Abstract
Background: The low zinc intake from human milk at ∼6 mo of age predicts the dependence on complementary foods (CF) to meet the zinc requirements of older breastfed-only infants.
Objective: The objective of this study was to compare major variables of zinc homeostasis and zinc status in 9-mo-old breastfed infants who were randomly assigned to different complementary food regimens.
Design: Forty-five exclusively breastfed 5-mo-old infants were randomly assigned to receive commercially available pureed meats, iron-and-zinc–fortified infant cereal (IZFC), or whole-grain, iron-only–fortified infant cereal (IFC) as the first and primary CF until completion of zinc metabolic studies between 9 and 10 mo of age. A zinc stable-isotope methodology was used to measure the fractional absorption of zinc (FAZ) in human milk and CF by dual-isotope ratios in urine. Calculated variables included the dietary intake from duplicate diets and 4-d test weighing, the total absorbed zinc (TAZ) from FAZ × diet zinc, and the exchangeable zinc pool size (EZP) from isotope enrichment in urine.
Results: Mean daily zinc intakes were significantly greater for the meat and IZFC groups than for the IFC group (P < 0.001); only intakes in meat and IZFC groups met estimated average requirements. Mean (±SEM) TAZ amounts were 0.80 ± 0.08, 0.71 ± 0.09, and 0.52 ± 0.05 mg/d for the meat, IZFC, and IFC groups, respectively (P = 0.027). Zinc from human milk contributed <25% of TAZ for all groups. The EZP correlated with both zinc intake (r = 0.43, P < 0.01) and TAZ (r = 0.54, P < 0.001).
Conclusion: Zinc requirements for older breastfed-only infants are unlikely to be met without the regular consumption of either meats or zinc-fortified foods.
INTRODUCTION
Because zinc concentrations in human milk (HM)5 sharply decline over the early months postpartum, the zinc intake of an exclusively breastfed infant also declines (1, 2). To meet physiologic requirements for zinc, the infant is progressively more dependent on complementary foods (CF) (3, 4). Despite recognition by the WHO and the American Academy of Pediatrics (5, 6) of the potential value of meats or fortified foods to provide an adequate zinc (and iron) intake, meats are not commonly consumed by infants in the United States (7) or in many low-resource settings (8). In the United States, not all commercial infant cereals are zinc fortified, and in many low-resource settings, no micronutrient-fortified products are readily available.
On the basis of a factorial approach, the Food and Nutrition Board of the Institute of Medicine estimated the physiologic requirement for zinc (the amount required to be absorbed to replace losses and to be retained for growth) for 7–12-mo-old infants to be 0.84 mg/d and the estimated average requirement (EAR) for dietary intake to be 2.5 mg/d (9). In 2 earlier studies of Denver infants who were breastfed only (ie, no formula), we reported the zinc intake from CF, including non–zinc-fortified infant cereal, at 7 mo to be ∼0.5 mg Zn/d, with an additional 0.5 mg/d contributed by breast milk (1, 10). These data indicated that traditional complementary feeding practices for older breastfed infants would not meet zinc requirements for many infants. Since our initial study, some commercial infant cereal products have been zinc fortified, whereas other brands are not fortified. To our knowledge, the bioavailability of the zinc in the zinc-fortified cereal has not been reported, and thus, the adequacy is unknown. In addition, the degree to which the zinc ingested from HM, with its favorable bioavailability, would compensate for a low intake from unfortified CF has also not been examined.
The primary objective of this study was to compare total daily zinc absorption and zinc status in older breastfed-only infants who were randomly assigned at 5 mo of age (while exclusively breastfed) to complementary feeding regimens that emphasized meat or commercial infant cereal with or without zinc fortification. We hypothesized that daily absorbed zinc would be significantly higher in infants who received meat than in infants randomly assigned to receive the unfortified cereal and that the daily zinc absorption for infants who received the zinc-fortified cereal would be intermediate. A secondary objective was to compare biomarkers of zinc status in the feeding groups.
SUBJECTS AND METHODS
Study design
This study was a randomized controlled intervention in which exclusively breastfed ∼5-mo-old infants were assigned to receive either commercially prepared pureed meats, iron-and-zinc–fortified infant cereal (IZFC), or organic, whole-grain iron-only–fortified infant cereal (IFC). These foods served as the first complementary food and were continued with 1 to 2 servings/d throughout the remainder of the study. Monthly visits were conducted to assess dietary compliance, obtain anthropometric measurements, and record infant morbidity.
