Abstract
Background
Emotional support and depression may influence adherence to risk factor management instructions after acute myocardial infarction (AMI), but their role requires further investigation.
Purpose
To examine the longitudinal association between perceived emotional support and risk factor management adherence and assess depressive symptoms as a moderator of this association.
Methods
Among 2,202 AMI patients, we assessed adherence to risk factor management instructions over the first recovery year. Modified Poisson mixed-effects regression evaluated associations, with adjustment for demographic and clinical factors.
Results
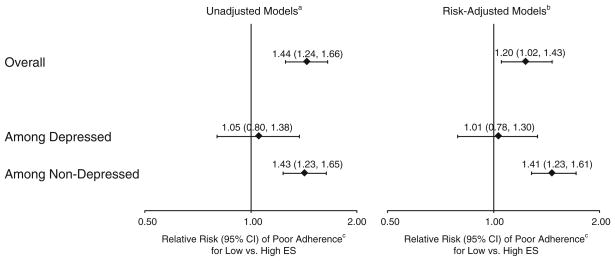
Patients with low baseline support had greater risk of poor adherence over the first year than patients with high baseline support (relative risk [RR]=1.20, 95% confidence interval [CI]=1.02–1.43). In stratified analyses, low support remained a significant predictor of poor adherence for non-depressed (RR=1.41, 95% CI=1.23–1.61) but not depressed (RR=1.01, 95% CI=0.78–1.30) patients (p for interaction<0.001).
Conclusions
Low emotional support is associated with poor risk factor management adherence after AMI. This relationship is moderated by depression, with a significant relationship observed only among non-depressed patients.
Keywords: Social support, Emotional support, Myocardial infarction, Adherence, Risk factor management, Depressive symptoms, Depression
Introduction
Although recent treatment advances for acute myocardial infarction (AMI) have led to significant reductions in hospital mortality, there remains substantial risk of poor health status, recurrent AMI, and subsequent mortality following hospital discharge [1]. Social support is a potentially modifiable psychosocial factor that has previously been associated with cardiac outcomes and is a possible target for interventions aiming to improve outcomes [2–5]. Although the specific mechanism linking social support to outcomes is not yet fully understood, patient adherence has been suggested as a possible mediator of the link between support and health outcomes [6]. For patients recovering from AMI, adherence to cardiac risk factor management instructions is particularly important [7]. Studies have linked greater levels of social support to better adherence to a number of risk factor management behaviors [6, 8–11]. In turn, risk factor management adherence has been associated with improved outcomes, including psychological well-being, quality of life, functional status, AMI recurrence, and mortality [10, 12–15]. Intensive risk factor management programs have also been linked to regression of coronary atherosclerosis among patients with moderate to severe coronary heart disease [16, 17].
Although risk factor management adherence seems a plausible mediator of the relationship between social support and health outcomes, the relationship between support and adherence itself remains unclear [6]. Few studies have longitudinally assessed this relationship among AMI patients. Adherence is likely to vary over the course of recovery and the relationship between social support at hospital presentation and adherence at different points in recovery (e.g., 1, 6, or 12 months) could vary as well. In addition, few studies have assessed potential psychosocial moderators of this relationship, such as depressive symptoms. Low social support and depression often co-occur, and both have been linked to poorer adherence [4]. Interaction between these states in predicting adherence could illuminate a need to address low support differently for depressed and non-depressed patients. The present study focused on perceived emotional support (one domain of social support related to feelings that love, affection, understanding, and guidance are available from others) and evaluated whether low levels assessed during the index hospitalization predict poorer adherence to risk factor management instructions during the first year after AMI using three assessment points for adherence (1, 6, and 12 months). A secondary aim was to examine the role of depressive symptoms as a potential moderator of the association between perceived emotional support and adherence.
Methods
Study Population
AMI patients were consecutively recruited between January 2003 and June 2004 from 19 United States centers to participate in the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER). The methods of PREMIER have been described previously [18]. Briefly, patients had to be age 18 years or older with increased troponin or creatine kinase-MB levels and additional evidence supporting AMI (>20 min of ischemic symptoms or electrocardiographic ST changes). Patients must have presented directly to the enrolling site or been transferred within the first 24 h of presentation. Patients who were incarcerated, developed elevated cardiac enzymes because of elective coronary revascularization, or did not speak English or Spanish were excluded. Of the 2,498 patients enrolled, 2,202 were included in the present study after excluding those who died during their index hospitalization (n=17), had incomplete baseline emotional support data (n=70), or had no data on adherence to risk factor management instructions during follow-up (n=230; categories not mutually exclusive).
