Abstract
Rationale and Objectives
To assess differences in excitatory (glutamate/glutamine or Glx) and inhibitory (γ-Aminobutyric acid or GABA) neurotransmitter levels using MR spectroscopy in pain processing regions of the brain in patients diabetic neuropathy (DN) and positive sensory symptoms and age-matched healthy control (HC) subjects.
Materials and Methods
Seven diabetic patients (5 males, 2 females, mean age = 57.0 ± 8.5 years) with confirmed DN and positive sensory symptoms and 7 age and sex matched HC subjects (mean age = 57.7 ± 3.2 years) underwent 3 Tesla MR spectroscopy. Glx and GABA levels were quantified in the right anterior and posterior insula, anterior cingulate cortex and right thalamus.
Results
Mean Glx levels were significantly higher and mean GABA levels were significantly lower within the posterior insula in the DN patients compared to HC (P = 0.005 and 0.012 respectively).
Conclusions
This pilot data demonstrates an excitatory/inhibitory neurotransmitter imbalance in the brain of in patients with DN and positive sensory symptoms compared to pain free HC subjects.
Keywords: Diabetes, neuropathy, MR spectroscopy, pain
SUMMARY
Diabetic neuropathy (DN) is a common complication of diabetes, affecting approximately 50% of diabetic patients and in at least half of these patients the neuropathy is associated with tingling, allodynia, hyperalgesia or frank pain. DN is traditionally considered a disease of the peripheral nervous system; however, there is emerging evidence for significant central nervous system modulation of symptoms of DN and painful DN in particular. Advanced neuroimaging methods provide a unique opportunity to study central nervous system factors in the setting of diabetic neuropathy, noninvasively and under physiologic conditions. Magnetic resonance (MR) spectroscopy in particular can provide important information regarding brain biochemistry. Alterations in N-acetyl aspartate within the thalamus have been reported in the context of diabetic neuropathy using conventional MR spectroscopy methods.
Glutamatergic (excitatory) and γ-aminobutyric acid (GABA)ergic (inhibitory) neurotransmission are thought to play a significant role within the pain processing pathways of the central nervous system. Glutamine (Glx) levels (combined glutamate and glutamine) can be measured using conventional MR techniques. GABA quantification using MR spectroscopy is not possible using conventional techniques because of signal overlap and low signal intensity. Elevated Glx levels within pain processing regions of the brain have been demonstrated in other chronic pain conditions such as fibromyalgia and migraine headaches. Until recently, there were no reports of brain GABA quantification in the setting of chronic pain; our group recently published the first report of GABA MR spectroscopy in fibromyalgia patients showing lower levels of GABA in the anterior insula in this patient population. Glx or GABA levels in the context of DN with or without associated pain have not been described in the literature. Our study provides the first report of Glx and GABA quantification in the brain of DN patients with positive sensory symptoms including pain at rest and reveals elevated Glx and reduced GABA levels in the insula, similar to previously reported findings in other chronic pain states.
In summary, our study is innovative in using both established and novel MR spectroscopy techniques to quantify excitatory and inhibitory neurotransmission in DN with positive sensory symptoms. Although the glutamatergic and GABAergic neurotransmitter systems are thought to play a role in DN, there are no reports of in vivo quantification of these metabolites in the human brain in the context of this disease entity. Our findings provide a contribution to the emerging literature pointing to a significant role of the CNS in the pathogenesis of DN. Better understanding of the pathogenesis of DN can result in much needed improved treatment strategies for this prevalent and debilitating disease entity. Our data may provide the first step towards longitudinal interventional studies that can assess the role of central neuro-transmitter phenotype in treatment response with established and novel agents. For example, pregabalin, one of the two Food and Drug Administration–approved medications for the treatment of painful diabetic neuropathy is thought to inhibit release of glutamate at the synapse. Assessing the relationship between baseline glutamatergic transmission and response to Pregabalin treatment could potentially allow for a priori selection of patients that would respond to the specific treatment.
