlapping proteomes. This problem can be overcome with repeated analysis and prefractionation or use of the last generation of MS-Orbitrap or Q-TOF (59).
A main objective of proteomic studies in nutrition and cancer prevention research is to develop predictive models of how pathways and protein complexes relay signals from food components. However, the cross-talk among pathways renders the dynamic prediction of protein network response to food components challenging. Sophisticated computational tools are now available to study protein-protein interaction networks (63) and for the proteomic-based analysis of cancer processes (64). Proteomic workflows should also include validation steps with various biochemical assays (65). Useful tools for the validation of MS data are protein microarrays, including forward- and reverse-phase protein arrays, which offer the advantage of high throughput. Some drawbacks of protein microarrays may be inability to fully inform about protein-protein interactions and complexity of spotting the complete proteome under study (66).
Future Areas of Proteomic Research and Needs
The complexity of protein wiring is a major challenge in the design of cancer prevention strategies based on individual bioactive components or food associations. Thousands of compounds present in the diet likely induce synergistic or opposing effects. Proteomic approaches are welcome to make an important paradigm shift. Specific research questions that should be addressed using proteomic approaches include: 1) how the timing and dose of exposure to bioactive compounds influence the activity of protein networks that contribute to cancer processes; 2) which are the protein networks and protein modifications that mediate the cell- and tissue-specific response to food components [global proteomic studies suggest that tissue specificity may be achieved by precise regulation of protein levels and modifications in space and time (67)]; 3) whether food components lead to sustained regulation of protein networks even after the original food exposure has been removed; and 4) which are the qualitative and quantitative proteomic modifications that discriminate between responders and nonresponders. Ideally, the systematic adoption of proteomic tools rather than a classical protein-by-protein approach should help isolate groups of proteins that can be targeted with individual food components or associations. However, the integration of proteomics with other complementary, high-throughput, “omic” approaches, such as genomics, epigenetics, and metabolomics, may offer the best insight into the mechanisms that determine the switch from normal to cancer phenotype and response to food components (68). This need for integration is perhaps best underlined by studies showing that interactions between inter-individual genotypic differences in metabolism and disposition influence the proteomic response to cruciferous vegetables (52).
To date, ~30,000 proteomic publications are available through a PubMed search. However, only ~6000 have reported on the use of proteomics in cancer research, and of the latter studies, only a small number (~120) focused on the effects of food components and diet. Also, many of the published nutrition proteomic and cancer studies do not report a comprehensive analysis of protein networks. It is clear that the adoption of proteomics tools in nutrition and cancer prevention research is lagging behind other research areas such as pharmacology, for which >5400 studies are available through PubMed. Several factors appear to be hindering the wide adoption of proteomic tools in nutrition and cancer prevention research and include: 1) limited accessibility to proteomic technologies; 2) insufficient preanalytical, sample handling, instrumentations, and sample processing training; and 3) insufficient cross-training in postanalytical bioinformatics, computational biology, structural biology, and system biology analyses. Progress in these areas may be accelerated by pre- and postdoctoral training, early-career awards, workshops, and conferences. The widespread utilization of proteomic tools could be facilitated by the availability of low-cost platforms. It is important that professional organizations and funding agencies develop targeted initiatives, foster collaborations, and support new funding mechanisms to support and encourage collaborative efforts among proteomic, nutrition, and cancer scientists.