Data Collection
Baseline patient data were collected by medical chart abstraction and in-person patient interviews administered within 24 to 72 h of hospital presentation. Follow-up patient interviews were conducted by telephone at 1, 6, and 12 months post-discharge by a national follow-up center. Institutional Review Board approval was obtained at each participating institution, and patients provided informed consent for both baseline and follow-up interviews.
Emotional Support Assessment
Perceived emotional support was measured at the index hospitalization (baseline) by five items from the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Social Support Inventory, a reliable and valid scale for cardiac populations [19, 20]. The full-length ENRICHD Social Support Inventory comprises seven items that prior studies have found individually predictive of mortality in cardiac patients [21–24]. The instrumental support and marital status items, however, were not used as part of the emotional support measure in our analyses as these items represent different domains of social support and could differentially impact outcomes. The remaining five items querying the perceived availability of different types of emotional support are measured on five-point scales and summed to create a single score ranging from 5 to 25, with higher scores indicating greater perceived support. This five-item scale has been used previously [5, 19, 25, 26], is highly correlated with the full-length seven-item scale [19], has itself been validated [19], and demonstrated strong internal consistency in our study population (Cronbach’s α=0.91). Consistent with ENRICHD [19], we defined low support as a score of ≤3 on at least two items and a total score of ≤18.
Depressive Symptoms Assessment
Depressive symptoms were assessed at the index hospitalization by the nine-item Primary Care Evaluation of Mental Disorders Patient Health Questionnaire (PHQ-9) [27], a valid and reliable measure for recognizing both major depression and sub-threshold depressive disorder in both general and cardiac populations [28–30]. Possible scores on the PHQ-9 range from 0 to 27, with higher scores indicating greater depressive symptomatology. The PHQ-9 was maintained as a continuous variable for inclusion in analytic models. For analyses stratified by depressive symptoms, depression was defined as a PHQ-9 score of 10 or higher since this score is indicative of at least moderately severe depressive symptoms and corresponds to the minimum number of reported symptoms needed for a diagnosis of major depression [31].
Outcome Assessment
At 1, 6, and 12 months post-AMI, patients were asked whether they received and adhered to 13 possible risk factor management instructions (medication adherence, warfarin use, follow-up plan/appointments, whom to call if symptoms worsen, cholesterol monitoring, cholesterol therapy, diabetes management, weight monitoring, weight loss, smoking cessation, diet, exercise, cardiac rehabilitation). The number of instructions received by individual patients was determined by asking each patient whether he or she had been instructed on any of the 13 activities since the last interview; the source and intensity of instruction were not captured. For each of the instructions received, the patient was then asked how well he or she had followed the instruction (“very carefully”, “fairly well”, “somewhat”, “not at all”, or “not able to for other reasons”). The degree of adherence was defined as the percentage of received instructions for which the patient reported that he or she had “very carefully” adhered. This definition was decided a priori and is in accordance with a prior study using PREMIER data that linked poor adherence to a greater likelihood of angina symptoms at 1 year post-AMI [10]. To ensure only those instructions that were relevant to the patient were included in the denominator for the adherence calculations, only diabetic patients were included in the assessment of diabetes management and only smokers were included in the assessment of smoking cessation. We dichotomized patients’ reports of adherence to their risk factor management instructions at the lowest quartile, defining poor adherence as “very carefully” adhering to <50% of instructions received. Sensitivity analyses examined thresholds as high as 80%, and results remained robust.
Covariates
Sociodemographic factors, clinical history and presentation, hospital care, and outpatient care were all assessed as part of the PREMIER study. The individual items were chosen for this study a priori according to clinical relevance and to help distinguish the relationships being examined; variables were included in models irrespective of statistical significance. Sociodemographic factors included age, sex, race, marital status, education greater than high school level, lack of primary insurance/self-pay, and avoidance of health care in the past because of cost. Clinical history and presentation included prior coronary artery disease (AMI, coronary artery bypass grafting, or percutaneous coronary intervention), diabetes, hypertension, hypercholesterolemia, prior stroke, congestive heart failure, chronic renal failure, chronic lung disease, smoking within past 30 days, daily alcohol consumption of greater than three drinks, obesity (body mass index ≥30), moderate or severe left ventricular systolic dysfunction, and final AMI diagnosis (ST-elevation versus non-ST-elevation). Hospital care included diagnostic catheterization and revascularization (coronary artery bypass grafting or percutaneous coronary intervention). Outpatient care included the mean number of relevant risk factor management instructions recalled by a patient during follow-up and whether a patient had visited a cardiologist or primary care physician during the 1-year follow-up period.