Diabetic neuropathy (DN) is one of the most common complications of diabetes mellitus, affecting up to 50% of diabetic patients. Sensory symptoms of neuropathy may be classified as negative (decreased ability to feel tactile stimuli, temperature changes, or other noxious stimuli) or positive (spontaneous sensory experiences that can be unpleasant or frankly painful). Painful DN affects 18%–27% of all diabetic patients (1–3). The condition is difficult to treat, with any single medication leading to pain relief in at most 30%–50% of patients (4–6). DN has a deleterious impact in many areas of a patient’s life including mood, sleep, ability to work and personal relationships (1). There are substantial direct and indirect health care costs associated with painful DN. One study showed that workers with painful DN lost 1.4 hours of work per week than patients with diabetes only, the equivalent of $3.65 billion per year in health-related lost productive time (2). Hospital admission and direct health care costs in painful DN patients are two to three times higher than diabetic patients without painful neuropathy (3).
Although DN is traditionally thought of as a disease of the peripheral nervous system, there is emerging evidence for central nervous system (CNS) modulation in diabetic neuropathy, especially when associated with pain. Advanced neuro-imaging techniques including magnetic resonance spectroscopy (MRS) have demonstrated alterations in CNS physiology and metabolism in the context of DN. Lower thalamic N-acetyl-aspartate (NAA) levels have been reported in patients with DN compared to diabetic patients without neuropathy (4). Sorensen et al showed decreased levels of thalamic NAA in patients with chronic neuropathic pain as opposed to diabetic subjects without pain (5). Lower cervical cord volumes have been reported in DN patients as opposed to diabetic subjects without neuropathy and healthy controls (6). Alterations in resting state attention networks have also been reported in the context of painful DN (7).
Glutamate (Glu), the main excitatory neurotransmitter, and γ-aminobutyric acid (GABA), the main inhibitory transmitter within the CNS are thought to play key roles in central pain processing. Elevated brain Glx (combined measure of Glu and glutamine) levels have been reported in other chronic pain conditions (8,9). In vivo measurements of GABA using MRS had not, until recently, been reported in the literature in the context of chronic pain due in part to technical hurdles. Conventional MRS techniques are not able to adequately resolve signals from the GABA molecule from those of other more concentrated metabolites. Our group successfully implemented a novel MRS spectral editing technique that, when combined with a conventional point resolved spectroscopy sequence (PRESS), allows reliable isolation and measurement of GABA concentration using a 3 T magnetic resonance imaging scanner (10). Using this MEGA-PRESS technique, we have recently demonstrated lower levels of GABA in the insula of fibromyalgia (FM) patients when compared to age-matched healthy controls (HC) (11). No human MRS studies assessing glutamatergic/GABAergic transmission in DN with or without associated pain are currently reported in the literature.
The aim of this study was to assess for baseline differences in Glx and GABA within pain processing regions of the brain in DN patients with positive sensory symptoms and HC subjects. We hypothesize that DN subjects with positive sensory symptoms will demonstrate elevated Glx and reduced GABA levels within the brain pain processing network, mirroring brain metabolic changes seen in other chronic pain states.
RESEARCH DESIGN AND METHODS
Seven adult diabetic patients (five males, two females, mean age = 57.0 ± 8.5 years) with an established diagnosis of peripheral DN (12) and positive neuropathic sensory symptoms (13) were studied. We also studied seven age- and sex-matched, pain-free control HC subjects (five males, two females, mean age = 57.7 ± 3.23 years). All participants were right handed and had no risk factors for other nondiabetic neuropathies.