Acknowledgments
D.F.R. and J.A.M. co-chaired the Nutrition Proteomics and Cancer Prevention session at the American Institute for Cancer Research Annual Research Conference on Food, Nutrition, Physical Activity and Cancer held in Washington, DC on October 21 and 22, 2010. Both authors wrote, read, and approved the final manuscript.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the 2010 American Institute for Cancer Research Annual Conference held in Washington, DC, October 21–22, 2010. The conference was organized by the American Institute for Cancer Research. This work was supported by an Intergovernmental Personnel Act from the Nutritional Sciences Research Group, Division of Cancer Prevention, National Cancer Institute, NIH to Donato F. Romagnolo, University of Arizona, Tucson. The views expressed in this publication are those of the authors and do not reflect the views or policies of the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition. The supplement coordinator for this supplement was Donato F. Romagnolo, University of Arizona, Tucson. Supplement Coordinator disclosures: D. F. Ramagnolo, no conflicts of interest. The supplement is the responsibility of the Guest Editor to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Harry D. Dawson, ARS/USDA. Guest Editor disclosure: H. D. Dawson, no conflicts of interest. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Abbreviations used: 2D, 2-dimensional gel electrophoresis; ESI, electrospray ionization; ITC, isothiocyanate; LC, light chromatography; MALDI, matrix-assisted laser desorption/ionization; MS/MS, tandem MS; Q, quadrupole; RAS, rat sarcoma; SILAC, stable isotope-based labeling; TOF, time-of-flight.
Literature Cited
- 1.Milner JA. Nutrition in the 'omics’ era. Forum Nutr. 2007;60:1–24 [DOI] [PubMed] [Google Scholar]
- 2.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequence analysis of the human genome. Nature. 2001;409:860–921 [DOI] [PubMed] [Google Scholar]
- 3.Conney AH. Tailoring cancer chemoprevention regimens to the individual. J Cell Biochem. 2004;91:277–86 [DOI] [PubMed] [Google Scholar]
- 4.Fortes C, Boffetta P. Nutritional epidemiological studies in cancer prevention: what went wrong, and how to move forwards. Eur J Cancer Prev. 2011;20:518–25 [DOI] [PubMed] [Google Scholar]
- 5.Ghadirian P, Narod S, Fafard E, Costa M, Robidoux A, Nkondjock A. Breast cancer risk in relation to the joint effect of BRCA mutations and diet diversity. Breast Cancer Res Treat. 2009;117:417–22 [DOI] [PubMed] [Google Scholar]
- 6.Jakubowska A, Gronwald J, Menkiszak J, Górski B, Huzarski T, Byrski T, Edler L, Lubiński J, Scott RJ, Hamann U. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res Treat. 2007;104:299–308 [DOI] [PubMed] [Google Scholar]
- 7.Kaput J, Rodriguez RL. Nutritional genomics: the next frontier in the postgenomic era. Physiol Genomics. 2004;16:166–77 [DOI] [PubMed] [Google Scholar]
- 8.A gene-centric human proteome project: Hupo, The Human Proteome Organization. Mol Cell Proteomics. 2010;9:427–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs D, Winkelmann I, Johnson IT, Mariman E, Wenzel U, Daniel H. Proteomics in nutrition research: principles, technologies and applications. Br J Nutr. 2005;94:302–14 [DOI] [PubMed] [Google Scholar]
- 10.Corthésy-Theulaz I, den Dunnen JT, Ferre P, Geurts JM, Müller M, van Belzen N, van Ommen B. . Nutrigenomics: the impact of biomics technology on nutrition research. Ann Nutr Metab. 2005;49:355–65 [DOI] [PubMed] [Google Scholar]
