Abstract
To investigate the role of donor-specific indirect pathway T cells in renal transplant tolerance, we analyzed responses in peripheral blood of 45 patients using the trans-vivo delayed-type hypersensitivity assay. Subjects were enrolled into five groups—identical twin, clinically tolerant (TOL), steroid monotherapy (MONO), standard immunosuppression (SI) and chronic rejection (CR)—based on transplant type, posttransplant immunosuppression and graft function. The indirect pathway was active in all groups except twins but distinct intergroup differences were evident, corresponding to clinical status. The antidonor indirect pathway T effector response increased across patient groups (TOL < MONO < SI < CR; p < 0.0001) whereas antidonor indirect pathway T regulatory response decreased (TOL > MONO = SI > CR; p < 0.005). This pattern differed from that seen in circulating naïve B-cell numbers and in a cross-platform biomarker analysis, where patients on monotherapy were not ranked closest to TOL patients, but rather were indistinguishable from chronically rejecting patients. Cross-sectional analysis of the indirect pathway revealed a spectrum in T-regulatory:T-effector balance, ranging from TOL patients having predominantly regulatory responses to CR patients having predominantly effector responses. Therefore, the indirect pathway measurements reflect a distinct aspect of tolerance from the recently reported elevation of circulating naïve B cells, which was apparent only in recipients off immunosuppression.
Keywords: DTH assay, human, immunological monitoring, indirect pathway, renal transplantation, transplant tolerance
Introduction
We have previously reported TGFβ- or IL10-producing regulatory indirect pathway T-cell responses in patients with long-term excellent graft function after kidney or liver transplantation and withdrawal of all immunosuppression (1). However, the Th3/TGFβ-dependent form of tolerance to a kidney transplant in monkeys and humans was found to be “metastable” (2–4) and eventually some grafts underwent acute cellular, antibody-mediated or chronic rejection (CR). It is now well established that Th17 responses are induced when cells are exposed to TGFβ in the presence of IL6, so metastable tolerance to allografts based on Th3/TGFβ cells may be prone to develop into Th17-driven CR, a phenomenon most clearly demonstrated in clinical lung transplants (5). A more stable form of tolerance may therefore involve both Th3/TGFβ- and Tr1/IL10-T regulatory (Treg)-cell responses (6).
How tolerance at the T- and B-cell levels are interconnected is not yet clear. Much interest in the possible role of B regulatory cells (Bregs; Ref. 7) has been generated by the recent discovery that renal transplant tolerance in rodents (8,9) and humans (10–12) is characterized by an increase in both intragraft and systemic B cells. Whether this increase is a driving force for tolerance induction or whether it simply helps to stabilize tolerance in the absence of immunosuppression has not been established.
We hypothesized that the regulatory indirect pathway T-cell response to donor alloantigens in kidney transplant recipients is a progressive indicator of tolerance, that is, one which becomes more pronounced in a patient population that receives less immunosuppression while maintaining excellent graft function. We studied patients in categories ranging from most tolerant (identical twin “isograft”) to least tolerant (chronic allograft rejection) and including three “in between” groups: patients with well-functioning renal allografts who were taking standard, minimal or zero (functionally tolerant) immunosuppressive medications. We compared the results of indirect pathway monitoring with the analysis of circulating B cells and B–cell-biased “probability of being tolerant” (POT) scores (10,11).
Materials and Methods
Source of cells and reagents
Renal transplant recipients and living donors from three transplant centers (Emory University, Atlanta, GA, USA; University of Wisconsin–Madison, WI, USA and Swedish Medical Center, Seattle, WA, USA) were enrolled in the ITN507ST observational trial, “Identification and Mechanistic Investigations of Tolerant Kidney Transplant Patients” (Clinicaltrials.gov identifier NCT01338779) using institutional review board-approved informed written consent procedures at each institution. Patients were assigned to groups based on clinical status as previously described (10). Patients enrolled in four of the groups—identical twin (TWIN—transplant from an identical twin donor), clinically tolerant (TOL—no immunosuppression medication for >1 year), steroid monotherapy (MONO, ≤10 mg/day of prednisone) and standard immunosuppression (SI—including any combination of standard post-transplant immunosuppression) all had stable and normal renal function (within 25% of baseline) at the time of enrollment. All patients in the CR group had moderately impaired or gradually deteriorating renal function (GFR < 40 mL/min or creatinine >50% above baseline value), had a previous biopsy showing chronic allograft nephropathy (BANFF 1997 criteria) and continued to receive immunosuppressive medications, but were not on dialysis. Peripheral blood samples were collected in citrate and processed using Ficoll-Hypaque to isolate peripheral blood mononuclear cells (PBMC). PBMC were either used immediately or cryopreserved before analysis.
Antigens used to test for indirect pathway responses included: donor PBMC or spleen cell sonicates, as described elsewhere (1); HLA-A1, HLA-B62 and HLA-B57 single antigen Luminex beads (One Lambda, Inc., Canoga Park, CA, USA), a kind gift of Dr. Junchao Cai, Paul Terasaki Foundation, Los Angeles, CA, USA and HLA-B allopeptides p37MA (DSDAASPRMAPRAPWIEQ) and p37TE (DSDAASPRTEPRAPWIEQ), described elsewhere (4).