MRS
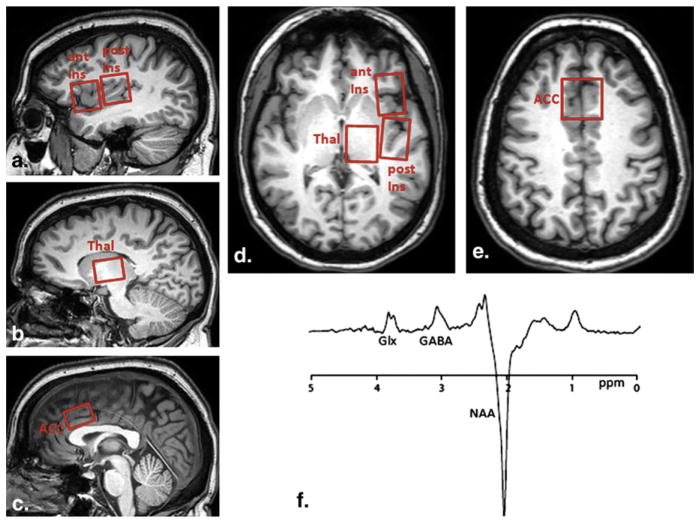
All subjects were imaged on a Philips Achieva 3T system (Best, Netherlands) using an eight-channel receive head coil. For each subject we performed a T1 weighted 3D-MPRAGE sequence with (0.9 mm3) isotropic voxel resolution for MRS voxel placement and subsequent tissue segmentation. MR spectra were acquired using 3.0 cm × 2.0 cm × 3.0 cm voxel in the right anterior insula (AI), right posterior insula (PI), anterior cingulate cortex (ACC), and thalamus (Fig 1). These brain regions of interest were a priori selected due to their known involvement in pain processing.
Figure 1.
Voxel placement and resulting spectrum. Sagittal (a–c) and axial (d,e) T1 images showing voxel placements for the right anterior insula (ant ins), right posterior insula (post ins), and anterior cingulate (ACC). (f) Representative magnetic resonance spectrum from the posterior insula using MEGA-PRESS spectral editing technique. Combined measure of glutamate and glutamine (Glx) is resolved at 3.8 ppm, γ-aminobutyric acid (GABA) at 3.0 ppm with an inverted N-acetylaspartate (NAA) peak at 2.0 ppm. DN, diabetic neuropathy; HC, healthy control; thal, thalamus.
Single-voxel PRESS spectra (repetition time/echo time = 2000/35 ms) were acquired from each region of interest using “vapor” water suppression with 32 averages and a total scan time of approximately 1 minute for each voxel. A MEGA-PRESS experiment optimized for GABA was performed with the following parameters: echo time = 68 ms (TE1 = 15 ms, TE2 = 53 ms); repetition time = 1.8 seconds; 256 transients of 2k datapoints; spectral width = 2 kHz; frequency selective editing pulses (14 ms) applied at 1.9 ppm (ON) and 7.46 ppm (OFF). Slice-selective refocusing was performed using amplitude-modulated pulse “GTST1203” (length = 7 ms, bandwidth = 1.2 kHz).
Postprocessing of MRS Data
Conventional PRESS spectroscopy was analyzed using LCModel. NAA values and Glx values from individual voxels were derived from the PRESS spectra. MEGA-PRESS spectroscopy was analyzed using in-house postprocessing software in Matlab with Gaussian curve fitting to the GABA and inverted NAA peaks. Concentration of GABA (in arbitrary institutional units) was then calculated by multiplying the ratio of GABA:NAA by the absolute NAA concentration determined from LCModel analysis of PRESS data. Metabolite concentrations were only used for statistical analysis from LCModel if the Cramér-Rao bounds were less than 20%.
Cerebral spinal fluid correction was performed for each voxel after tissue segmentation of MPRAGE images using statistical parameter mapping software (SPM, Wellcome Trust Centre for Neuroimaging, London, UK).
Statistical Analysis
Two-tailed independent sample t-tests were used to assess for differences in GABA, Glx, and GABA/Glx ratios between groups.
All participants provided written informed consent and all study protocols were approved by our institutional review board.
