Abstract
Background: Factors affecting bone calcium deposition across pregnancy and lactation are not well characterized.
Objective: The impact of maternal age, calcium intake, race-ethnicity, and vitamin D status on the rate of bone calcium deposition (VO+) was assessed across pregnancy and lactation.
Design: Stable calcium isotopes were given to 46 women at pre- or early pregnancy (trimester 1), late pregnancy (trimester 3), and 3–10 wk postpartum. Three cohorts were included: 23 adolescents from Baltimore (MD), aged 16.5 ± 1.4 y (mean ± SD; Baltimore cohort); 13 adults from California, aged 29.5 ± 2.6 y (California cohort); and 10 adults from Brazil, aged 30.4 ± 4.0 y (Brazil cohort). The total exchangeable calcium pool, VO+, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], parathyroid hormone, and calcium intake were evaluated.
Results: At trimester 3, inverse associations between 1,25(OH)2D and VO+ were evident in the Baltimore (P = 0.059) and Brazil (P = 0.008) cohorts and in the whole group (P = 0.029); calcium intake was not a significant determinant of VO+ in any group during pregnancy. At postpartum, a significant positive association was evident between VO+ and calcium intake (P ≤ 0.002) and between VO+ and African ethnicity (P ≤ 0.004) in the whole group and within the Baltimore and Brazil cohorts.
Conclusions: Elevated 1,25(OH)2D was associated with decreased rates of bone calcium deposition during late pregnancy, a finding that was particularly evident in pregnant adolescents and adult women with low calcium intakes. Higher dietary calcium intakes and African ethnicity were associated with elevated rates of bone calcium deposition in the postpartum period.
INTRODUCTION
Significant adaptations in maternal calcium homeostasis and bone turnover occur to support the demands of pregnancy and lactation. To date, these physiologic alterations and their possible determinants have not been fully characterized. The human fetus acquires ∼30 g Ca across gestation (1), which increases from only several milligrams per day in the first trimester to >250 mg/d during the last trimester (2). During lactation, calcium losses into human breast milk represent ∼260 mg/L (3). These increased demands may involve alterations in calcium absorption and excretion and rates of bone calcium turnover.
Racial differences in rates of bone calcium deposition between nonpregnant white and African American females have been reported (4, 5), but possible differences during pregnancy are largely unexplored. Alterations in bone turnover markers support an impact of age on bone turnover between pregnant and lactating adolescent and adult women (6). Similarly, longitudinal bone density studies in adult women have reported bone mineral density losses of 3.2–4.6% at trabecular sites across pregnancy (7, 8), with losses being more pronounced among adolescents (9). Although trabecular losses are frequently evident, increases in bone density at cortical bone sites may occur during pregnancy (10). During lactation, temporal losses of trabecular bone mineral at the spine and hip (∼3–5%) occur over the first 3–6 mo of lactation (11), and these losses appear to be unaffected by calcium supplementation (12).
Studies assessing longitudinal changes in 1,25-dihydroxyvitamin D [1,25(OH)2D]4 across pregnancy and lactation have supported the concept that this hormone typically increases across pregnancy (13–17). Determinants of this increase are less well studied as are possible relations between serum 1,25(OH)2D and rates of bone calcium deposition across pregnancy and lactation. Longitudinal measures of parathyroid hormone (PTH) during pregnancy have reported decreases, increases, or no change in this hormone across gestation (6, 18, 19). Recent data highlight the increased risk of suboptimal 25-hydroxyvitamin D [25(OH)D] concentrations among pregnant women, with increased insufficiency being evident among minorities (16, 17, 20). Increasing data also link suboptimal concentrations of this prohormone with elevated concentrations of parathyroid hormone and/or 1,25(OH)2D (16, 17, 20), although all data do not support these associations (2, 21). The possible impact of these hormonal changes on bone calcium deposition during pregnancy remains largely uncharacterized.