Statistical Analysis
Sociodemographic characteristics, clinical history and presentation, hospital care, outpatient care, and depressive symptoms were compared between patients with low and high levels of perceived emotional support using Pearson chi-squared or Fisher’s exact tests for categorical variables and t tests for continuous variables. The frequency with which patients recalled receiving the 13 individual risk factor management instructions and the percentage of patients reporting they “very carefully” adhered to these instructions at 1, 6, and 12 months were determined and also compared between the emotional support categories. Secondary analyses examined these associations after stratification by depressive symptoms.
Modified Poisson mixed-effects regression [32] examined whether low perceived emotional support at hospital presentation was associated with poor adherence to risk factor management instructions during recovery. This form of regression was used to directly calculate relative risks for poor adherence according to level of support because poor adherence was not rare in our study. In such instances, odds ratios generated from logistic regression may not provide accurate representations of effects. For this analysis, the model included three repeated adherence measurements within-subjects over time (1, 6, and 12 months) and a random effect for site to account for patient clustering within hospitals. The model sequentially adjusted for groups of related variables (sociodemographic characteristics, clinical history and presentation, hospital care, outpatient care, and depressive symptoms). Variation over time in the relationship between perceived emotional support and risk factor management adherence was evaluated by inclusion of a support-by-time interaction term. As this term was nonsignificant at the p=0.10 level, it was not included in the final model, and thus the relative risks presented represent the association of support with adherence during the follow-up period, averaged across all time points (1, 6, and 12 months). To assess whether the presence of at least moderately severe depressive symptoms at hospital presentation modifies the association between perceived emotional support and risk factor management adherence, the risk-adjusted model was stratified by depressive symptom status, and a formal test of interaction was performed by including a support-by-depression interaction term in the final risk-adjusted model. Two-way interactions between emotional support and sex and between emotional support and marital status were also examined.
All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Tests for statistical significance were 2-tailed with α=0.05.
Results
The 2,202 patients included in this study were, on average, 61 years old and were predominately male (68%), white (76%), and married (62%). Approximately 21% of patients experienced a previous AMI, and comorbidities were relatively common, including diabetes (28%), chronic lung disease (12%), and chronic renal failure (9%). At hospital presentation, 33% of patients were smokers, 22% were depressed, and 14% reported low emotional support. Comparisons of patient characteristics by baseline emotional support revealed that those with low support were significantly more likely to be unmarried, current smokers, current drinkers, and depressed (Table 1). They were also more likely to be unemployed, lack health insurance, and have avoided health care in the past due to cost. They were less likely to have visited a cardiologist or cardiac surgeon during follow-up.
Table 1.
Patient characteristics by baseline emotional support
| Characteristic | High ES n=1890 | Low ES n=312 | p value |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, y | 60.8±12.9 | 59.6±12.1 | 0.119 |
| Female | 32.3 | 33.7 | 0.643 |
| Caucasiana | 76.2 | 73.3 | 0.269 |
| Marrieda | 66.2 | 38.4 | <0.001 |
| Working full- or part-timea | 46.3 | 39.0 | 0.018 |
| Greater than high school educationa | 49.0 | 49.5 | 0.871 |
| No health insurance/self-paya | 11.8 | 16.2 | 0.035 |
| Avoided care because of costa | 15.6 | 32.0 | <0.001 |
| Clinical history | |||
| Prior MI | 20.5 | 21.8 | 0.609 |
| Prior revascularization | 25.0 | 24.0 | 0.723 |
| CHF | 10.1 | 12.2 | 0.253 |
| Hypertension | 62.6 | 65.4 | 0.344 |
| Diabetes | 27.3 | 29.8 | 0.359 |
| Hypercholesterolemia | 49.4 | 51.0 | 0.601 |
| Chronic lung disease | 11.9 | 14.4 | 0.209 |
| Chronic renal failure | 8.5 | 11.5 | 0.077 |
| Prior stroke/TIA | 7.9 | 10.6 | 0.110 |
| Current smoker (past 30 days)a | 31.5 | 43.6 | <0.001 |
| Daily alcohol consumption >3 drinksa | 12.1 | 16.1 | 0.048 |
| Obese (BMI ≥30)a | 39.3 | 39.8 | 0.861 |
| Clinical presentation | |||
| Acute SBP, mmHga | 138.9±30.6 | 142.2±33.7 | 0.106 |
| Acute heart ratea | 80.6±21.4 | 82.2±21.0 | 0.225 |
| LVSD: moderate/severea | 25.2 | 23.4 | 0.508 |
| Final MI diagnosis: STEMI | 45.1 | 42.6 | 0.420 |
| In-hospital care | |||
| Diagnostic catheterization | 41.4 | 46.2 | 0.113 |
| Revascularization | 73.8 | 68.9 | 0.071 |
| Outpatient care | |||
| Visited primary care clinician during follow-up | 72.5 | 69.2 | 0.228 |
| Visited cardiologist/cardiac surgeon during follow-up | 82.2 | 74.0 | <0.001 |
| Psychosocial moderator | |||
| Depressed (PHQ-9 ≥10)a | 18.4 | 42.5 | <0.001 |
| RFM Instructions | |||
| Mean # applicable RFM instructions | 5.7±2.1 | 5.3±2.3 | 0.005 |
Data presented as mean ± standard deviation for continuous variables (comparisons via t test) and column-wise percentages for categorical variables (comparisons via Pearson chi-squared test or Fisher’s exact test).