RESULTS
For the DN group, mean diabetes mellitus duration was 24.8 × 9.2 years (range, 1.5–48.0) and mean hemoglobin A1c was 8.6 ± 2.3% at the time of imaging. All DN subjects in the study cohort carried a diagnosis of severe peripheral DN based on established criteria including positive symptoms, signs, and abnormal electrophysiology (12). Regarding positive neuropathic sensory symptoms, four of seven DN patients had pain at rest, two of seven reported persistent tingling sensation in the feet, and one of seven had allodynia in the feet. None of the DN subjects took any pharmacologic agents for their neuropathy in the 5 days preceding the study.
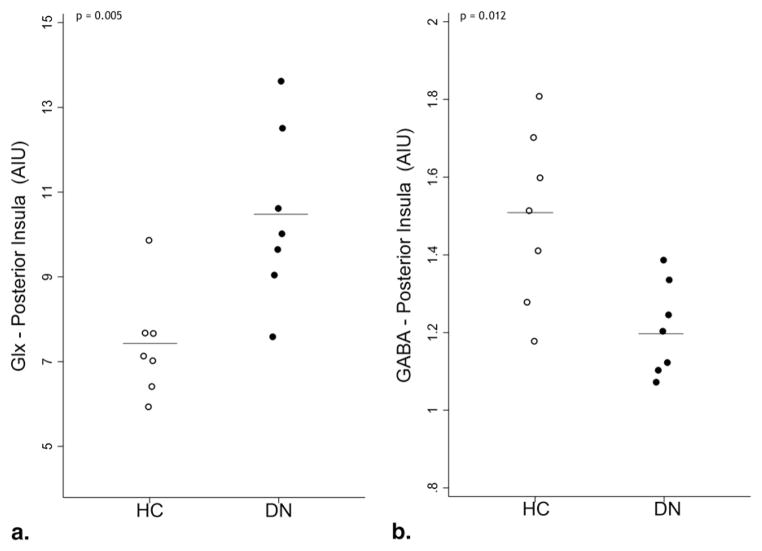
Significantly higher mean Glx levels were present in the posterior insula of DN subjects compared to HC. (Mean DN Glx =10.5 ± 1.9 arbitrary institutional units [AIU], mean HC Glx = 7.4 ± 1.2 AIU, P = .005, Fig 2a). Within the same region, mean GABA levels were significantly lower in DN subjects compared to HC (mean DN GABA =1.2 ± 0.1 AIU, mean HC GABA = 1.5 ± 0.1 AIU, P = .012, Fig 2b). Mean Glx or GABA levels were not significantly different between patients and HC in any of the other brain regions examined (Table 1).
Figure 2.
Glutamate/glutamine (Glx) (a) and γ-aminobutyric acid (GABA) (b) levels in the posterior insula of patients with diabetic neuropathy and positive sensory symptoms (DN) and healthy controls (HC). Horizontal bars indicate group mean metabolite levels.
TABLE 1.