To date, calcium isotopic studies across pregnancy and into the early postpartum/lactation period have focused primarily on changes in intestinal calcium absorption (6, 22–25). To our knowledge, only one longitudinal kinetic study has been published on bone calcium turnover across pregnancy and lactation (26). To address rates of bone deposition in response to the calcium challenges of pregnancy and lactation, we compiled our existing unpublished and published (26) longitudinal data with the use of intravenous stable calcium isotopes during both pregnancy and lactation as a function of habitual calcium intake, race-ethnicity, maternal age, and 1,25(OH)2D concentration.
SUBJECTS AND METHODS
Subject recruitment
All women who were recruited were healthy nonsmokers. In the Baltimore cohort, pregnant adolescents (n = 23; ≤18 y of age) were recruited from 1996 to 2002 from a Johns Hopkins Hospital clinic providing prenatal care to adolescents. Of the 23 adolescents, all were non-Hispanic; 20 were African American, and 3 were white. Adolescents were eligible to participate if they had a parity of 0 and intended to breastfeed their infant. Characteristics of the population at this clinic have been previously reported (27–29). The Johns Hopkins University Institutional Review Board approved the protocol, and informed written consent was obtained from all participants. Data on calcium absorption in this cohort have been published (30).
In the California cohort, women (n = 13; 25–34 y of age) were recruited from the San Francisco Bay area from 1991 to 1993. Women were not eligible to participate if they were vegetarian, their prepregnancy BMI (in kg/m2) was <17 or >27, their calcium intake was <800 mg/d, or they consumed >3 caffeine-containing beverages daily. All women were white, 2 women were Hispanic (15%), and all were of upper-middle-income economic status. Informed written consent was obtained, and the study was approved by the Committee of Human Subjects at the University of California. Calcium absorption data from this cohort have been published (25).
In the Brazil cohort, pregnant women (n = 10; 20–35 y of age) were recruited from the Maternidade Escola of the Federal University of Rio de Janeiro from 1997 to 1999. All women were Hispanic, 4 women were white, and 6 were of African ethnicity; all were of low socioeconomic status. The study was approved by the Committee of Human Subjects at the University of California and by the Ethical Committee of the Maternidade Escola of the Federal University of Rio de Janeiro, Brazil. Data on calcium absorption and rates of bone calcium turnover from this cohort have been previously published (26, 31).
Calcium kinetic studies
Studies were undertaken in a research unit after an overnight fast. Baseline anthropometric measures were obtained, fasting blood was collected, and women were given a standardized breakfast. In the Baltimore cohort, breakfast was self-selected to provide approximately one-third of the habitual daily calcium intake; in the California cohort, breakfast consisted of a toasted English muffin and peanut butter; and in the Brazil cohort, breakfast consisted of white bread, butter, milk, and coffee. Midway through breakfast, women ingested an oral stable calcium isotope. Baltimore adolescents received either 46Ca (0.0075 μmol/kg) or 44Ca (0.005 mmol/kg) in ∼60 mL milk; California women received 44Ca (0.43 ± 0.05 mmol) in a small glass of a nonnutritive beverage (Crystal Light; Kraft General Foods); and Brazil women received 46Ca (0.25 μmol) in water. After breakfast, each woman received an intravenous injection of 42Ca. Intravenous amounts of stable calcium isotope given varied in relation to the analytic precision of the mass spectrometer and because of the increased quantity of stable calcium isotope required in adolescents (32). Intravenous amounts of stable calcium isotope administered were 0.025 mmol/kg or ∼1.75 mmol (Baltimore cohort), 0.12 ± 0.03 mmol (California cohort), and 0.12 mmol (Brazil cohort). In all studies, timed blood samples were collected for 8 h after calcium isotope administration, a complete 24-h urine collection was obtained after administration, and then 3 daily spot urine samples were obtained 120 h after administration. In the Baltimore cohort, studies were undertaken during the third trimester of pregnancy (32–36 wk gestation) and in the early postpartum period (3–7 wk postpartum). In the California cohort, studies were undertaken at prepregnancy, during the second trimester (23–26 wk gestation), during the the third trimester (34–36 wk gestation), and in the early postpartum period (6–10 wk postpartum). In the Brazil cohort, studies were undertaken during the first (10–12 wk gestation) and third (34–36 wk gestation) trimesters of pregnancy and again in the postpartum period (7–8 wk postpartum).