ES emotional support; MI myocardial infarction; CHF congestive heart failure; TIA transient ischemic attack; BMI body max index, kg/m2; PHQ Patient Health Questionnaire; SBP systolic blood pressure; LVSD left ventricular systolic dysfunction; STEMI ST-segment elevation myocardial infarction; RFM risk factor management.
Missing data present: <1% for Caucasian, married, working full- or part-time, current smoker, LVSD; 1%–3% for greater than high school education, avoided care because of cost, daily alcohol consumption >3 drinks, acute SBP, acute heart rate; 3%–5% for no health insurance/self-pay, obese, depressed.
On average, patients reported receiving approximately six risk factor management instructions during the first year of recovery, with low perceived emotional support patients reporting having received fewer instructions (Table 1). The overall number of instructions decreased slightly over time from six instructions received between baseline and 1 month to five instructions received between 6 and 12 months. This decrease over time was observed for each of the individual risk factor management instructions, except cholesterol monitoring, cholesterol therapy, and diabetes monitoring which increased with time (Table 2). There were few significant differences in the reported receipt of instructions between the emotional support groups.
Table 2.
Frequency of receiving individual risk factor management instructions by time and baseline emotional support
| Instruction | 1 month
|
6 months
|
12 months
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| High ES n=1715 | Low ES n=270 | p value | High ES n=1614 | Low ES n=252 | p value | High ES n=1449 | Low ES n=212 | p value | |
| Medications | 89.8 | 90.7 | 0.632 | 73.7 | 71.4 | 0.442 | 64.5 | 60.4 | 0.248 |
| Warfarin | 17.4 | 16.3 | 0.646 | 12.8 | 12.3 | 0.838 | 11.6 | 9.9 | 0.258 |
| Follow-up plan | 83.3 | 83.3 | 0.997 | 79.6 | 74.2 | 0.053 | 82.1 | 77.8 | 0.132 |
| Whom to call | 73.1 | 67.0 | 0.038 | 58.9 | 56.4 | 0.452 | 58.4 | 48.1 | 0.005 |
| Cholesterol monitoring | 40.6 | 41.5 | 0.780 | 64.8 | 58.7 | 0.064 | 70.2 | 58.0 | <0.001 |
| Cholesterol therapy | 42.2 | 34.1 | 0.012 | 48.1 | 46.8 | 0.711 | 45.3 | 45.3 | 0.987 |
| Diabetes management | 20.6 | 20.0 | 0.825 | 23.0 | 21.2 | 0.511 | 21.9 | 22.2 | 0.923 |
| Weight monitoring | 38.9 | 33.7 | 0.103 | 38.4 | 34.1 | 0.198 | 32.1 | 33.4 | 0.702 |
| Weight loss | 31.9 | 31.1 | 0.797 | 32.3 | 31.8 | 0.851 | 29.1 | 27.4 | 0.611 |
| Smoking cessation | 28.3 | 38.5 | <0.001 | 20.1 | 32.1 | <0.001 | 17.0 | 29.3 | <0.001 |
| Diet | 65.4 | 58.9 | 0.037 | 59.1 | 54.0 | 0.124 | 46.5 | 43.4 | 0.395 |
| Exercise | 71.9 | 66.3 | 0.059 | 72.2 | 64.3 | 0.010 | 63.8 | 53.3 | 0.003 |
| Cardiac rehabilitation | 62.5 | 55.1 | 0.021 | 56.6 | 46.6 | 0.003 | 21.7 | 15.4 | 0.036 |
Data presented as column-wise percentages (comparisons via Pearson chi-squared test or Fisher’s exact test)
ES emotional support
Adherence at 1 month was low for many of the individual risk factor management instructions, with less than two-thirds of patients reporting that they “very carefully” adhered to their instructions for weight loss and monitoring, smoking cessation, diet, exercise, and cardiac rehabilitation (Table 3). With the exception of cardiac rehabilitation, adherence to these six instructions decreased over time. Adherence to the remaining seven instructions also generally decreased over time, particularly for patients with low perceived emotional support; however, slight improvements in adherence over time were observed for several of these instructions between 1 and 6 months. With few exceptions (warfarin at 1 month and diabetes management at 6 months), patients reporting low emotional support were less likely to have “very carefully” adhered to their individual risk factor management instructions than patients reporting high emotional support.