- 11.Veenstra TD, Zhou M. Tissue proteomics and metabolomics: an excellent start and a promising future. J Proteome Res. 2009;8:1617. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z, Mi L, Chung F-L, Veenstra TD. Proteomic analysis of covalent modifications of tubulins by isothiocyanates. J Nutr. 2012;142:1377S–81S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Roos B, Romagnolo DF. Proteomic approaches to predict bioavailability of fatty acids and their influence on cancer and chronic disease prevention. J Nutr. 2012;142:1370S–76S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betancourt AM, Wang J, Jenkins S, Mobley J, Russo J, Lamartiniere C. Altered carcinogenesis and proteome in mammary glands of rats after prepubertal exposures to the hormonally active chemicals bisphenol A and genistein. J Nutr. 2012;142:1382S–88S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ideker T, Sharan R. Protein networks in disease. Genome Res. 2008;18:644–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamet-Payrastre L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Curr Cancer Drug Targets. 2006;6:135–45 [DOI] [PubMed] [Google Scholar]
- 17.Kemp MQ, Jeffy BD, Romagnolo DF. Conjugated linoleic acid inhibits cell proliferation through a p53-dependent mechanism: effects on the expression of G1-restriction points in breast and colon cancer cells. J Nutr. 2003;133:3670–7 [DOI] [PubMed] [Google Scholar]
- 18.Rahal OM, Simmen RC. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis. 2010;31:1491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afaq F, Khan N, Syed DN, Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol. 2010;86:1318–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousa AS, Mousa SA. Anti-angiogenesis efficacy of the garlic ingredient alliin and antioxidants: role of nitric oxide and p53. Nutr Cancer. 2005;53:104–10 [DOI] [PubMed] [Google Scholar]
- 21.Kang SW, Choi JS, Choi YJ, Bae JY, Li J, Kim DS, Kim JL, Shin SY, Lee YJ, Kwun IS, et al. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J Nutr Biochem. 2010;21:55–65 [DOI] [PubMed] [Google Scholar]
- 22.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10 [DOI] [PubMed] [Google Scholar]
- 23.Kastan MB, Bartek J. Cell-cycle checkpoints, cancer. Nature. 2004;432:316–23 [DOI] [PubMed] [Google Scholar]
- 24.Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14–3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11 [DOI] [PubMed] [Google Scholar]
- 25.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15 [DOI] [PubMed] [Google Scholar]
- 26.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–48 [DOI] [PubMed] [Google Scholar]
- 28.Jensen ON. Interpreting the protein language using proteomics. Nat Rev Mol Cell Biol. 2006;7:391–403 [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18 [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–26 [DOI] [PubMed] [Google Scholar]
- 31.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48 [DOI] [PubMed] [Google Scholar]
- 32.Sung MK, Yeon JY, Park SY, Park JH, Choi MS. Obesity-induced metabolic stresses in breast and colon cancer. Ann N Y Acad Sci. 2011;1229:61–8 [DOI] [PubMed] [Google Scholar]
- 33.Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat Rev Genet. 2010;11:60–74 [DOI] [PubMed] [Google Scholar]
- 34.Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J. 2005;392:249–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leroy C, Fialin C, Sirvent A, Simon V, Urbach S, Poncet J, Robert B, Jouin P, Roche S. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–86 [DOI] [PubMed] [Google Scholar]
- 36.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58 [DOI] [PubMed] [Google Scholar]
- 37.Yan GR, Xiao CL, He GW, Yin XF, Chen NP, Cao Y, He QY. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics. 2010;10:976–86 [DOI] [PubMed] [Google Scholar]
- 38.Fuchs D, Vafeiadou K, Hall WL, Daniel H, Williams CM, Schroot JH, Wenzel U. Proteomic biomarkers of peripheral blood mononuclear cells obtained from postmenopausal women undergoing an intervention with soy isoflavones. Am J Clin Nutr. 2007;86:1369–75 [DOI] [PubMed] [Google Scholar]