Between 9 and 10 mo of age, each infant had his or her blood drawn to assess biochemical markers of zinc status and participated in a metabolic study that applied a zinc stable-isotope methodology to measure key variables of zinc homeostasis, including the fractional absorption of zinc (FAZ) (11), total absorbed zinc (TAZ), and exchangeable zinc pool size (EZP). Daily intakes of HM and milk zinc concentrations were also obtained during the metabolic period.
Subjects
Forty-five infants (18 boys and 27 girls) between the ages of 5 and 6 mo from the Denver metropolitan area were enrolled in the study. Recruitment for this study began 16 January 2008, and methods included advertisements sent through the university e-mail system, flyers in lactation clinics, and word of mouth. Infants were included in the study if the mother could show an intent or history of exclusive breastfeeding (no formula use) through 1 y and the infant was born at term (37–42 wk gestation) with birth weight appropriate for gestational age. Infants were excluded if they had current or planned formula use, had low birth weight, used vitamin-mineral supplements (except vitamin D), or had significant congenital anomalies or known chronic conditions that would affect feeding, growth, or developmental potential.
The study was approved by the Colorado Multiple Institutional Review Board, and written and informed consent was obtained from the mothers or fathers of the infants.
Sample-size calculations were based on previous data available from published data from our group (12) and unpublished data for 2–5-mo-old exclusively and partially breastfed infants. Summary statistics from the 2 available data sets (n = 9 and n = 16, respectively) included the mean (±SD) TAZ of 0.57 ± 0.19 mg/d. With the use of independent sample t tests, we used PASS software (NCSS 2007 and PASS 2008; Number Cruncher Statistical Systems) to estimate that we would have an 82–93% power (depending on absolute amounts of TAZ) to detect a difference of 0.20 mg TAZ/d between the 3 complementary food groups with 15 subjects per group.
Diets
The nutritional composition of the study foods as measured in the laboratory (zinc and phytate) and obtained from the product labels (energy) is shown in Table 1. The phytate content of the IZFC was relatively low, whereas that of the whole-grain IFC was several-fold higher.
TABLE 1.
Nutritional content per serving of study foods1
| IFC | IZFC | Pureed meat2 | |
| Serving size (g) | 14 (1/4 cup) | 15 (1/4 cup) | 71 (1 jar) |
| Energy (kcal)3 | 60 | 60 | 70 |
| Zinc (mg)4 | 0.3 | 1.2 | 2.1 |
| Phytate (mg)4 | 107 | 23 | 0 |
| Phytate:zinc molar ratio | 35.4 | 1.9 | — |
IFC, iron-only–fortified infant cereal (dry); IZFC, iron-and-zinc–fortified infant cereal (dry).
Values for pureed beef and gravy.
From the label.
From laboratory analyses.
At enrollment, infants were randomly assigned to receive one of the 3 dietary regimens previously described (meat, IZFC, or IFC). Parents were asked to introduce CF at ∼6 mo, at which point the assigned food was initiated. The main dietary components (cereal or meat purees) were provided at monthly visits to help ensure dietary compliance. The mothers were instructed to feed their infants ad libitum but were given monthly written guidelines on amounts of either meat or cereal that would be appropriate for the age of the infant on the basis of current recommendations (5). The meat group was encouraged to aim for the consumption of one jar (71 g) of pureed meat and gravy per day by 7 mo of age and >1 to ≤2 jars/d by 9 mo. Cereal groups were encouraged to offer 1 serving/d (15 g dry weight according to the manufacturer's label) by 7 mo and 2 servings/d by 9 mo. These instructions were provided as feeding guidelines, and parents were encouraged to let their infant's appetite dictate the actual amount of intake. The guidelines also contained information about foods to avoid such as iron- and/or zinc-fortified finger foods and infant formula or foods that contained formula. Mothers were given a nonfortified ready-to-eat cereal to use as a finger food and nonfortified teething biscuits to encourage developmentally appropriate feeding without significantly affecting zinc and iron intakes. Infants in the cereal groups (IZFC and IFC) were instructed to avoid single-ingredient meats; at 8 mo of age, commercial infant dinners were allowed [one or fewer 4-oz (113 g) jars/d]. The meat group was instructed to avoid fortified infant cereals but was allowed to use unfortified cereals such as quick oats or commercial unfortified cooked rice starting at 7 mo (≤15 g/d). For all groups, unfortified fruit, vegetables, yogurt, and cheese were allowed ad libitum as developmentally appropriate. Three-day diet records were completed by the mothers before monthly visits and were reviewed with the research coordinator at each visit (complete intake data to be presented separately in a future article).