NAA, Glx, and GABA Levels as well as Glx/GABA Ratios in Brain Regions within the Pain Processing Network for DN Subjects with Positive Sensory Symptoms and Age- and Gender-matched HC
| DN (n = 7) | HC (n = 7) | P Value | |
|---|---|---|---|
| Right anterior insula | |||
| Mean NAA (SD) | 6.69 (0.65) | 7.15 (0.30) | .14 |
| Mean Glx (SD) | 9.39 (1.26) | 8.75 (1.77) | .49 |
| Mean GABA (SD) | 1.20 (0.21) | 1.26 (31) | .69 |
| Mean Glx/GABA (SD) | 8.21 (2.35) | 7.58 (2.80) | .68 |
| Right posterior insula | |||
| Mean NAA (SD) | 6.87 (0.57) | 6.99 (0.36) | .69 |
| Mean Glx (SD) | 10.45 (1.90) | 7.40 (1.17) | .005 |
| Mean GABA (SD) | 1.21 (0.11) | 1.50 (0.09) | .012 |
| Mean Glx/GABA (SD) | 8.64 (1.55) | 5.12 (1.83) | .002 |
| Right thalamus | |||
| Mean NAA (SD) | 7.40 (1.32) | 7.62 (0.59) | .74 |
| Mean Glx (SD) | 8.92 (2.70) | 7.56 (2.26) | .39 |
| Mean GABA (SD) | 1.38 (0.54) | 1.84 (0.54) | .19 |
| Mean Glx/GABA (SD) | 7.64 (3.48) | 4.20 (1.14) | .06 |
| Anterior cingulate cortex | |||
| Mean NAA (SD) | 7.49 (1.1) | 7.59 (0.44) | .84 |
| Mean Glx (SD) | 7.62 (2.57) | 8.26 (4.0) | .74 |
| Mean GABA (SD) | 1.33 (0.34) | 1.26 (0.30) | .70 |
| Mean Glx/GABA (SD) | 6.12 (2.49) | 7.19 (3.62) | .56 |
GABA, γ-aminobutyric acid; Glx, glutamine; NAA, N-acetylaspartate; SD, standard deviation.
Parameters showing significant differences between groups are marked in bold.
Glx/GABA ratios were also significantly elevated in the posterior insula in DN subjects compared to HC (8.6 ± 1.6 versus 5.1 ± 1.8, P = .0017). Glx/GABA ratios in the thalamus were also higher in subjects with DN and positive sensory symptoms (7.6 ± 3.5 versus 4.2 ± 1.1) with a trend toward statistical significance (P = .06).
NAA values were not significantly different between groups for any of the interrogated brain regions.
DISCUSSION
Our study demonstrates an imbalance of excitatory and inhibitory neurotransmitters in pain processing brain regions in the context of DN with positive sensory symptoms including pain at rest. This is the first report of in vivo measurements of Glx and GABA in patients with confirmed DN.
Elevated levels of Glx in the posterior insula are consistent with similar observations in other chronic pain states. The insula is recognized as a key part of the pain processing brain network with the posterior insula in particular being involved in the sensory perception and processing of pain. Our group previously showed elevated levels of Glx in the posterior insula in FM patients as compared to pain-free controls as well as significant correlations of Glx levels with evoked pain sensitivity (8). We also previously showed that FM patients displayed highly significant correlations between changes in posterior insula Glx levels and changes in multiple pain domains (14). Patients with greater reductions in Glx displayed greater improvements in both clinical and experimental pain. This “phenotype” of elevated Glx within the FM brain has been subsequently reported in other brain regions including the amygdala (15). Pregabalin, a medication used in the treatment of chronic pain and one of the two Food and Drug Administration–approved pharmacologic agents for the treatment of painful diabetic neuropathy is thought to decrease release of glutamate into the synapse. Our findings of elevated insular Glx levels may in part explain the mode of action of Pregabalin in attenuating the symptoms of painful DN.
Reduced GABA levels in the insula in the DN group are consistent with our group’s findings of lower insular GABA in FM patients (11). A reduced level of GABA may reflect lack of inhibitory response that can lead to heightened neuronal activity and result in the amplified nociceptive response characteristic of FM patients as well as the “positive” sensory symptoms including pain at rest, tingling, and allodynia present in our cohort of DN patients. This reduced inhibitory response is also consistent with findings from animal models of DN that have shown spinal cord and thalamic neuronal hyperexcitability (16). A recent study demonstrated reductions in GABA-B receptor mRNA and protein in the spinal cord in a rat model of diabetic neuropathic pain, suggesting that attenuation of GABAergic neurotransmission may contribute to neuronal hyperexcitability and the pathogenesis of DN pain (17).