- 39.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. [DOI] [PubMed] [Google Scholar]
- 40.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–2 [DOI] [PubMed] [Google Scholar]
- 42.Mallick P, Kuster B. Proteomics: a pragmatic perspective. Nat Biotechnol. 2010;28:695–709 [DOI] [PubMed] [Google Scholar]
- 43.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat Biotechnol. 2010;28:710–21 [DOI] [PubMed] [Google Scholar]
- 44.Choudhary C, Mann M. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol. 2010;11:427–39 [DOI] [PubMed] [Google Scholar]
- 45.Cecconi D, Zamò A, Parisi A, Bianchi E, Parolini C, Timperio AM, Zolla L, Chilosi M. Induction of apoptosis in Jeko-1 mantle cell lymphoma cell line by resveratrol: a proteomic analysis. J Proteome Res. 2008;7:2670–80 [DOI] [PubMed] [Google Scholar]
- 46.Mastrangelo L, Cassidy A, Mulholland F, Wang W, Bao Y. Serotonin receptors, novel targets of sulforaphane identified by proteomic analysis in Caco-2 cells. Cancer Res. 2008;68:5487–91 [DOI] [PubMed] [Google Scholar]
- 47.Lee CH, Jeong SJ, Yun SM, Kim JH, Lee HJ, Ahn KS, Won SH, Kim HS, Lee HJ, Ahn KS, et al. Down-regulation of phosphoglucomutase 3 mediates sulforaphane-induced cell death in LNCaP prostate cancer cells. Proteome Sci. 2010;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Betancourt AM, Mobley JA, Lamartiniere CA. Proteomic discovery of genistein action in the rat mammary gland. J Proteome Res. 2011;10:1621–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi L, Xiao Z, Veenstra TD, Chung FL. Proteomic identification of binding targets of isothiocyanates: a perspective on techniques. J Proteomics. 2011;74:1036–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67 [DOI] [PubMed] [Google Scholar]
- 52.Brauer HA, Libby TE, Mitchell BL, Li L, Chen C, Randolph TW, Yasui YY, Lampe JW, Lampe PD. Cruciferous vegetable supplementation in a controlled diet study alters the serum peptidome in a GSTM1-genotype dependent manner. Nutr J. 2011;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan GR, Yin XF, Xiao CL, Tan ZL, Xu SH, He QY. Identification of novel signaling components in genistein-regulated signaling pathways by quantitative phosphoproteomics. J Proteomics. 2011;75:695–707 [DOI] [PubMed] [Google Scholar]
- 54.Macek B, Mann M, Olsen JV. Global and site-specific quantitative phosphoproteomics: principles and applications. Annu Rev Pharmacol Toxicol. 2009;49:199–221 [DOI] [PubMed] [Google Scholar]
- 55.Albrethsen J. The first decade of MALDI protein profiling: a lesson in translational biomarker research. J Proteomics. 2011;74:765–73 [DOI] [PubMed] [Google Scholar]
- 56.Liuni P, Wilson DJ. Understanding and optimizing electrospray ionization techniques for proteomic analysis. Expert Rev Proteomics. 2011;8:197–209 [DOI] [PubMed] [Google Scholar]
- 57.Elschenbroich S, Kislinger T. Targeted proteomics by selected reaction monitoring mass spectrometry: applications to systems biology and biomarker discovery. Mol Biosyst. 2011;7:292–303 [DOI] [PubMed] [Google Scholar]
- 58. The Human Protein Atlas [cited April 15, 2012]. Available from: www.proteinatlas.org.
- 59.Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat. 2012;132:175–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kassie F, Anderson LB, Scherber R, Yu N, Lahti D, Upadhyaya P, Hecht SS. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67:6502–11 [DOI] [PubMed] [Google Scholar]
- 61.Wabnitz GH, Goursot C, Jahraus B, Kirchgessner H, Hellwig A, Klemke M, Konstandin MH, Samstag Y. Mitochondrial translocation of oxidized cofilin induces caspase-independent necrotic-like programmed cell death of T cells. Cell Death Dis. 2010;1:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabidó E, Selevsek N, Aebersold R. Mass spectrometry-based proteomics for systems biology. Curr Opin Biotechnol. 2011;23:1–7 [DOI] [PubMed] [Google Scholar]