Of the 23 teens in the Baltimore cohort, 15 returned for the postpartum study (3–7 wk after delivery). Of these, 8 of 15 (53%, all of whom were African American) were either partially (n = 4) or exclusively (n = 4) breastfeeding. In the California cohort, 10 women returned for the postpartum study (6–10 wk postdelivery). In the California cohort, 6 participants were exclusively breastfeeding and 4 were partially breastfeeding at postpartum. In the Brazil cohort, all women returned for the postpartum study from 7 to 8 wk after delivery. All Brazil women were exclusively breastfeeding at postpartum.
Dietary intakes
The Baltimore adolescents were inpatients over the entire 120-h study. All dietary intakes were weighed before and after the study and analyzed for nutrient content with the use of the Minnesota Nutrient Database System (version 2.91; University of Minnesota). In the California cohort, a standardized diet was provided on the day before dosing, and a standardized breakfast, morning snack, and lunch were provided on the day of dosing. After lunch, California women adhered to their usual calcium intakes, and weighed food intakes were collected on 2 weekdays and on 1 weekend. Diet analysis was undertaken by using the Nutritionist III database (software version 8.5, Nutritionist III; N-Squared Computing). In the Brazil cohort, women were inpatients for 24 h after dosing then maintained on their usual calcium intakes after discharge. Dietary intakes in the Brazil cohort were weighed for 3 consecutive days before the study and were analyzed by using a Brazilian adaptation of the Food Processor nutrient database (33).
Breast-milk calcium losses
The calcium content of the breast milk was measured in the Baltimore cohort by the nursing staff who weighed the infant before and after every feeding over one 24-h interval. The weight of milk produced was multiplied by the typical calcium content of breast milk (260 mg/L) (3). Of the 8 lactating adolescents, one did not complete the 120-h kinetic study; therefore, data from this individual were not available. In the California cohort, breast-milk intake was quantified by test-weighing the infant and by analysis of total calcium content of a complete 24-h breast-milk collection (25). In the Brazil cohort, breast-milk calcium was estimated (200 mg/d) by using Brazilian data from similar stages of lactation (34).
Stable-isotope analysis and calculations
Calcium isotopes were measured in the Baltimore and Brazil cohorts by using magnetic sector thermal ionization mass spectrometry (Triton TI mass spectrometer; Thermoquest), and an earlier model of this mass spectrometer (MAT 261; Thermoquest) was used for the California cohort. The ratio of each administered tracer to either 48Ca or 43Ca was measured, and the degree to which this ratio increased over the natural abundance ratio was calculated (35).