Table 3.
“Very careful” reported adherence to risk factor management instructions by time and baseline emotional support
| Instruction | 1 month
|
6 months
|
12 months
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| High ES | Low ES | p value | High ES | Low ES | p value | High ES | Low ES | p value | |
| Medications | 94.6 | 89.4 | 0.002 | 90.7 | 81.1 | <0.001 | 91.7 | 78.9 | <0.001 |
| Warfarin | 93.4 | 95.0 | 0.999 | 92.2 | 89.7 | 0.714 | 92.1 | 89.5 | 0.657 |
| Follow-up plan | 89.7 | 87.0 | 0.274 | 89.9 | 84.7 | 0.032 | 90.8 | 84.1 | 0.007 |
| Whom to call | 89.6 | 84.6 | 0.071 | 90.3 | 87.6 | 0.335 | 88.8 | 83.2 | 0.096 |
| Cholesterol monitoring | 80.2 | 75.9 | 0.359 | 85.7 | 76.9 | 0.006 | 84.6 | 75.0 | 0.008 |
| Cholesterol therapy | 71.2 | 65.2 | 0.233 | 73.7 | 62.7 | 0.013 | 70.8 | 56.3 | 0.004 |
| Diabetes management | 72.5 | 70.4 | 0.742 | 68.4 | 69.0 | 0.934 | 69.7 | 63.8 | 0.416 |
| Weight monitoring | 62.7 | 47.3 | 0.005 | 56.9 | 40.7 | 0.005 | 46.5 | 29.4 | 0.008 |
| Weight loss | 43.9 | 34.5 | 0.107 | 36.0 | 27.5 | 0.137 | 27.1 | 19.0 | 0.187 |
| Smoking cessation | 54.1 | 46.2 | 0.140 | 34.0 | 29.6 | 0.460 | 21.5 | 8.1 | 0.015 |
| Diet | 51.3 | 41.5 | 0.020 | 40.7 | 30.9 | 0.029 | 32.8 | 27.2 | 0.279 |
| Exercise | 54.4 | 44.1 | 0.010 | 45.8 | 37.0 | 0.037 | 34.2 | 23.0 | 0.017 |
| Cardiac rehabilitation | 33.9 | 31.3 | 0.536 | 68.9 | 67.5 | 0.761 | 55.5 | 53.1 | 0.801 |
Data presented as column-wise percentages (comparisons via Pearson chi-squared test or Fisher’s exact test)
ES emotional support
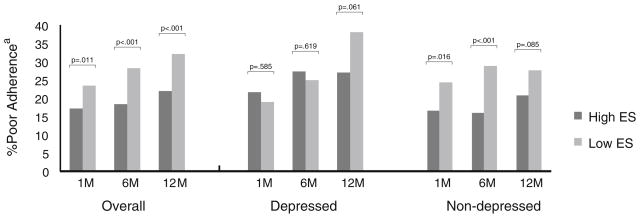
For the composite measure of adherence, 354 (18%) patients at 1 month, 366 (20%) patients at 6 months, and 383 (23%) patients at 12 months reported that they “very carefully” adhered to fewer than half of their relevant risk factor management instructions. Although patients reporting low emotional support were more likely to report poor adherence at each time point than patients reporting high emotional support, stratification of the population by depressive symptoms status revealed that these differences were primarily limited to non-depressed patients (Fig. 1). Among depressed patients, level of perceived emotional support was not significantly associated with adherence at 1 and 6 months. By 12 months, however, differences in adherence by emotional support status began to emerge among depressed patients, with those reporting low emotional support also reporting poorer adherence.
Fig. 1.
Percent poor adherence by time and baseline emotional support: overall and stratified by depressive symptoms. Abbreviations: ES emotional support; M months. aPoor adherence defined as “very carefully” adhering to <50% of reported risk factor management instructions
In unadjusted regression models, patients reporting low emotional support at baseline had 44% higher risk of poor adherence to risk factor management instructions than patients reporting high emotional support at baseline (Fig. 2). With adjustment, this relationship was slightly attenuated but remained significant. The relationship was moderated by depressive symptoms (p<0.001 for support-by-depression interaction), with low perceived emotional support associated with a 41% higher risk of poor adherence among non-depressed patients, but not associated with poor adherence among depressed patients. No statistically significant two-way interactions of perceived emotional support with time, sex, or marital status were observed (all p for interaction >0.1).
Fig. 2.