Antibodies co-injected with PBMC: rabbit anti-human TGF-β, goat anti-human IL-10 or control normal rabbit IgG or goat IgG (all R&D Systems, Minneapolis, MN, USA, all at 25 μg/injection), anti-human IFNγ (eBioscience, San Diego, CA, USA, 5 μg/injection) or anti-human IL17 (R&D Systems, 5 μg/injection).
Indirect pathway analysis
Human PBMC (7–9 × 106 cells) were mixed with either donor or recall (tetanus/diphtheria toxoid [TT/DT] or Eptstein-Barr virus [EBV]) antigen or with a combination of donor plus recall antigen and injected into CB.17 SCID mouse footpads (1,13). Footpad thickness was measured using a spring-loaded caliper at time 0 and 24 h. Net swelling is determined by subtracting the background swelling from an injection of the same cells in phosphate-buffered saline (PBS). This trans-vivo delayed-type hypersensitivity (tvDTH) reaction is associated with edema, human cell retention and mouse neutrophil recruitment in the mouse footpad that is initiated by human T cells in response to processed antigens (14). Inhibition of recall responses in the presence of donor antigens was determined by comparing the net footpad swelling with each injection using the formula
and is a measure of indirect pathway donor antigen-specific regulatory activity (1).
B-cell depletion
B-cell depletion was performed using anti-CD19 immuno-magnetic beads (Miltenyi Biotec, Auburn, CA, USA) and the autoMACS system. Subsequent flow cytometry for CD19+ cells verified removal of 99% of the B cells from the PBMC. In the “add-back” experiments, B cells were combined with the B-cell-depleted sample at the same ratio of B to non-B cells as was determined by flow cytometry of the whole PBMC.
Statistical analysis
Kruskal–Wallis test, a nonparametric equivalent to the one-way analysis of variance, was used to compare results across all groups; the Dunn’s correction was used for pair-wise comparisons. For some analyses, an unadjusted Mann–Whitney U test was used for individual pair-wise comparisons. Paired t-tests were used to compare within subject responses. The p-value less than 0.05 was considered statistically significant. Statistical analysis was performed with Prism GraphPad software.
Results
The spectrum of responses to soluble donor alloantigens correlates with graft status
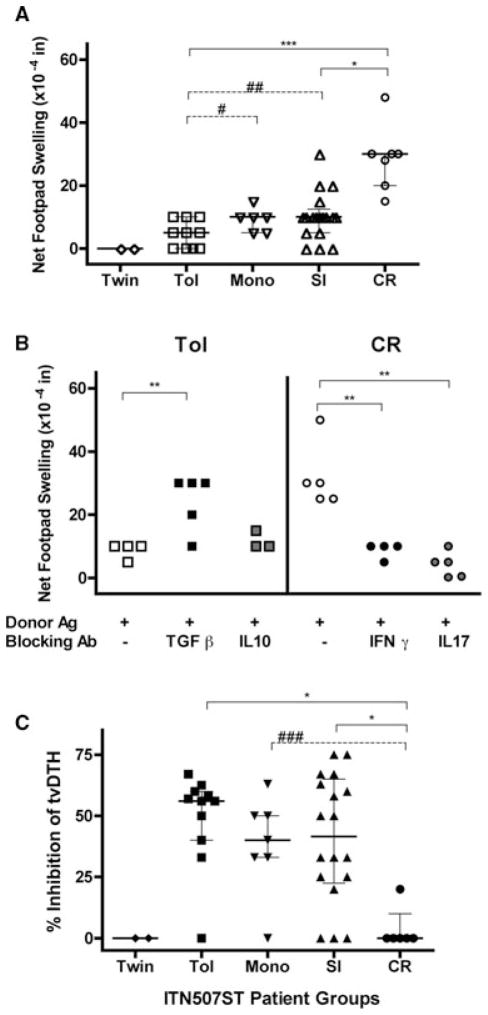
PBMC from half of the subjects (n = 45, selected because of cell availability) enrolled in the ITN507ST trial were tested for donor-specific indirect T effector (Teff) and Treg responses using the tvDTH assay and donor (or donor HLA-matched surrogate) cell-free lysates. As shown in Figure 1A, indirect Teff responses to donor antigens reveals a distinct spectrum across the enrollment groups (p < 0.001); footpad swelling increased as the patients’ clinical status moves from those that are tolerant to those that are chronically rejecting. Identical twins did not respond to donor antigen whereas patients in the TOL group had a median net swelling response of 5 × 10−4 inches (mean of 4.5 ± 4.2). The MONO group had a higher median response of 10 × 10−4 inches (mean of 9.3 ± 3.4), as did the SI group (mean of 10.3 ± 7.6). Finally, responses of patients in the CR group were 30 × 10−4 inches (mean of 28.7 ± 10.3), which was significantly higher than all the others. Analysis using a single pair-wise comparison of TOL to either MONO (p = 0.04) or SI (p = 0.03) groups revealed significantly lower Teff response in TOL patients.
Figure 1. Indirect pathway T-cell analysis.