Metabolic study
Between 9 and 10 mo of age, infants underwent an in-home zinc stable-isotope metabolic study to measure variables of zinc homeostasis. On study day 1, the following 2 different oral zinc isotopes were given to the infant as extrinsic labels: 70Zn to label breast milk and 67Zn to label all CF in a 24-h period. On study day 2, infants came to the outpatient Clinical and Translational Research Center, Children's Hospital Colorado, to have blood drawn and a third zinc isotope tracer (68Zn) intravenously infused by trained nursing staff. Spot urine samples (10–20 mL each) were collected twice daily on study days 5–8 of the metabolic study. Duplicate diets were collected to determine total dietary zinc intake on days 1 and 5–8. Four-day (96-h) test weighing to measure breast-milk intakes was also conducted on study days 5–8; midfeed milk samples were also collected with each feeding (12).
Isotope-dose preparation and administration
Enriched stable isotopes of zinc were obtained from Trace Science International. Accurately weighed quantities of each isotopically enriched preparation were dissolved in 0.5 mol H2SO4/L and then diluted with deionized water to prepare stock solutions. To prepare oral doses, stock solutions of 70Zn (95.5% enrichment) and 67Zn (94.2% enrichment) were diluted with purified water and titrated to a pH 6.0 with metal-free ammonium hydroxide. The solution was subsequently filtered through a 0.22-μm filter to ensure sterility. Zinc concentrations of solutions were determined by triplicate measure of the preparation by using atomic absorption spectrometry with adjustment of concentration measurements for different atomic weights of the preparation. Isotope-enriched doses were prepared individually for each infant.
HM was labeled with the 70Zn isotope, and the number of doses for each infant was based on the number of daily nursing episodes that the mother reported. To label the breast milk, an accurately weighed amount of ∼75 μg 70Zn was added to a weighed amount of HM and was placed in a refrigerator to equilibrate for ∼4 h. The 70Zn-labeled HM was then drawn up into accurately weighed 3-mL syringes for administration to the infant. Two extra doses were made and reserved for zinc-isotope analysis by using inductively coupled plasma mass spectrometry (ICP-MS) to determine the total amount of 70Zn given to the infant.
A similar method was used to prepare 67Zn oral doses administered with each complementary feeding (11). An accurately weighed amount of ∼125 μg (IFC) or ∼250 μg (meat and IZFC) 67Zn solution was added to a weighed amount (∼30 g) of infant applesauce and allowed to equilibrate for 4 h. The 67Zn-labeled applesauce was divided into accurately weighed doses. The amount of 67Zn isotope in each dose was proportionate to the percentage of daily zinc intake consumed at that meal. Two extra labeled aliquots were accurately weighed and analyzed by using ICP-MS to determine the total dose given to the infant.
To prepare the intravenous dose, a stock solution of 68Zn (99.4% enrichment) was diluted with 0.45% saline, adjusted to a pH 6.0, and filtered through a 0.22-μm filter to ensure sterility. The pharmaceutical quality of the sterile solution (ie, sterility and pyrogenicity) was certified by the University of Colorado Hospital pharmacy and the core laboratory of the General Clinical Research Center, respectively.
70Zn-labeled breast milk was given at the midpoint of each breastfeeding episode. The 67Zn-labeled applesauce was given with each complementary feeding by alternating between bites of the prepared meal and the labeled applesauce until all of the labeled applesauce was consumed. All losses of doses from drooling or spits were collected on ashless filter paper during the dose administration. These were analyzed by using ICP-MS, and the amount of isotope lost during administration was subtracted from the calculated dose to give a final administered isotope dose.
Immediately after blood collection for biomarker analyses on the morning of study day 2, ∼800 μg 68Zn isotope was administered intravenously over 2–3 min via a 3-way stopcock attached to a butterfly needle placed in a superficial forearm vein; isotope infusion was followed by 2 equal-volume rinses of sterile saline through the syringe and tubing. Filter paper was used to collect any losses during administration. After the infusion, the needle, tubing, and syringes used to administer the dose were rinsed with 0.5 mol saline/L, and total zinc and isotopic enrichment was measured to calculate any zinc stable-isotope losses during dose administration.