Association of baseline low emotional support with poor risk factor management adherence: overall and stratified by depressive symptoms. Abbreviations: CI confidence interval; ES emotional support. aAccounts for site (using a random effect) and repeated adherence measures over time (1, 6, 12 months). bAccounts for all of the above and risk adjusts for sociodemographics (age, sex, race, marital status, education, primary insurance, avoiding health care because of cost), clinical factors (prior coronary artery disease [myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention], diabetes, hypertension, hypercholesterolemia, prior stroke, congestive heart failure, chronic renal failure, chronic lung disease, smoking status, alcohol use, obesity, left ventricular systolic dysfunction, final myocardial infarction diagnosis), in-hospital care (diagnostic catheterization, revascularization [coronary artery bypass grafting or percutaneous coronary intervention]), and outpatient care (mean number of relevant risk factor management instructions recalled by patient during follow-up, visited cardiologist during follow-up, visited primary care physician during follow-up). The overall model additionally adjusts for depressive symptoms. cPoor adherence defined as “very carefully” adhering to <50% of reported risk factor management instructions
Discussion
Adherence to risk factor management instructions is an important component of AMI recovery. In this prospective study, we found a significant association between low perceived emotional support and poor adherence to risk factor management instructions over the first year after an AMI. The strength of this relationship did not vary significantly over the course of recovery, but was moderated by depressive symptoms status, with a significant relationship observed principally among the non-depressed patients.
Our finding of poorer adherence to risk factor management instructions among those reporting low emotional support compared to high emotional support is consistent with previous studies assessing relationships between various domains of social support and adherence. A meta-analysis of such studies conducted in a variety of patient and community samples showed that those without emotional support had 1.35 times the risk of nonadherence to medical treatment compared to those with emotional support [6]. The relationship of practical support to adherence was even stronger, while the relation of more structural aspects of social support (e.g., marital status and living arrangements) was somewhat weaker but still statistically significant [6]. A significant support–adherence relationship has also been noted among AMI patients, with aspects of social support linked to individual behaviors such as medication adherence [8], participation in cardiac rehabilitation [9], and smoking cessation [11].
Although a number of studies have examined the relationship between social support and one particular risk factor management behavior [6, 8, 9, 11], few have examined the relationship between social support and the multitude of instructions for risk factor management provided to patients following AMI for the purpose of reducing risk for subsequent cardiac events and other adverse outcomes. In a previous study using data from PREMIER, perceived social support (as assessed by the seven-item ENRICHD Social Support Inventory) at hospital presentation was associated with adherence to a composite measure of 13 risk factor management instructions at 1 month post-AMI in bivariate analyses [10]. As social support was not the focus of the study, the authors did not perform more in-depth analyses. Our study expands upon this previous finding by focusing on the emotional support domain of social support and evaluating whether the support–adherence relationship persisted throughout the entire first year of recovery, was independent of clinical variables, or was moderated by other psychosocial variables.
Using data from three assessment points during the recovery process (1, 6, and 12 months after AMI), we found that the relationship between perceived emotional support at hospital presentation and adherence to risk factor management instructions during follow-up did not appear to vary over time. We also observed that this support–adherence relationship was independent of sociodemographic characteristics, clinical history and presentation, hospital inpatient and outpatient care, and depressive symptoms. The consideration of depressive symptoms is particularly important as a strong and complex association exists between these two variables [5, 33, 34]. In studies not controlling for depressive symptoms, it is unclear whether an observed relationship between support and adherence may be partly, or wholly, attributable to depression. Our results show that controlling for these symptoms slightly attenuated the relationship between emotional support and adherence, although low emotional support remained a significant predictor of poor adherence.
There are several ways in which emotional support could influence risk factor management adherence [6, 35, 36]. Emotional support could act as a buffer to extreme emotions or stressors that interfere with adherence. Persons providing support may provide encouragement to adhere to instructions or directly provide resources or travel assistance. Emotional support may also help patients maintain the self-esteem that is important to maintaining the drastic lifestyle changes needed following AMI. Medication adherence, weight monitoring, diet, and exercise are areas of risk factor management instruction that may be particularly sensitive to emotional support during the early recovery period, according to our study results. Later in recovery, these areas of instruction remain linked to level of support, and cholesterol monitoring, cholesterol therapy, and adherence to a follow-up plan also become sensitive to emotional support. To better understand the role of depressive symptoms in these relationships, we explored a potential interaction between low perceived emotional support and depressive symptoms. Interestingly, we observed a significant relationship between low support and adherence among non-depressed patients, but not among depressed patients. These differences in the role of emotional support by depressive symptoms are likely related to factors at both the patient- and support provider-level. Depressed patients may have distorted perceptions of their available support, or they may be less affected by low support because their adherence is already very poor due to the depression, leaving little opportunity for these patients’ adherence to decrease further. It is also possible that individuals providing support to depressed patients may alter the intensity or type of support they provide; they may focus their efforts on relieving the immediate problem of the depression rather than risk factor management adherence.