(A) The tvDTH responses to donor antigen (Teff responses) are significantly different between subject groups (p < 0.0001). PBMC from patients (n = 45) were co-injected into the mouse footpad with cell-free donor antigen. Swelling was measured before and 24 h after each injection. Net swelling was determined by subtracting the swelling response obtained from the injection of the same PBMC in PBS. Patient groups: TWIN, identical twin donor (n = 2); TOL, clinically tolerant, no immunosuppression for >1 year (n = 11); MONO, prednisone monotherapy (n = 7); SI, standard immunosuppression (n = 18) and CR, chronic rejection (n = 7). (B) Antibody blocking of indirect pathway T-cell responses. PBMC from patients with CR (n = 5) were injected into the footpad with cell-free donor antigen and blocking antibodies to IFNγ or IL-17 (right side), whereas PBMC from patients in the TOL group (n = 5) were injected with cell-free donor antigen and antibodies to block TGFβ or IL-10 (left side). The net swelling response to donor antigen was significantly elevated in the TOL patients when TGFβ was neutralized (p < 0.01) but not when IL10 was neutralized whereas blocking of either IFNγ or IL17 significantly decreased the swelling response to donor antigen in the CR patients tested (p < 0.01). Net swelling response for each subject with donor antigen and a given test antibody were compared to that with PBMC + donor antigen and isotype control Ab (−) using a paired t-test. (C) Indirect pathway Treg responses are significantly different between subject groups (p = 0.005). PBMC from patients in the same groups as described in (A) were injected with recall antigen (TT/DT or EBV) alone and with recall plus cell-free donor antigen into the mouse footpad. Net swelling was determined as in (A). The percentage inhibition of recall alone response in the presence of donor antigen was calculated as described in the methods. All panels: Individual results from each subject (individual symbols) and group median (horizontal line) with quartiles are shown. Each patient was tested one or two times. Significant differences between groups using pair-wise comparisons (Dunn’s correction for multiple comparisons shown in solid lines); *p < 0.05, **p < 0.01 and ***p < 0.001; (Mann–Whitney U test unadjusted for multiple comparisons shown with dotted lines) #p = 0.04, ##p = 0.03 and ###p = 0.008.
Antibodies to IFNγ and IL17 significantly blocked the indirect pathway Teff-cell responses to donor antigens in the CR patients tested (p < 0.01, compared to IgG control, Figure 1B, right panel). In contrast, an indirect pathway Teff response was revealed (net footpad swelling of 20–30 × 104 inches) in four of five TOL patients when TGFβ-mediated suppression was blocked by co-injection of anti-TGFβ antibody with PBMC and donor antigen (Figure 1B, left panel). In contrast, IL10 did not appear to contribute to the antidonor Treg-cell response as neutralizing IL10 did not affect the footpad swelling to donor antigen. Neither anti-TGFβ nor anti-IL10 increased the footpad swelling when co-injected with PBMC from CR patients and donor antigen (data not shown).
An antidonor indirect pathway Treg response can also be measured by the linked-suppression assay. When donor antigen and a recall antigen are co-injected with the PBMC into the mouse footpad, inhibition of the recall response in the presence of the donor antigen indicates the action of allospecific CD4 or CD8 Treg cells (4,6,15), but unlike the uncovering of an antidonor Teff response shown in Figure 1B, it does not require the presence of a donor specific Teff cell. Figure 1C shows that the pattern of indirect pathway T-cell regulation to donor alloantigen mirrors the pattern of antidonor Teff response across the patient groups; higher percentage inhibition of recall responses defines increased regulation. As expected, neither TWIN had evidence of linked-suppression to their donor as there were no alloantigens to stimulate Treg cells. In contrast, the TOL group, with one exception, all inhibited at least 30% of the recall antigen response in the presence of donor antigen with a median inhibition of 60% (mean of 49 ± 19). The MONO group had a lower median inhibition of 40% but displayed a similar clustering with a single outlier who did not regulate (mean of 38 ± 20). The SI group had the same median inhibition as the MONO group; however, similar to the Teff responses (Figure 1C, panel A), the range of indirect pathway regulation in the SI patients was large (mean of 41 ± 26). The CR group was significantly lower in its regulation scores (mean of 3.3 ± 8) compared to TOL and MONO groups. Overall, the data in Figure 1 are consistent with a spectrum of increasing cell-mediated immune response to donor soluble antigens and a decrease in regulatory response over the range of most (TOL) through intermediate (MONO and SI) to least tolerant (CR) patients.
Analysis of naive B-cell counts and POT index
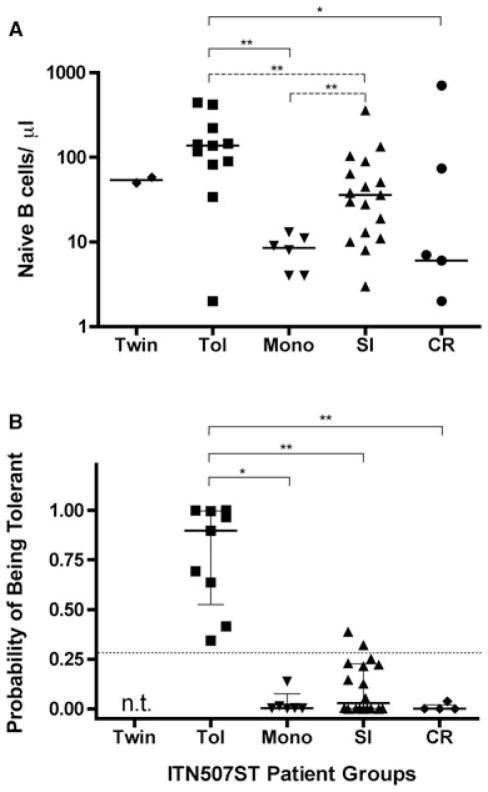
We verified that the 45 subjects in this study were representative of the entire 91 subjects in the ITN507ST, specifically in regards to the characteristics we and others have previously found to describe tolerant renal transplant recipients (10–12). As shown in Figure 2A, circulating naïve B-cell numbers were significantly different among patient groups (p = 0.005) with patients in the TOL group having significantly more compared to those in the SI group when single pair-wise Mann–Whitney U test was performed. In this way, our TOL and SI subgroups are representative of those same groups in the complete ITN507ST (10). The TOL patients also had significantly higher median naïve B-cell counts than those in the MONO and CR groups. Interestingly, the MONO group median most closely resembled the CR group median and was significantly lower (not higher) than the SI median using the unadjusted Mann–Whitney U test.