Sample collections
Weighed duplicate meals, adjusted for plate waste, were collected on study days 1 and 5–8 of the stable-isotope study to determine daily intakes of zinc. Breast-milk samples (∼3–5 mL) were collected midway through each feeding to measure zinc concentrations. Urine was collected in the morning and evening for 4 d (days 5–8) by using adhesive, pediatric urine-collection bags (Briggs) and was subsequently transferred to a zinc-free polypropylene container until analysis. Blood was drawn at ∼0900 in the pediatric Clinical and Translational Research Center for all subjects. A 1-mL blood sample was collected into a zinc-free heparinized microtube, and the plasma was separated within 30 min. Samples were stored at −20°C in freezers in the Pediatric Nutrition Laboratory, University of Colorado Denver.
Sample processing and analysis
Duplicate diet and breast-milk samples from the metabolic study were individually dried in an electric drying oven before digestion at 450°C in a muffle furnace for 24 h. A few drops of concentrated nitric acid were added to each sample, and the sample was dried on a hot plate and ashed again at 450°C for 24 h. Ashed samples were quantitatively reconstituted with 25 mL 6 mol/L HCl (diet) or 10 mL 0.1 mol/L HCl (breast milk), and the total zinc concentration was measured by using atomic absorption spectrophotometry fitted with a deuterium arc background lamp (model AAnalyst 400; Perkin-Elmer Corp).
Urine samples were digested by using a MARS microwave sample-preparation system (CEM Corp). A 5-mL urine sample was placed into the HP500 vessel system (CEM Corp) with 3 mL concentrated HNO3. The microwave gradually ramped up the temperature and pressure in the vessel to about 200°C and 120 psi and digested the sample in ∼60 min. The remaining liquid from the digested sample was placed into a 50-mL beaker and evaporated to dryness on a hot plate. The dried sample was reconstituted in 2 mL ammonia acetate buffer (pH 5.6), and zinc was purified by first chelating it with trifluoroacetylacetone and then extracting the chelate with hexane.
Isotope enrichment was determined by measurement of isotope ratios 67Zn:64Zn, 68Zn:64Zn and 70Zn:64Zn by using ICP-MS (ICP-MS, VG Plasma Quad 3, VG Elemental; Thermo Electron Corp) and the conversion of ratios to enrichment by using a mathematical matrix. Tracer enrichment was defined as all of the zinc in the sample that was derived from the isotopically enriched tracer preparation divided by the total zinc in the sample (13).
Plasma zinc concentrations were determined in the Pediatric Nutrition Laboratory by using flame atomic absorption spectrometry. The precision of the plasma zinc analysis was determined by concurrent measurement of a plasma pool. Accuracy was established by analysis of a certified reference material [SRM-1598 bovine serum; National Institute of Standards and Technology; mean (±SD) certified value: 89.0 ± 6.0 μg/dL] and comparison with an analyzed value of 89.0 ± 1.7 μg/dL.
Data processing and statistical analyses
Data processing
The total dietary intake of zinc at the time of metabolic studies was determined from the zinc intake in the duplicate diet added to the zinc intake from breast milk. The zinc intake from breast milk was determined by multiplying the zinc concentration of an aliquot of breast milk by grams of breast milk consumed at the same feeding on the basis of test weights (12).
The FAZ from CF and breast milk was determined by using a dual-isotope tracer-ratio technique (12, 14), which compared isotopic ratios of the intravenously administered 68Zn to the orally administered 67Zn (CF) and 70Zn (breast milk), respectively, by using the following equation:
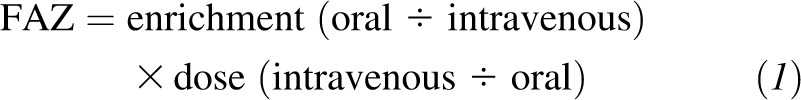 |
For each subject, the mean FAZ from CF and HM was calculated from each of the 8–12 individual spot-urine samples collected during the metabolic period. The TAZ (mg/d) was calculated as the sum of the products of the FAZ and dietary zinc from CF and HM as follows:
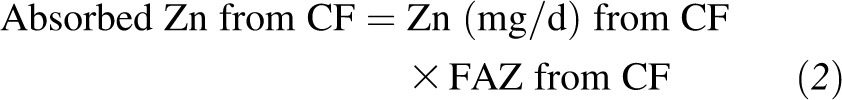 |
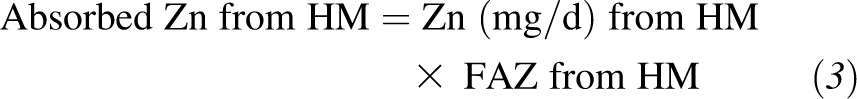 |
The EZP, which was defined as the estimate of the total size of the combined pools of zinc that exchange with zinc in plasma within ∼2–3 d, was calculated by dividing the dose of the intravenous isotope (68Zn) infused by the enrichment value at the y intercept of the linear regression of a semilog plot of urine-enrichment data from study days 5–8 after isotope administration (15).