Interventions aimed at improving low emotional support, and potentially other types of social support, may be a means to also improve adherence to risk factor management behaviors. Studies across a range of disciplines, with interventions varying by delivery format (group or individual), focus (social network expansion, support provision, or skills training), and support source (family, friends, peers, or professional), have generally shown that support can be increased via intervention [2]. However, intervention effects among AMI patients, specifically, have been more modest [37, 38], and more efficacious intervention strategies aimed at improving perceptions of support are needed. For depressed patients, emotional support intervention may not be as effective, unless the concomitant depressive symptoms are also identified and treated. Indeed, depression has previously been linked to poor adherence to both individual risk factor management behaviors [39, 40] and composite measures of risk factor management instructions [10, 41]. Closer monitoring of patients with low perceived emotional support, with or without concomitant depression, may serve as a first step in improving adherence behaviors.
Several limitations should be noted in the interpretation of our results. The receipt of risk factor management instructions was measured by self-report, and we could not assess the source or intensity of the instructions provided to patients. Although a standardized interview and centralized follow-up center were used to assess the receipt of the instructions from patients, and we accounted for site in our analyses, the delivery of the instructions or recall may vary among patients and by psychosocial factors under examination in this study. Adherence to these instructions was also measured by self-report. Accordingly, adherence may have been exaggerated due to reasons related to social desirability; however, research suggests such self-reports are positively related to other methods of adherence assessment [42]. Our use of the emotional support items from the ENRICHD Social Support Inventory does not provide a thorough examination of other forms of social support (e.g., structural aspects, tangible support, received support, support quality), which might also influence adherence. We cannot rule out the possibility of unmeasured residual confounding in our observational study as it is not possible to randomize patients to high or low emotional support at the time of their AMI. Finally, results from this multi-site study, which included a variety of institution types and geographic locations across the United States, may not generalize to AMI patients seen at other institutions or AMI patients who do not speak English or Spanish.
Our study has shown that low perceived emotional support predicts poorer adherence to cardiac risk factor management instructions during the first year after AMI. This relationship was moderated by depressive symptoms, with level of emotional support predicting adherence only among non-depressed patients. It will be important for future research to identify methods and opportunities to successfully intervene on emotional support among non-depressed patients and to further evaluate why depressed patients may not experience comparable benefits from such support.
Acknowledgments
As a doctoral student, Dr. Leifheit-Limson was supported by National Institute on Aging training grant T32AG00153. PREMIER was principally supported by CV Therapeutics, Inc., with funding for all analyses from CV Outcomes, Inc.
Footnotes
Conflict of Interest The authors have no conflict of interest to disclose.
Contributor Information
Erica C. Leifheit-Limson, Yale University School of Medicine, New Haven, CT, USA.
Stanislav V. Kasl, Yale University School of Medicine, New Haven, CT, USA.
Haiqun Lin, Yale University School of Medicine, New Haven, CT, USA.
Donna M. Buchanan, Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA.
Pamela N. Peterson, University of Colorado-Denver, Aurora, CO, USA. Denver Health Medical Center, Denver, CO, USA.
John A. Spertus, Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA. University of Missouri—Kansas City, Kansas City, MO, USA.
Judith H. Lichtman, Yale University School of Medicine, New Haven, CT, USA.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: A Report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hogen B, Linden W, Najarian B. Social support interventions: Do they work? Clin Psychol Rev. 2002;22:381–440. doi: 10.1016/s0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- 3.Barth J, Schneider S, von Kanel R. Lack of social support in the etiology and the prognosis of coronary heart disease: A systematic review and meta-analysis. Psychosom Med. 2010;72:229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- 4.Lett HS, Blumenthal JA, Babyak MA, et al. Social support and coronary heart disease: Epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67:869–878. doi: 10.1097/01.psy.0000188393.73571.0a. [DOI] [PubMed] [Google Scholar]
- 5.Leifheit-Limson EC, Reid KJ, Kasl SV, et al. The role of social support in health status and depressive symptoms after acute myocardial infarction: Evidence for a stronger relationship among women. Circ Cardiovasc Qual Outcomes. 2010;3:143–150. doi: 10.1161/CIRCOUTCOMES.109.899815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMatteo MR. Social support and patient adherence to medical treatment: A meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 7.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 8.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 9.Daly J, Sindone AP, Thompson DR, et al. Barriers to participation in and adherence to cardiac rehabilitation programs: A critical literature review. Prog Cardiovasc Nurs. 2002;17:8–17. doi: 10.1111/j.0889-7204.2002.00614.x. [DOI] [PubMed] [Google Scholar]