Figure 2. Naive B-cell counts and cross-platform probabilitÿ of being tolerant scores.
(A) Naïve B-cell number (CD19+CD27-IgM+IgD+) in fresh peripheral blood of the 45 patients analyzed in Figure 1. (B) Probability of being tolerant, estimated according to the Indices of Tolerance model of 14 biomarkers, including B-cell parameters and direct pathway T-cell parameters (11). Subjects in the TWIN group were not tested (n.t.). All panels: Individual results from each subject (individual symbols) and group median (horizontal line) with quartiles (panel B only) are shown. Significant differences between groups using pair-wise comparisons, *p < 0.05 and **p < 0.01; Dunn’s correction for multiple comparisons shown in solid lines, unadjusted Mann–Whitney U test comparisons shown with dotted lines.
Three TOL patients scored at the low end of the range of donor-specific indirect pathway Treg-driven linked-suppression (0%, 33% and 40% inhibition, Figure 1C). Circulating naïve B cells (138, 2 and 34 cells/μL, respectively, Figure 2A) were also low for two of these three individuals. Importantly, there was no correlation between the naïve B-cell numbers and indirect pathway analysis for patients in any of the groups (data not shown).
The POT scores are based on 14 biomarkers identified from a multiparameter analysis of 11 tolerant renal transplant patients enrolled in the Indices of Tolerance trial in Europe and 60 subjects in comparison groups (11). Samples from patients in the ITN507ST study (n = 89; TOL group = 25) were used as a test set and a tolerance signature threshold of >0.27 was established (11). As shown in Figure 2B, POT scores were available for 9 of the 11 TOL patients analyzed in Figure 1. Although the scores ranged widely, they were all >0.27. The TOL group had significantly higher median POT scores than the MONO, SI and CR groups. Two patients in the SI group but, remarkably, no patients in the MONO group had a POT score above the threshold of 0.27. Thus the data in Figures 1 and 2 reflect distinct aspects of the immunologic status of renal transplant recipients. TOL and MONO patients exhibit very similar indirect pathway responses whereas the levels of circulating naïve B cells were very different.
The tvDTH assay measures indirect pathway T-cell regulation in a B-cell-independent manner
Because naïve B cells are elevated and linked-suppression is strongest in the TOL patients, we next sought to determine if B cells played any role in linked-suppression of cell-mediated immunity detected in the tvDTH assay. We depleted B cells from patients 002055 (SI group, 75% inhibition) and 002002 (TOL group, 67% inhibition) and then retested for linked-suppression using donor antigen (data not shown) or allopeptide (HA1-H or p37MA) in the presence of a recall antigen. As shown in Figure 3, neither patient’s inhibitory response to allopeptide was affected by depletion (open triangles) or add-back of B cells (gray circles). Notably, both of these patients had high numbers of circulating naïve B cells in their PBMC (360 cells/μL for 002055 and 142 cells/μL for 002002).
Figure 3. B cells do not impact tvDTH suppression via indirect pathway.
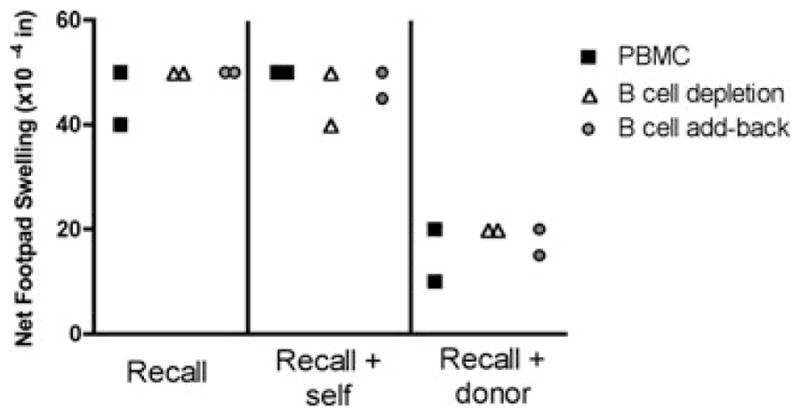
PBMC from two patients with high circulating naive B-cell counts, TOL patient 002002 and SI patient 002055 were assayed directly (PBMC, filled squares), after being depleted of CD19+ B cells (open triangles) or after depletion and add-back of B-cell fraction (gray circles). Cell fractions were injected into the footpad with PBS (negative control, data not shown) with recall antigen (TT/DT or EBV, left panel), recall antigen and self-peptide (p37TE or HA-1R, middle panel) or recall antigen and donor peptide (p37MA, or HA-1H, right panel). Swelling was measured before and 24 h after each injection. Net swelling was determined by subtracting the swelling response obtained from the injection of the same cell fractions in PBS.