Values for plasma zinc concentrations for infants with elevated high-sensitivity C-reactive protein were not included in the analyses because inflammation potentially confounds the interpretation.
Statistical analysis
Results were analyzed with GraphPad Prism version 5.00 for Windows (GraphPad Software). Group data sets were first assessed for normality. If data were normally distributed, 1-factor ANOVA with Tukey's multiple comparisons posttest were used to compare group means. Data are presented as means ± SEMs unless otherwise noted. Significance was defined at the α = 0.05 level.
RESULTS
Forty-two subjects (17 boys and 25 girls) completed the study with 14 infants in each feeding group. Three additional infants (one infant per group) dropped out from the study at 8 mo because the mother was unable to continue breastfeeding without formula supplementation. Two infants were unable to complete the metabolic study at 9 mo because the intravenous zinc-isotope dose was not successfully administered. No adverse effects were reported from any of the 3 dietary regimens or study procedures.
Dietary intake
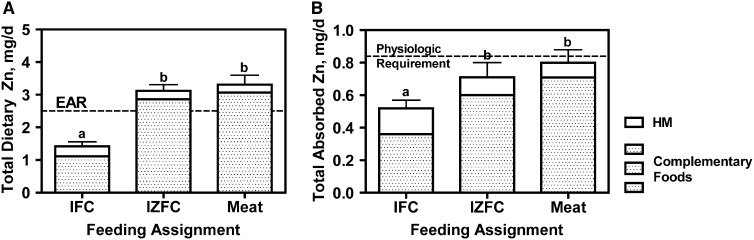
Data of mean total dietary zinc intakes at the time of the metabolic study are presented in Figure 1A. All groups had similar HM intakes, which provided relatively modest contributions to daily zinc intakes of <10% of the total for meat and IZFC groups and 22% of the total for subjects in the IFC group. The mean total dietary zinc intake was significantly higher in the meat and IZFC groups than in the IFC group (P < 0.001). As shown in Figure 1A, the mean dietary zinc of infants in the meat and IZFC groups exceeded the EAR for zinc of 2.5 mg/d (9), whereas the mean intake of the IFC group was ∼50% of the EAR. Only one of the infants in the IFC group met the EAR for zinc intake, whereas 86% and 79% of infants in the meat and IZFC groups, respectively, met or exceeded the EAR.
FIGURE 1.
Mean (±SEM) total dietary Zn (A), including intakes from HM and complementary foods, and total absorbed zinc (B) of infants by group [meat (n = 13), IZFC (n = 14), and IFC (n = 13)]. Different lowercase letters between groups indicate significance in the pairwise comparison (P < 0.001; ANOVA with Tukey's multiple comparisons posttest). Dashed lines represent the EAR ( ) and physiologic requirement ( ) for infants of this age. EAR, estimated average requirement; HM, human milk; IFC, iron-only–fortified infant cereal; IZFC, iron-and-zinc–fortified infant cereal.
Variables of zinc homeostasis
The FAZ from CF was significantly higher in the IFC group than in the meat and IZFC groups (P = 0.003; Table 2). The FAZ from HM did not differ significantly by group.
TABLE 2.
Fractional absorption of zinc from complementary foods and human milk1
| Fractional absorption of zinc |
||
| Feeding assignment | Complementary foods | Human milk |
| Meat (n = 13) | 0.22 ± 0.01a | 0.42 ± 0.04 |
| IZFC (n = 14) | 0.21 ± 0.02a | 0.43 ± 0.03 |
| IFC (n = 13) | 0.33 ± 0.04b | 0.52 ± 0.04 |
All values are means ± SEMs. Different lowercase letters between groups indicate significance in pairwise comparison (P = 0.003; 1-factor ANOVA with Tukey's multiple comparison posttest). IFC, iron-only–fortified infant cereal (dry); IZFC, iron-and-zinc–fortified infant cereal (dry).