- 10.Decker C, Ahmad H, Moreng KL, et al. Risk factor management after myocardial infarction: Reported adherence and outcomes. Am Heart J. 2009;157:556–562. doi: 10.1016/j.ahj.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Dawood N, Vaccarino V, Reid KJ, et al. Predictors of smoking cessation after a myocardial infarction: The role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168:1961–1967. doi: 10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- 12.Pischke CR, Scherwitz L, Weidner G, Ornish D. Long-term effects of lifestyle changes on well-being and cardiac variables among coronary heart disease patients. Health Psychol. 2008;27:584–592. doi: 10.1037/0278-6133.27.5.584. [DOI] [PubMed] [Google Scholar]
- 13.Aldana SG, Whitmer WR, Greenlaw R, et al. Cardiovascular risk reductions associated with aggressive lifestyle modification and cardiac rehabilitation. Heart Lung. 2003;32:374–382. doi: 10.1016/s0147-9563(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 14.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: Secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143:659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Iestra JAR, Kromhout DMPHP, van der Schouw YTP, et al. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: A systematic review. Circulation. 2005;112:924–934. doi: 10.1161/CIRCULATIONAHA.104.503995. [DOI] [PubMed] [Google Scholar]
- 16.Ornish D, Brown SE, Billings JH, et al. Can lifestyle changes reverse coronary heart disease?: The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 17.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 18.Spertus JA, Peterson E, Rumsfeld JS, et al. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)—Evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.The ENRICHD Investigators. Enhancing recovery in coronary heart disease patients (ENRICHD): Study design and methods. Am Heart J. 2000;139:1–9. doi: 10.1016/s0002-8703(00)90301-6. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: The ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Williams RB, Barefoot JC, Califf RM, et al. Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA. 1992;267:520–524. [PubMed] [Google Scholar]
- 22.Berkman LF, Leo-Summers L, Horwitz RI. Emotional support and survival after myocardial infarction: A prospective, population-based study of the elderly. Ann Intern Med. 1992;117:1003–1009. doi: 10.7326/0003-4819-117-12-1003. [DOI] [PubMed] [Google Scholar]
- 23.Gorkin L, Schron EB, Brooks MM, et al. Psychosocial predictors of mortality in the Cardiac Arrhythmia Suppression Trial-1 (CAST-1) Am J Cardiol. 1993;71:263–267. doi: 10.1016/0002-9149(93)90788-e. [DOI] [PubMed] [Google Scholar]
- 24.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 25.Barry LC, Kasl SV, Lichtman J, Vaccarino V, Krumholz HM. Social support and change in health-related quality of life 6 months after coronary artery bypass grafting. J Psychosom Res. 2006;60:185–193. doi: 10.1016/j.jpsychores.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 26.Mendes de Leon CF, Czajkowski SM, Freedland KE, et al. The effect of a psychosocial intervention and quality of life after acute myocardial infarction: The Enhancing Recovery in Coronary Heart Disease (ENRICHD) clinical trial. J Cardiopulm Rehabil. 2006;26:9–15. doi: 10.1097/00008483-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JBW Group at PHQPCS. Validation and utility of a self-report version of PRIME-MD. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): A diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417–424. doi: 10.1016/j.genhosppsych.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Bosworth HB, Hays JC, George LK, Steffens DC. Psychosocial and clinical predictors of unipolar depression outcome in older adults. Int J Geriatr Psychiatry. 2002;17:238–246. doi: 10.1002/gps.590. [DOI] [PubMed] [Google Scholar]
- 34.Oxman TE, Berkman LF, Kasl S, Freeman DH, Jr, Barrett J. Social support and depressive symptoms in the elderly. Am J Epidemiol. 1992;135:356–368. doi: 10.1093/oxfordjournals.aje.a116297. [DOI] [PubMed] [Google Scholar]
- 35.Schumaker SA, Hill DR. Gender differences in social support and physical health. Health Psychol. 1991;10:102–111. doi: 10.1037//0278-6133.10.2.102. [DOI] [PubMed] [Google Scholar]
- 36.Wallston BS, Alagna SW, DeVellis BM, DeVellis RF. Social support and physical health. Health Psychol. 1983;2:367–391. [Google Scholar]
- 37.Cowan MJ, Freedland KE, Burg MM, et al. Predictors of treatment response for depression and inadequate social support—The ENRICHD randomized clinical trial. Psychother Psychosom. 2008;77:27–37. doi: 10.1159/000110057. [DOI] [PubMed] [Google Scholar]
- 38.Berkman L, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 39.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 40.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: Findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegelstein RC, Fauerbach JA, Stevens SS, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 42.Becker MH. Patient adherence to prescribed therapies. Med Care. 1985;23:539–555. doi: 10.1097/00005650-198505000-00014. [DOI] [PubMed] [Google Scholar]