Indirect pathway T-cell responses analyzed using HLA single-antigen beads
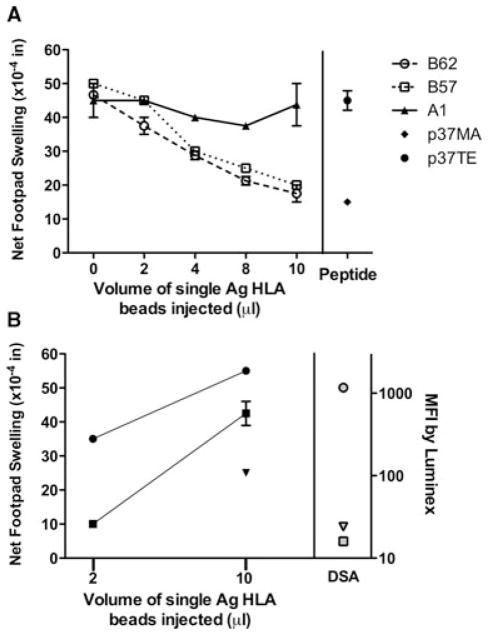
To exclude the possibility that the donor antigen lysates contained nonspecific factors responsible for either inhibition or stimulation of tvDTH responses, we selected informative patients and challenged their PBMC with single antigen HLA-coated beads or with HLA allopeptide antigens rather than whole cell lysates. PBMC from TOL patient 002002 was obtained 14 years after transplant from an HLA class II well-matched and HLA-A-matched deceased donor. This subject had not taken any immuno-suppression for 12 years. We previously reported that this patient had a DQ3-restricted CD4 T-cell response to a single immuno-dominant allopeptide, p37MA, found in the heavy chain of donor-type HLA-B62; this epitope is shared with HLA-B57 but not with other HLA-A or B proteins (4,16). As shown in Figure 4A, we observed a dose-dependent inhibition of tvDTH response to recall antigen challenge when PBMC were injected with B62 (open circles) or B57 beads (open squares), resulting in a maximum inhibition of 60–63%. In contrast, over the same dose range of beads coated with HLA-A1 (filled triangles), a shared antigen, we found little-to-no inhibition of the footpad swelling response to recall antigen. When self HLA-B37 peptide p37TE (filled circle) was compared with the allopeptide p37MA (filled diamond, co-injection of 1000 ng with recall antigen), we saw similar strong (68%) inhibition of the recall antigen response with the p37MA allopeptide but no effect was seen with the autologous p37TE peptide (Figure 4A, peptide). The equivalent dose-response to the B57- and the B62-bead indicates that the p37MA peptide provides the critical component for response, and not the intact antigen.
Figure 4. Donor-specific indirect pathway responses using HLA-single antigen beads.
(A) PBMC of tolerant patient 002002 were mixed with varying amounts (0–10 μL) of donor type HLA-B62 (open circle, p37MA+), third party HLA–B57 (open square, p37MA+) or self HLA-A1 (filled triangle, p37MA−)-coated single antigen beads before addition of TT/DT and injected into the mouse footpad. Net swelling was determined as in Figure 1A. The maximum percentage inhibition of response with recall TT/DT (0 beads) versus the response with 10 μL of single antigen beads was calculated as described in the methods and is 60% with B57 beads and 63% with B62 beads. Peptides: PBMC were co-injected into the mouse footpad with recall antigen TT/DT and 1000 ng of HLA-B62 allopeptide p37MA (DSDAASPRMAPRAPWIEQ) or HLA-B37 self peptide p37TE (DSDAASPRTEPRAPWIEQ). The percentage inhibition of recall antigen response with donor peptide is 68%. Patient 002002 = HLA A1, 2; B37, 60; DR 4, 13; donor type: A1, 2; B44, 62; DR 4, 13. (B) Indirect pathway tvDTH response to single antigen-beads detects stronger sensitization to a DSA+ as compared with DSA− donor HLA antigens. PBMC from CR patient 002048 were mixed with donor HLA-A2 (circles), HLA-A1 (squares) or HLA-B57 (inverted triangle)-coated single antigen beads and injected into a CB17 SCID mouse footpad. Net swelling was determined as in Figure 1A. Mean fluorescent intensity (MFI) values for a contemporaneous serum sample of the same subject tested using HLA-A2 (gray circle)-, HLA-A1 (gray square)- or HLA-B57 (gray inverted triangle)-coated single antigen beads are shown on the right. Patient 002048 = HLA-A11, 25; B18, 51;DR 9, 15 Donor = HLA-A1, 2; B7, 57; DR 11,15 (mismatch in bold).