- 63.Sardiu ME, Washburn MP. Building protein-protein interaction networks with proteomics and informatics tools. J Biol Chem. 2011;286:23645–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang GL, DeLuca DS, Brusic V. Database resources for proteomics-based analysis of cancer. Methods Mol Biol. 2011;723:349–64 [DOI] [PubMed] [Google Scholar]
- 65.Pontén F, Jirström K, Uhlen M. The human protein atlas: a tool for pathology. J Pathol. 2008;216:387–93 [DOI] [PubMed] [Google Scholar]
- 66.Boja E, Hiltke T, Rivers R, Kinsinger C, Rahbar A, Mesri M, Rodriguez H. Evolution of clinical proteomics and its role in medicine. J Proteome Res. 2011;10:66–84 [DOI] [PubMed] [Google Scholar]
- 67.Pontén F, Gry M, Fagerberg L, Lundberg E, Asplund A, Berglund L, Oksvold P, Björling E, Hober S, Kampf C, et al. A global view of protein expression in human cells, tissues, and organs. Mol Syst Biol. 2009;5:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Britton LM, Gonzales-Cope M, Zee BM, Garcia BA. Breaking the histone code with quantitative mass spectrometry. Expert Rev Proteomics. 2011;8:631–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIα. J Biol Chem. 2011;286:33591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeon YK, Yoo DR, Jang YH, Jang SY, Nam MJ. Sulforaphane induces apoptosis in human hepatic cancer cells through inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4, mediated by hypoxia inducible factor-1-dependent pathway. Biochim Biophys Act. 2011;1814:1340–8 [DOI] [PubMed] [Google Scholar]
- 71.Neo JC, Rose P, Ong CN, Chung MC. β-Phenylethyl isothiocyanate mediated apoptosis: a proteomic investigation of early apoptotic protein changes. Proteomics. 2005;5:1075–82 [DOI] [PubMed] [Google Scholar]
- 72.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103:571–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cross JV, Rady JM, Foss FW, Lyons CE, Macdonald TL, Templeton DJ. Nutrient isothiocyanates covalently modify and inhibit the inflammatory cytokine macrophage migration inhibitory factor (MIF). Biochem J. 2009;423:315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoelzl C, Lorenz O, Haudek V, Gundacker N, Knasmüller S, Gerner C. Proteome alterations induced in human white blood cells by consumption of Brussels sprouts: results of a pilot intervention study. Proteomics Clin Appl. 2008;2:108–17 [DOI] [PubMed] [Google Scholar]
- 75.Mitchell BL, Yasui Y, Lampe JW, Gafken PR, Lampe PD. Evaluation of matrix-assisted laser desorption/ionization-time of flight mass spectrometry proteomic profiling: identification of alpha 2-HS glycoprotein B-chain as a biomarker of diet. Proteomics. 2005;5:2238–46 [DOI] [PubMed] [Google Scholar]
- 76.Breikers G, van Breda SG, Bouwman FG, van Herwijnen MH, Renes J, Mariman EC, Kleinjans JC, van Delft JH. Potential protein markers for nutritional health effects on colorectal cancer in the mouse as revealed by proteomics analysis. Proteomics. 2006;6:2844–52 [DOI] [PubMed] [Google Scholar]
- 77.Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer's disease. Neurobiol Aging. 2008;29:51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr. 2005;135:S2953–9 [DOI] [PubMed] [Google Scholar]
- 79.Zhang D, Tai YC, Wong CH, Tai LK, Koay ES, Chen CS. Molecular response of leukemia HL-60 cells to genistein treatment, a proteomics study. Leuk Res. 2007;31:75–82 [DOI] [PubMed] [Google Scholar]
- 80.Fuchs D, Erhard P, Rimbach G, Daniel H, Wenzel U. Genistein blocks homocysteine-induced alterations in the proteome of human endothelial cells. Proteomics. 2005;5:2808–18 [DOI] [PubMed] [Google Scholar]