The TAZ varied significantly in the 3 groups (Figure 1B). The proportions of TAZ attributable to HM were similar to those observed for dietary zinc intakes for each group. Also similar to the dietary intake results, the mean TAZ was significantly higher for the meat group than for the IFC group (P < 0.05) but not compared with the IZFC. A comparison of the TAZ to the physiologic requirement of 0.84 mg/d showed that the TAZ for the IFC group was significantly lower than the physiologic requirement (P < 0.0001), whereas the TAZ for the IZFC and meat groups were not different from the physiologic requirement (P = 0.09 and P = 0.34, respectively).
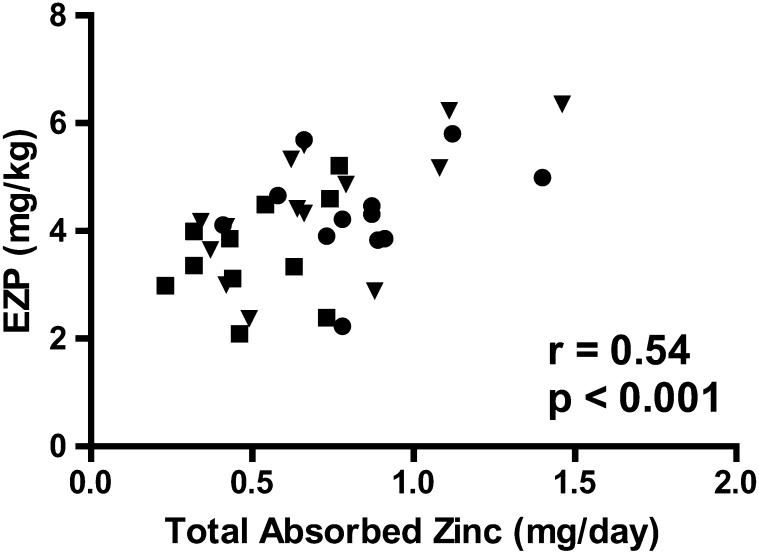
The mean ± SEM (n) EZP did not differ according to groups [meat: 4.34 ± 0.27 mg/kg (12); IZFC: 4.46 ± 0.33mg/kg (14); and IFC: 3.42 ± 0.26 mg/kg (11)]. However, significant correlations were observed between the EZP and individual total dietary zinc intakes (r = 0.43, P < 0.01) and the TAZ (Figure 2). Plasma zinc concentrations also did not differ significantly (P = 0.53) in groups with means (n) of 83.5 ± 4.0 μg/dL (11), 71.8 ± 3.5 μg/dL (11), and 78.7 ± 3.9 μg/dL (10) for meat, IZFC, and IFC groups, respectively. Unlike for the EZP, no significant correlations were observed between plasma zinc concentrations and either dietary zinc intake or TAZ. Plasma zinc and EZP size were also not correlated.
FIGURE 2.
Total absorbed zinc of infants by group [meat (n = 13), IZFC (n = 14), and IFC (n = 13)] at 9–10 mo of age correlated with the EZP (Pearson's correlation). Squares represent subjects in the IFC group, inverted triangles represent subjects in the IZFC group, and circles represent subjects in the meat group. EZP, exchangeable zinc pool size; IFC, iron-only–fortified infant cereal (dry); IZFC, iron-and-zinc–fortified infant cereal (dry).
DISCUSSION
To our knowledge, this study was the first to provide a detailed examination of zinc absorption from different complementary feeding regimens in older breastfed infants. The data convincingly demonstrate that, without consumption of either foods naturally high in zinc, such as meats, or micronutrient-fortified products, the daily absorbed zinc of fully breastfed infants is well below the estimated physiologic requirement of 0.84 mg/d for this age (9). The average amount of pureed meat consumed by the infants in the meat group represented ∼60 g meat/d, when the amounts of added water and gravy in the commercial purees were considered. A similar amount of daily zinc was consumed by the IZFC group, and the average intake for both the meat and IZFC groups modestly exceeded the EAR of 2.5 mg/d (9). For similar total zinc intake, the amount of absorbed zinc of the fortified-cereal group was not significantly lower than that of the meat group. This result was consistent with the relatively low phytate: zinc molar ratio in the product, which would be presumed to only modestly lower the zinc bioavailability from the commercial cereal (16).