To compare the indirect pathway Teff response with B-cell responses in the same individual to the identical donor HLA protein, subjects in the CR group were tested in tvDTH and Luminex (per clinical follow-up) using HLA single antigen beads. Data from one such patient (#002048) is shown in Figure 4B. Footpad swelling responses to donor HLA-A1 (squares), HLA-A2 (circles) and HLA-B57 (inverted triangle) were detected when HLA-coated single antigen beads were injected with patient PBMC. Donor specific antibody (DSA) analysis from the same patient (right panel) revealed a specific humoral response to HLA-A2 (gray circle, mean fluorescent intensity [MFI] = 1168) but not to HLA-A1 (gray square, MFI = 16, Luminex lower limit of detection) or HLA-B57 (gray inverted triangle, MFI = 24) mismatched donor antigen. The hierarchy of indirect pathway cellular reactivity, HLA-A2 ≫ HLA-A1 or HLA-B57, reflected in dose–response to single antigen beads parallels the DSA response in serum detected using the same substrate, while indicating a broader antigen sensitization at the cellular versus humoral immunity level.
Discussion
Given the critical importance of indirect pathway alloreactivity in tolerance (17,18) and in both cellular and humoral (19) immunity to allografts, many groups including our own (20) have sought to develop in vitro methods of monitoring human indirect pathway T cells as a guide to tolerance induction and successful immunosuppressive drug withdrawal and the early diagnosis of CR (21). However, weak lymphoproliferative and cytokine responses by low frequency indirect pathway T cells have hampered the clinical application of indirect pathway assays. Taking advantage of the unique characteristics of the tvDTH assay to detect both indirect pathway regulation and effector function (15,22–24) and using the original design of the ITN507ST cross-sectional trial, we found a pattern of indirect pathway T-cell responses consistent with a spectrum of immune responsiveness in 45 renal transplant recipients of various clinical status. At the extreme ends of the spectrum, identical twins showed no indirect pathway T-cell responses at all, consistent with having received a kidney isograft, whereas allosensitized, chronic rejectors demonstrated strong IFNγ and IL17-dependent indirect pathway Teff and B-cell (DSA) responses to donor HLA, with no evidence of indirect pathway Treg-cell-mediated bystander suppression. The TOL group displayed the lowest indirect pathway Teff-cell response to donor and the highest regulatory response. Although not all patients in the TOL group were analyzed, the regulatory response appeared to be mainly Th3-type because TGFβ and not IL10 blocking antibodies revealed a latent indirect pathway Teff DTH response. This finding is consistent with the TGFβ bias in indirect pathway Treg responses to kidney transplants previously reported in mouse (25) and monkey (3) tolerance models. Interestingly, the steroid MONO group had a slightly higher baseline indirect pathway T-cell response to donor antigen and less donor-specific regulation, but otherwise closely resembled the TOL group, particularly in the range and distribution of individual responses. The SI group was also in between the TOL and CR groups in both regulation and Teff antidonor tvDTH assays.
The existence of a progressive spectrum in the indirect pathway means that analysis of this parameter may yield insights into potential candidates for partial withdrawal of immunosuppression, as has been recently demonstrated (26). It is unclear why a similarly progressive spectrum was not seen in circulating B-cell counts or in the B-cell-influenced “POT” signature (Figure 2). It is possible that corticosteroid monotherapy is toxic to B cells. It is also possible that naïve and transitional B cells are required to stabilize tolerance once it is established but are less critical during the development of tolerance. This hypothesis predicts that B cells would accumulate only after some initial tolerance threshold has been reached and the subject is free of immunosuppression. When we depleted B cells from PBMC of two patients (one in TOL, one in SI) with high naïve B-cell counts, the strong indirect pathway T-cell regulation was unaffected, evidence that the cellular immunity-based indirect pathway function as detected by the tvDTH assay is not directly influenced by the presence of B cells. It is theoretically possible that donor antigen-specific Breg cells acting as an antigen presenting cells could be involved in suppression of the indirect pathway. This would require intact donor HLA, not allopeptide. In fact, we could detect no difference in dose–response of inhibition between intact donor HLA-B62-coated beads and third party HLA-B57 (p37MA+)-coated beads (Figure 4A), arguing against this idea, at least in one TOL patient. However, there was a suggestive piece of evidence of a connection between the two parameters within the TOL group itself—namely, two of the three TOL patients with the lowest regulation to donor antigen (Figure 1C) also had the lowest number of B cells (Figure 2A). It is possible that the maintenance of tolerance in these two individuals is independent of either of these two factors or that both patients are in a category of “unstable tolerance”.
In addition to a significantly elevated CD20 mRNA signal observed in urine samples of TOL versus SI patients in the ITN507ST trial (10), recent data demonstrates a rise in in-tragraft B cells in spontaneous kidney allograft tolerance in mice (8) and in a rat model of allograft tolerance induced using LF15–0195, a deoxyspergualine analog (8,9). Weak or suppressed indirect pathway in TOL patients may result in failure of intragraft and lymph node B cells to receive adequate T-follicular help for transition to plasma cells, preventing isotype switch from IgM to IgG (8,9). Without such help, naïve and transitional B cells accumulate in the graft and peripheral blood instead of emigrating to the bone marrow where they could produce alloantibodies (8). An alternative hypothesis is that once all immuno-suppression has been removed, the B cells that emerge are not simply “help-less” or help-resistant (27) but are in fact “Bregs” that actively contribute to tolerance stability (7). Although indirect pathway T-cell-mediated regulation of cell-mediated immunity may be common to tolerance in different organ transplant contexts such as liver (1,28), kidney (1,3) and heart (15,23), the role of Breg cells in allo-graft tolerance in mice (29,30) and in humans (31) seems to be highly organ/tissue-dependent.