- 81.Pakalapati G, Li L, Gretz N, Koch E, Wink M. Influence of red clover (Trifolium pratense) isoflavones on gene and protein expression profiles in liver of ovariectomized rats. Phytomedicine. 2009;16:845–55 [DOI] [PubMed] [Google Scholar]
- 82.Wenzel U, Herzog A, Kuntz S, Daniel H. Protein expression profiling identifies molecular targets of quercetin as a major dietary flavonoid in human colon cancer cells. Proteomics. 2004;4:2160–74 [DOI] [PubMed] [Google Scholar]
- 83.Dihal AA, van der Woude H, Hendriksen PJ, Charif H, Dekker LJ, Ijsselstijn L, de Boer VC, Alink GM, Burgers PC, Rietjens IM, et al. Transcriptome and proteome profiling of colon mucosa from quercetin fed F344 rats point to tumor preventive mechanisms, increased mitochondrial fatty acid degradation and decreased glycolysis. Proteomics. 2008;8:45–61 [DOI] [PubMed] [Google Scholar]
- 84.Mouat MF, Kolli K, Orlando R, Hargrove JL, Grider A. The effects of quercetin on SW480 human colon carcinoma cells: a proteomic study. Nutr J. 2005;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou J, Liang S, Fang L, Chen L, Tang M, Xu Y, Fu A, Yang J, Wei Y. Quantitative proteomic analysis of HepG2 cells treated with quercetin suggests IQGAP1 involved in quercetin-induced regulation of cell proliferation and migration. OMICS. 2009;13:93–103 [DOI] [PubMed] [Google Scholar]
- 86.Zanini C, Giribaldi G, Mandili G, Carta F, Crescenzio N, Bisaro B, Doria A, Foglia L, di Montezemolo LC, Timeus F, et al. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing's sarcoma cell lines. J Neurochem. 2007;103:1344–54 [DOI] [PubMed] [Google Scholar]
- 87.Hu W, Wu W, Verschraegen CF, Chen L, Mao L, Yeung SC, Kudelka AP, Freedman RS, Kavanagh JJ. Proteomic identification of heat shock protein 70 as a candidate target for enhancing apoptosis induced by farnesyl transferase inhibitor. Proteomics. 2003;3:1904–11 [DOI] [PubMed] [Google Scholar]
- 88.Aalinkeel R, Bindukumar B, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, Schwartz SA. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68:1773–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herzog A, Kindermann B, Döring F, Daniel H, Wenzel U. Pleiotropic molecular effects of the pro-apoptotic dietary constituent flavone in human colon cancer cells identified by protein and mRNA expression profiling. Proteomics. 2004;4:2455–64 [DOI] [PubMed] [Google Scholar]
- 90.de Roos B, Geelen A, Ross K, Rucklidge G, Reid M, Duncan G, Caslake M, Horgan G, Brouwer IA. Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics. 2008;8:1965–74 [DOI] [PubMed] [Google Scholar]
- 91.de Roos B, Duivenvoorden I, Rucklidge G, Reid M, Ross K, Lamers RJ, Voshol PJ, Havekes LM, Teusink B. Response of apolipoprotein E*3-Leiden transgenic mice to dietary fatty acids: combining liver proteomics with physiological data. FASEB J. 2005;19:813–5 [DOI] [PubMed] [Google Scholar]
- 92.de Roos B, Rucklidge G, Reid M, Ross K, Duncan G, Navarro MA, Arbones-Mainar JM, Guzman-Garcia MA, Osada J, Browne J, et al. Divergent mechanisms of cis9, trans11-and trans10, cis12-conjugated linoleic acid affecting insulin resistance and inflammation in apolipoprotein E knockout mice: a proteomics approach. FASEB J. 2005;19:1746–8 [DOI] [PubMed] [Google Scholar]
- 93.Tan HT, Tan S, Lin Q, Lim TK, Hew CL, Chung MC. Quantitative and temporal proteome analysis of butyrate-treated colorectal cancer cells. Mol Cell Proteomics. 2008;7:1174–85 [DOI] [PubMed] [Google Scholar]
- 94.Tan S, Seow TK, Liang RC, Koh S, Lee CP, Chung MC, Hooi SC. Proteome analysis of butyrate-treated human colon cancer cells (HT-29). Int J Cancer. 2002;98:523–31 [DOI] [PubMed] [Google Scholar]
- 95.Fung KY, Lewanowitsch T, Henderson ST, Priebe I, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove LJ. Proteomic analysis of butyrate effects and loss of butyrate sensitivity in HT29 colorectal cancer cells. J Proteome Res. 2009;8:1220–7 [DOI] [PubMed] [Google Scholar]