Chronic pain, including neuropathic pain pathophysiology is currently incompletely understood, which partially accounts for the fact that currently available treatments are at best of moderate efficacy. In vivo studies of central nervous system metabolism and its relationship to clinical symptoms are particularly important in furthering our understanding of the mechanisms underlying chronic pain. In addition, chronic pain is a very heterogeneous entity with variable response to pharmacologic and nonpharmacologic interventions. Understanding the mechanisms underlying a particular individual’s chronic pain symptoms would be a key step in developing personalized and therefore more effective treatment strategies.
Alterations in thalamic NAA levels have been previously described in the context of DN (4,5). Specifically, reductions in thalamic NAA were described in patients with DN compared to diabetic patients without neuropathy and healthy controls (4). Furthermore, reductions in NAA were also noted in the thalamus in subjects with diabetes and chronic neuropathic pain compared to diabetic subjects without pain and healthy controls (5). We did not find any differences in NAA in any of the regions interrogated (including the right thalamus) between DN subjects and HCs. It is possible that the difference in findings relates to size of voxel used (we used 18 cm3 voxels to be able to adequately quantify GABA as opposed to 8 cm3 voxels used previously). We also had smaller numbers of subjects in our study compared to these prior reports. It is also possible that prior studies “overestimated” NAA reduction as they did not correct for cerebrospinal fluid/volume loss; correcting for cerebrospinal fluid did result in substantial attenuation of the NAA differences between DN subjects and HCs in our dataset. Nevertheless, the trend observed in our cohort toward higher Glx/GABA values in the thalamus of DN patients may provide further evidence for a significant role of the thalamus in altered processing of painful stimuli in the context of DN with resultant allodynia/hyperalgesia.
It is important to note that our described alterations in Glx and GABA levels may reflect concentration differences in the presynaptic terminals, synaptic vesicles, or neurotransmitter uptake mechanisms. Although our MRS method cannot discern these possibilities, at a mechanistic level, we believe that the MRS findings of elevated Glx and/or decreased GABA are related to neurotransmission with localization in the presynaptic neurons as well as the synaptic vesicles. MRS Glx (glutamate and glutamine) measurements are thought to be highly correlated with glutamatergic transmission as most glutamate is synthesized from glucose within the CNS (18), and MRS ignores the larger macromolecules that contain glutamate/glutamine only resolving the small and mobile nonassociated glutamate/glutamate independent molecules (19). MRS have also established that there is a close coupling of overall neuronal activity and glutamine-glutamate-GABA fluxes suggesting that MRS measures of Glx and GABA do indeed reflect glutamatergic excitatory and GABAergic inhibitory neurotransmitter activity during brain activation (20).
Given the cross-sectional design of this study, we cannot determine if the higher Glx/lower GABA levels are a cause of positive sensory symptoms or if they represent sequelae of disease pathology. Furthermore, our study cohort is small and the positive sensory symptoms in the patient group are heterogeneous with varying degrees of pain at rest, tingling, and allodynia. Nevertheless, even the small set of subjects included in the study provide supporting evidence for our a priori hypothesis of DN subjects with pain/positive sensory symptoms showing alterations in Glx and GABA levels within the brain processing network that resemble other chronic pain states. As such, our findings provide further substantial evidence for central modulation of DN and neuropathy associated with positive sensory symptoms in particular. A prospective cohort study in which diabetic patients are followed longitudinally would be an ideal way to define evolution of neuroimaging findings, symptoms, and clinical diagnosis of neuropathy and assess causality. This would however be a very costly and not practically feasible study design. In an attempt to partially reconstruct the temporal sequence of events in CNS metabolism during the development of DN with positive sensory symptoms, we are in the process of using a similar experimental paradigm and including subjects with a diagnosis of diabetes without neuropathy, a group with a diagnosis of neuropathy and negative sensory symptoms and a group with a diagnosis of DN and positive sensory symptoms/pain at rest. Examining the effect of pharmacologic intervention on central metabolism and clinical symptoms and the relationship of the changes in these parameters can also be used in a proof of concept, mechanistic study that can then be used for a therapeutic trial incorporating neuroimaging parameters as response predictors.