The unfortified-cereal group's intakes were low, and despite a higher fractional absorption, the total amount of absorbed zinc averaged only ∼60% of the estimated physiologic requirement, and none of the infants in the IFC group achieved this amount of daily absorbed zinc. The significantly lower absorbed zinc showed limited compensation for a low dietary intake, especially from foods that are relatively high in phytate, as was the IFC product. For infants in this group, a near doubling of the absorption from CF would have been required to approximate the physiologic requirement. The observed results for fractional and total absorbed daily zinc were consistent with current concepts of zinc homeostasis that the primary determinants of fractional absorption of zinc are the amount of zinc ingested and the dietary phytate and not the zinc status or need of the host (16, 17). These results are highly relevant to predominantly breastfed infants in the United States for whom emphasis has traditionally been placed on meeting iron requirements through iron-fortified cereals with less emphasis on food choices to meet zinc requirements (18). For older infants in low-resource settings who often do not have access to fortified products and for whom traditional CF consist primarily of high-phytate, unfortified cereal and grain staples that are poor sources of bioavailable zinc (8, 19), risk of zinc deficiency is high.
With respect to zinc status, for which sensitive biomarkers are limited, the EZP data represent, to our knowledge, the largest published set of results combined with complete dietary data. The group EZP means and significant correlations between the EZP of individuals and both dietary intake and absorbed zinc suggested that zinc status was more favorable in the meat and iron-and-zinc–fortified cereal groups. The mean plasma zinc concentration tended to be higher for the meat group, but the low sensitivity of this biomarker, small sample sizes in each group, and substantial variability support conclusions that plasma zinc concentrations are primarily useful for assessment of risk of zinc deficiency of populations (20). For clinical settings, these results confirmed the value of a good diet history to judge risk of deficiency for older infants and toddlers who are predominantly breastfed.
The limitations of this study included the relatively small sample size and, thus, limited power to assess differences in any functional outcomes related to differences in zinc status. Comparisons of such outcomes as growth, appetite, neurocognitive development, and infectious morbidity were beyond the scope of this study. The constraints placed on diet patterns by the study design may be construed as artificially biased toward extremes of zinc intake that would not be observed in reality in the United States. However, the limited intake data available suggest otherwise. The most recent cross-sectional survey of infant-feeding practices in the United States indicated that <5% of 6–12-mo-old infants consume infant meats, and the percentage has sharply declined for 9–12-mo-olds. The reduction in meat intake was accompanied by increases in intakes of yogurt and protein-vegetable-starch mixtures (7). This same report also showed a sharp decline in recent years in the consumption of fortified infant cereals (7). The emphasis from national surveys of the feeding patterns of US infants and toddlers is on the high percentage of those whose zinc intakes are above the upper limits (9, 21). Such reports reflect zinc intakes of the majority of infants and older toddlers who consume many fortified products, including infant formulas (21). This emphasis, however, fails to account for the dependence of older breastfed infants on choices of CF to meet zinc and iron requirements (22). According to the most recent national data on breastfeeding practices, ∼15% of infants in the United States are exclusively breastfed at 6 mo of age (23), and ∼24% of infants in the United States are still breastfed at 12 mo of age (24). For these infants, recommendations for complementary feeding practices should ideally be tailored to address micronutrient needs of older breastfed infants.
In conclusion, this study represents a uniquely in-depth examination of the impact of different complementary feeding patterns on zinc intake and homeostasis in older breastfed infants in the United States. The unfortified, whole-grain cereal had both a low zinc content and a high phytate:zinc molar ratio. Even combined with zinc from HM, the total daily absorbed zinc of the IFC group was well below estimated requirements for this age. We conclude that the incorporation of meats into the dietary regimen at the time of initiation of complementary feeding is well accepted and provides an intake of zinc that meets estimated dietary requirements in a form that is well absorbed. The results of this study also indicate that zinc-fortified commercial infant cereals provide zinc intake and daily absorbed zinc similar to that of the meat group. The CDC (25) and the American Academy of Pediatrics (26) have recognized the importance of these dietary sources to avoid deficiencies of zinc and iron for older breastfed infants. Furthermore, these results support complementary feeding recommendations by the WHO for settings with low resources (6) where deficiencies of zinc and iron in infants and toddlers are extremely widespread.
Acknowledgments
The authors’ responsibilities were as follows—NFK, KMH, and JEW: designed the research protocol; DLC, JEW, and LS: conducted the research and provided sample analyses; DLC, LVM, and JEW: analyzed the data and performed the statistical analyses; NFK: wrote and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CF, complementary foods; EAR, estimated average requirement; EZP, exchangeable zinc pool size; FAZ, fractional absorption of zinc; HM, human milk; ICP-MS, inductively coupled plasma mass spectrometry; IFC, iron-only–fortified infant cereal; IZFC, iron-and-zinc–fortified infant cereal; TAZ, total absorbed zinc.