- 96.Gharbi S, Garzón B, Gayarre J, Timms J, Pérez-Sala D. Study of protein targets for covalent modification by the antitumoral and anti-inflammatory prostaglandin PGA1: focus on vimentin. J Mass Spectrom. 2007;42:1474–84 [DOI] [PubMed] [Google Scholar]
- 97.Deshane J, Chaves L, Sarikonda KV, Isbell S, Wilson L, Kirk M, Grubbs C, Barnes S, Meleth S, Kim H. Proteomics analysis of rat brain protein modulations by grape seed extract. J Agric Food Chem. 2004;52:7872–83 [DOI] [PubMed] [Google Scholar]
- 98.Lee SC, Chan J, Clement MV, Pervaiz S. Functional proteomics of resveratrol-induced colon cancer cell apoptosis: caspase-6-mediated cleavage of lamin A is a major signaling loop. Proteomics. 2006;6:2386–94 [DOI] [PubMed] [Google Scholar]
- 99.Narayanan NK, Narayanan BA, Nixon DW. Resveratrol-induced cell growth inhibition and apoptosis is associated with modulation of phosphoglycerate mutase B in human prostate cancer cells: two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mass spectrometry evaluation. Cancer Detect Prev. 2004;28:443–52 [DOI] [PubMed] [Google Scholar]
- 100.Li N, Guo R, Li W, Shao J, Li S, Zhao K, Chen X, Xu N, Liu S, Lu Y. A proteomic investigation into a human gastric cancer cell line BGC823 treated with diallyl trisulfide. Carcinogenesis. 2006;27:1222–31 [DOI] [PubMed] [Google Scholar]
- 101.Zhang YK, Zhang XH, Li JM, Sun de S, Yang Q, Diao DM. A proteomic study on a human osteosarcoma cell line Saos-2 treated with diallyl trisulfide. Anticancer Drugs. 2009;20:702–12 [DOI] [PubMed] [Google Scholar]
- 102.Lu QY, Yang Y, Jin YS, Zhang ZF, Heber D, Li FP, Dubinett SM, Sondej MA, Loo JA, Rao JY. Effects of green tea extract on lung cancer A549 cells: proteomic identification of proteins associated with cell migration. Proteomics. 2009;9:757–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007;43:546–56 [DOI] [PubMed] [Google Scholar]
- 104.Ramljak D, Romanczyk LJ, Metheny-Barlow LJ, Thompson N, Knezevic V, Galperin M, Ramesh A, Dickson RB. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol Cancer Ther. 2005;4:537–46 [DOI] [PubMed] [Google Scholar]
- 105.Mahn AV, Toledo HM, Ruz M. Dietary supplementation with selenomethylselenocysteine produces a differential proteomic response. J Nutr Biochem. 2009;20:791–9 [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, He QY, Chen H, Chiu JF. Synergistic effects of retinoic acid and tamoxifen on human breast cancer cells: proteomic characterization. Exp Cell Res. 2007;313:357–68 [DOI] [PubMed] [Google Scholar]
- 107.Laserna EJ, Valero ML, Sanz L, del Pino MM, Calvete JJ, Barettino D. Proteomic analysis of phosphorylated nuclear proteins underscores novel roles for rapid actions of retinoic acid in the regulation of mRNA splicing and translation. Mol Endocrinol. 2009;23:1799–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park S, Lee J, Yeom CH. A proteomic approach to the identification of early molecular targets changed by L-ascorbic acid in NB4 human leukemia cells. J Cell Biochem. 2006;99:1628–41 [DOI] [PubMed] [Google Scholar]
- 109.Duthie SJ, Horgan G, de Roos B, Rucklidge G, Reid M, Duncan G, Pirie L, Basten GP, Powers HJ. Blood folate status and expression of proteins involved in immune function, inflammation, and coagulation: biochemical and proteomic changes in the plasma of humans in response to long-term synthetic folic acid supplementation. J Proteome Res. 2010;9:1941–50 [DOI] [PubMed] [Google Scholar]
- 110.Duthie SJ, Mavrommatis Y, Rucklidge G, Reid M, Duncan G, Moyer MP, Pirie LP, Bestwick CS. The response of human colonocytes to folate deficiency in vitro: functional and proteomic analyses. J Proteome Res. 2008;7:3254–66 [DOI] [PubMed] [Google Scholar]