The use of single antigen HLA beads to probe the T-cell indirect pathway in transplant recipients represents an important step toward standardization of indirect pathway T-cell assays, because single antigen beads are now widely used in monitoring DSA by HLA labs around the world. Studies are underway in our laboratory to probe indirect pathway T-cell responses in second-generation indirect pathway T-cell in vitro assays using the single antigen bead substrate, requiring no detailed knowledge of the allopeptides or host class II restriction involved. The example of a CR group patient shown in Figure 4B suggests that a strong cellular indirect pathway T-cell response to a particular donor HLA antigen (e.g. HLA-A2) may give rise to sufficient T follicular helper cells to enable naïve B cells to transition to IgG alloantibody producers. The weaker antidonor indirect pathway responses to HLA-A1 and HLA-B57 in the same patient resulted in DTH reactivity that may have also contributed to the CR, but were not sufficient to support helper activity needed for B-cell activation.
A hypothetical model to account for the findings of this study is diagrammed in Figure 5. In this view, as a patient progresses toward drug independence, one sees a higher incidence of the regulatory response (Th3/TGFβ and Tr1/IL10) and better control of indirect pathway Th17 and Th1 responses to donor alloantigen. Whether the increased incidence and robustness of Treg-cell responses is cause or effect, this pattern probably means that at each stage of reduction in immunosuppression, “regulator” patients persist and perhaps even strengthen their regulation level both in the graft and peripherally. Conversely, there is attrition of nonregulators due to either recurrence of disease or breakthrough rejection, leading to resumption of multidrug immunosuppression (26). At the extreme ends of the spectrum, we hypothesize that B cells play a critical role in sustaining tolerance or CR. In the condition of CR, Th1 and Th17 cells are less constrained by Tr1 and Th3 cells and cellular immunity is joined by humoral immunity as very strong indirect pathway T-cell responses trigger germinal center B cells to isotype-switch and develop into plasma cells secreting C′-fixing DSA (left). In the state of complete immunosuppression withdrawal and tolerance, there is not only a higher median level of indirect pathway T regulation measured by linked-suppression mediated through the dendritic cell (center), but also additional factors critical to maintaining this high level of indirect pathway regulation appear in a tissue-appropriate manner (32). For kidney transplantation, this may mean increased numbers of circulating B cells, possibly including Bregs, and accumulation of Breg cells in the graft.
Figure 5. Proposed model of indirect pathway in renal transplant recipients.
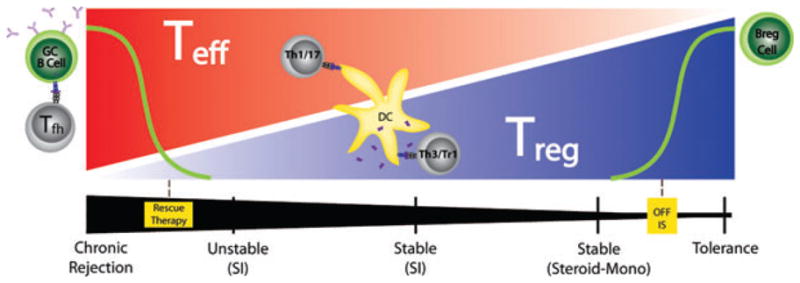
Red intensity indicates relative frequency of donor specific indirect pathway Teff cells, which may be Th1 or Th17 type; blue intensity denotes relative frequency of indirect pathway Treg cells, which may be Th3 or Tr1 type. Dendritic cells (DC) or germinal center (GC) B cells present donor-derived peptides (pink lines), which arise from processed soluble donor HLA (sHLA) or minor H antigens, to indirect pathway T cells. The black intensity (bottom) represents the relative amount of the immunosuppressive medication of patients, ranging from steroid monotherapy to standard immunosuppression (SI; middle), to chronic rejection (left). Rescue therapy refers to emergency measures such as plasmapheresis and high dose steroids applied in cases of unstable and decreasing graft function, along with increased Teff cells and increased DSA-secreting B cells. Decreased immunosuppression is associated with increased indirect pathway Treg cells and, once the patient is off of all immunosuppression, increased numbers of naïve B, possibly Breg, cells.
Acknowledgments
This work was supported by ITN507ST grant from the NIH-supported Immune Tolerance Network, by NIH grant #1R01- AI066219 (to WJB) and by the EU-sponsored One Study (WJB, LDH and MHF). M.H.F. and R.I.L. acknowledge support from ITN503ST, EU FP6 grant: QLRT–2002–02127 and from the UK Department of Health by National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors wish to thank Glen Leverson for his critical help with statistical analysis.
Funding source: No part of this manuscript was prepared by any commercial organization nor was it funded by any commercial organization.
Abbreviations
- Breg
B regulatory cell
- CR
chronic rejector group
- DSA
donor specific antibody
- EBV
Epstein Barr virus
- MFI
mean fluorescent intensity
- MONO
monotherapy group
- PBS
phosphate buffered saline
- PBMC
peripheral blood mononuclear cells
- POT
probability of being tolerant
- SI
standard immunosuppression group
- Teff
T effector cells
- TOL
clinically tolerant group
- Treg
T regulatory cells
- TT/DT
tetanus toxoid and diphtheria toxoid pediatric vaccine
- tvDTH
trans-vivo delayed-type hypersensitivity assay
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Note added in proof: While the data indicates that TGF-beta plays a role in the regulation of indirect pathway observed in tolerant group patients, we cannot rule out a role for IL35 and hence iTR35 T cells.