It is important to note that edited measurements of GABA do not excite pure GABA signal and suffer from co-editing of macromolecular signal. Although it is possible that the effects we observe are driven by changes in macromolecular component, the study is based on a strong association between central pain processing and GABAergic function. Given these findings, we therefore interpret our observations as representing true alterations in GABAergic neurotransmission. An additional limitation of the study is the relatively large prescribed voxel size, which is required to achieve adequate signal to noise for quantification of GABA using the MEGA-PRESS technique at 3T field strength. Blood glucose levels were not assessed immediately before or during the imaging session, which could also potentially influence brain metabolite levels.
CONCLUSION
Elevated Glx and reduced GABA levels are present within the posterior insula of DN patients with positive sensory symptoms compared to pain free, age- and gender-matched HCs. Furthermore, higher Glx/GABA ratios are present within the right thalamus in the patient compared to the control group. Overall, findings are suggestive of altered excitation/inhibition within the pain processing network in DN patients with positive sensory symptoms, a finding also reported in other chronic pain states. Larger studies are required to further define the role of Glx and GABA in patients with DN and positive and negative sensory symptoms as well as diabetic patients without evidence of neuropathy. Given the known action of Pregabalin on glutamatergic neurotransmission, this work also provides foundation for longitudinal interventional studies examining alterations in glutamatergic neuro-transmission within the CNS in response to treatment.
Acknowledgments
Supported by the Program in Neurology Research and Discovery at the University of Michigan, the Juvenile Diabetes Research Foundation (JDRF) and the Radiological Society of North America (RSNA).
Footnotes
No conflicts of interest relevant to this article were reported.
M.P. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. R.P.B. and B.R.F. researched data, contributed to discussion and reviewed and edited the manuscript. R.A.E.E., B.C.C., S.E.H., R.E.H., D.J.C., and E.L.F. contributed to the discussion and reviewed and edited the manuscript.
References
- 1.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–128. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Ricci JA, Chee E, et al. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. J Occup Environ Med. 2007;49:672–679. doi: 10.1097/JOM.0b013e318065b83a. [DOI] [PubMed] [Google Scholar]
- 3.Ritzwoller DP, Ellis JL, Korner EJ, et al. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin. 2009;25:1319–1328. doi: 10.1185/03007990902864749. [DOI] [PubMed] [Google Scholar]
- 4.Selvarajah D, Wilkinson ID, Emery CJ, et al. Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia. 2008;51:2088–2092. doi: 10.1007/s00125-008-1139-0. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen L, Siddall PJ, Trenell MI, et al. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–981. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- 6.Selvarajah D, Wilkinson ID, Emery CJ, et al. Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care. 2006;29:2664–2669. doi: 10.2337/dc06-0650. [DOI] [PubMed] [Google Scholar]
- 7.Cauda F, Sacco K, Duca S, et al. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescot A, Becerra L, Pendse G, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Molel Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Foerster BR, Petrou M, Edden RA, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apfel SC, Asbury AK, Bril V, et al. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. J Neurol Sci. 2001;189(1–2):3–5. doi: 10.1016/s0022-510x(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 14.Harris RE, Sundgren PC, Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 15.Valdes M, Collado A, Bargallo N, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 2010;62:1829–1836. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- 16.Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009;1268:154–161. doi: 10.1016/j.brainres.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 17.Wang XL, Zhang Q, Zhang YZ, et al. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett. 2011;490:112–115. doi: 10.1016/j.neulet.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Hertz L, Dringen R, Schousboe A, et al. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- 19.Sachdev PS, McBride R, Loo C, et al. Effects of different frequencies of transcranial magnetic stimulation (TMS) on the forced swim test model of depression in rats. Biol Psychiatry. 2002;51:474–479. doi: 10.1016/s0006-3223(01)01298-7. [DOI] [PubMed] [Google Scholar]
- 20.Rothman DL, Behar KL, Hyder F, et al. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]