REFERENCES
- 1.Krebs NF, Reidinger CJ, Robertson AD, Hambidge KM. Growth and intakes of energy and zinc in infants fed human milk. J Pediatr 1994;124:32–9 [DOI] [PubMed] [Google Scholar]
- 2.Krebs NF, Reidinger CJ, Hartley S, Robertson AD, Hambidge KM. Zinc supplementation during lactation: effects on maternal status and milk zinc concentrations. Am J Clin Nutr 1995;61:1030–6 [DOI] [PubMed] [Google Scholar]
- 3.Krebs NF, Hambidge KM. Zinc requirements and zinc intakes of breast-fed infants. Am J Clin Nutr 1986;43:288–92 [DOI] [PubMed] [Google Scholar]
- 4.Gibson RS, Ferguson EL. Assessment of dietary zinc in a population. Am J Clin Nutr 1998;68(suppl):430S–4S [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Pediatric nutrition handbook. 6th ed. Elk Grove, IL: American Academy of Pediatrics, 2009 [Google Scholar]
- 6. PAHO/WHO. Guiding principles for complementary feeding of the breastfed child. Washington, DC: PAHO, WHO, 2003.
- 7.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc 2010;110(suppl):S38–51. [DOI] [PubMed] [Google Scholar]
- 8.Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, Carlo W, Goco N, Kindem M, Wright LL, et al. Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr Bull 2011;32:185–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin a, vitamin k, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 10.Krebs NF, Abebe Y, Stoecker BJ, Gibson RS, Westcott JE, Hambidge KM. Intake of zinc from human milk and complementary foods by 7 month old infants from the Sidama Zone in Southern Ethiopia. FASEB J 2006;20(suppl):A985 [Google Scholar]
- 11.Sheng XY, Hambidge KM, Miller LV, Westcott JE, Lei S, Krebs NF. Measurement of zinc absorption from meals: comparison of extrinsic zinc labeling and independent measurements of dietary zinc absorption. Int J Vitam Nutr Res 2009;79:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs NF, Reidinger CJ, Miller LV, Hambidge KM. Zinc homeostasis in breast-fed infants. Pediatr Res 1996;39:661–5 [DOI] [PubMed] [Google Scholar]
- 13.Hambidge KM, Huffer JW, Raboy V, Grunwald GK, Westcott JL, Sian L, Miller LV, Dorsch JA, Krebs NF. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids. Am J Clin Nutr 2004;79:1053–9 [DOI] [PubMed] [Google Scholar]
- 14.Friel JK, Naake VL, Jr, Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr 1992;55:473–7 [DOI] [PubMed] [Google Scholar]
- 15.Miller LV, Hambidge KM, Naake VL, Hong Z, Westcott JL, Fennessey PV. Size of the zinc pools that exchange rapidly with plasma zinc in humans: alternative techniques for measuring and relation to dietary zinc intake. J Nutr 1994;124:268–76 [DOI] [PubMed] [Google Scholar]
- 16.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007;137:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9 [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Pediatric nutrition handbook. 5th ed. Elk Grove, IL: American Academy of Pediatrics, 2003 [Google Scholar]
- 19.World Health Organization Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva, Switzerland: World Health Organization, 1998 [Google Scholar]
- 20.de Benoist B, Darnton-Hill I, Davidsson L, Fontaine O, Hotz C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr Bull 2007;28:S480–79 [DOI] [PubMed] [Google Scholar]
- 21.Butte NF, Fox MK, Briefel RR, Siega-Riz AM, Dwyer JT, Deming DM, Reidy KC. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J Am Diet Assoc 2010;110(suppl):S27–37 [DOI] [PubMed] [Google Scholar]
- 22.Krebs NF, Hambidge KM. Complementary feeding: clinically relevant factors affecting timing and composition. Am J Clin Nutr 2007;85:639S–45S [DOI] [PubMed] [Google Scholar]
- 23.Jones JR, Kogan MD, Singh GK, Dee DL, Grummer-Strawn LM. Factors associated with exclusive breastfeeding in the United States. Pediatrics 2011;128:1117–25 [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control Breastfeeding Report Card - United States, 2011. Available from: http://www.cdc.gov/breastfeeding/data/reportcard.htm (cited 24 January 2011).
- 25.Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics . 2008;122(suppl 2):S36–42 [DOI] [PubMed] [Google Scholar]
- 26.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]