References
- 1.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafferty KJ, Babcock SK, Gill RG. Prevention of rejection by treatment of the graft: An overview. Prog Clin Biol Res. 1986;224:87–117. [PubMed] [Google Scholar]
- 3.Torrealba JR, Katayama M, Fechner JH, Jr, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J Immunol. 2004;172:5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q, Lee J, Jankowska-Gan E, et al. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178:3983–3995. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 5.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Lee J, Jankowska-Gan E, et al. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017–1023. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Cordoba S, Hu M, et al. Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol. 2011;24:149–156. doi: 10.1016/j.trim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Le Texier L, Thebault P, Lavault A, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11:429–438. doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 10.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 13.Burlingham WJ, Jankowska-Gan E. Mouse strain and injection site are crucial for detecting linked suppression in transplant recipients by trans-vivo DTH assay. Am J Transplant. 2007;7:466–470. doi: 10.1111/j.1600-6143.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 14.Hegde S, Jankowska-Gan E, Roenneburg DA, Torrealba J, Burlingham WJ, Gumperz JE. Human NKT cells promote monocyte differentiation into suppressive myeloid antigen-presenting cells. J Leukoc Biol. 2009;86:757–768. doi: 10.1189/jlb.0209059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanBuskirk AM, Wakely ME, Sirak JH, Orosz CG. Patterns of allosensitization in allograft recipients: Long-term cardiac allograft acceptance is associated with active alloantibody production in conjunction with active inhibition of alloreactive delayed-type hypersensitivity. Transplantation. 1998;65:1115–1123. doi: 10.1097/00007890-199804270-00017. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Lee J, Keller M, Burlingham WJ. Analysis of indirect pathway CD4+ T cells in a patient with metastable tolerance to a kidney allograft: Possible relevance to superior graft survival of HLA class II closely matched renal allografts. Transpl Immunol. 2009;20:203–208. doi: 10.1016/j.trim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H., Jr Recipient MHC class II expression is required to achieve long-term survival of murine cardiac allografts after costimulatory blockade. J Immunol. 2001;167:5522–5526. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto K, Yuan X, Auchincloss H, Jr, Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of action of donor-specific transfusion in inducing tolerance: Role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. J Am Soc Nephrol. 2004;15:2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 19.Steele DJ, Laufer TM, Smiley ST, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burlingham WJ, Fechner JH, DeVito LD, Sollinger HW, Knechtle SJ, Grailer AP. Human interleukin-2 and lymphoproliferative (T-helper cell) responses to soluble HLA class I antigens in vitro: I. Specificity for polymorphic domains. Tissue Antigens. 1993;42:35–38. doi: 10.1111/j.1399-0039.1993.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 21.Poggio ED, Clemente M, Riley J, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:1952–1960. doi: 10.1097/01.asn.0000129980.83334.79. [DOI] [PubMed] [Google Scholar]
- 22.Niederkorn JY, Streilein JW. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. J Immunol. 1983;131:2670–2674. [PubMed] [Google Scholar]
- 23.Warnecke G, Chapman SJ, Bushell A, Hernandez-Fuentes M, Wood KJ. Dependency of the trans vivo delayed type hypersensitivity response on the action of regulatory T cells: Implications for monitoring transplant tolerance. Transplantation. 2007;84:392–399. doi: 10.1097/01.tp.0000269705.94545.3a. [DOI] [PubMed] [Google Scholar]
- 24.Molitor-Dart ML, Andrassy J, Kwun J, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: Relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 25.Cook CH, Bickerstaff AA, Wang JJ, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180:3103–3112. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowska-Gan E, Sollinger HW, Pirsch JD, et al. Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor-specific regulation. Transplantation. 2009;88:533–541. doi: 10.1097/TP.0b013e3181b0f92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callaghan CJ, Rouhani FJ, Negus MC, et al. Abrogation of antibody-mediated allograft rejection by regulatory CD4 T cells with indirect allospecificity. J Immunol. 2007;178:2221–2228. doi: 10.4049/jimmunol.178.4.2221. [DOI] [PubMed] [Google Scholar]
- 28.Jankowska-Gan E, Rhein T, Haynes LD, et al. Human liver allograft acceptance and the “tolerance assay”. II. Donor HLA-A, -B but not DR antigens are able to trigger regulation of DTH. Hum Immunol. 2002;63:862–870. doi: 10.1016/s0198-8859(02)00450-0. [DOI] [PubMed] [Google Scholar]
- 29.DiLillo DJ, Griffiths R, Seshan SV, et al. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol. 2011;186:2643–2654. doi: 10.4049/jimmunol.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarkhin V, Chalasani G, Sarwal MM. The yin and yang of B cells in graft rejection and tolerance. Transplant Rev (Orlando) 2010;24:67–78. doi: 10.1016/j.trre.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Llordella M, Lozano JJ, Puig-Pey I, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matzinger P, Kamala T. Tissue-based class control: The other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]