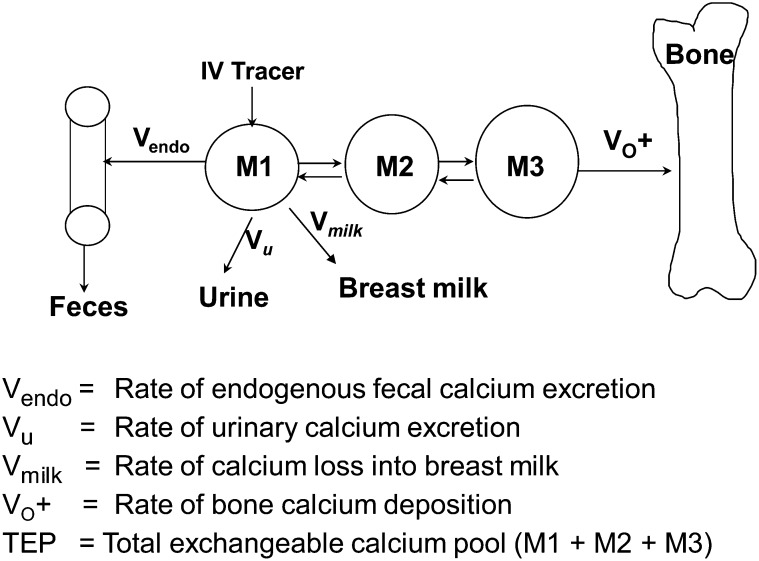
Rates of bone calcium deposition (VO+) were determined by the same individual (KOO) by using a multicompartmental model (Figure 1) and the Simulation, Analysis, and Modeling program as previously detailed (36). VO+ was obtained by longitudinal measures of the change in the enrichment of the intravenous tracer in serum and urine over the 120-h postdosing period, after urinary and endogenous fecal calcium losses (and breast-milk calcium losses in lactating subjects) were accounted for. VO+ was calculated as the product of the mass of compartment M3 multiplied by the fractional transfer coefficient of calcium loss from this compartment. We chose not to measure rates of bone calcium resorption in this study because this indirect measure is dependent on calcium absorption data and the variable study designs would limit interpretation between cohorts.
FIGURE 1.
To assess VO+ and calcium absorption longitudinally across pregnancy and lactation, a stable calcium isotope (42Ca) was injected directly into compartment M1, which represents the mass of rapidly exchanging calcium pool. Clearance of the intravenous tracer in serum and urine over the 120-h interval postdosing was used to assess VO+. Compartments M2 and M3 are additional pools of calcium that represent time intermediaries in bone matrix formation. During pregnancy, VO+ reflects the loss of calcium into both maternal and fetal bone. During lactation, additional calcium loss from compartment M1 is added into the model to account for calcium loss into breast milk. The TEP reflects the pool sizes of compartments M1, M2, and M3. The fraction of the TEP that is used for bone calcium deposition is defined as VO+/TEP.
The total exchangeable pool (TEP) reflects the mass of calcium in metabolically active bone or along bone surfaces, which are in equilibrium with plasma and extracellular fluid. TEP was calculated as the sum of compartments M1, M2, and M3. The metabolic activity of bone was estimated on the basis of the amount of calcium transferred to bone as a fraction of the TEP (VO+/TEP) (37, 38).
Endogenous fecal calcium losses were set at 1.5 mg · kg−1 · d−1. These losses are similar in adults and adolescents (39–41) and appear to be unaffected by pregnancy (42). Estimates of these losses will affect measures of net skeletal retention and bone calcium resorption but only minimally affect VO+ as determined by following the disappearance of intravenous isotope over the 120-h interval after dosing. Calcium in 24-h urine was measured by using atomic absorption spectrophotometry.
Hormone analysis
In the Baltimore cohort, 25(OH)D (sensitivity: 1.5 ng/mL; expected normal values: 9–37.6 ng/mL) and 1,25(OH)2D (sensitivity: 2 pg/mL; 95% CI: 25.1–66.1 pg/mL) were measured by using radioimmunoassays (Diasorin Inc); PTH analyses were not undertaken. In the California cohort, 1,25(OH)2D was measured by using a radioreceptor assay (Nichols Institute; 95% CI: 18–62 pg/mL; sensitivity: 2 pg/mL), serum 25(OH)D was determined by using a competitive protein binding assay (Nichols Institute; normal range: 16–74 ng/mL; sensitivity: 2.2 ng/mL), and PTH was measured by using an intact PTH assay (sensitivity: 2.82 pg/mL; Allegro; Nichols Institute) (25). In the Brazil cohort, 1,25(OH)2D was measured by using the same assay as for the California cohort (Nichols Institute Diagnostics), PTH was measured by ELISA (sensitivity: 1 pg/mL; normal range: 13–176 pg/mL; Diagnostic Systems Laboratories), and serum 25(OH)D was not measured.
Statistical analyses
Data were analyzed by using SAS (version 9.2, 2007; SAS Institute). Descriptive statistics were computed for each of the variables by cohort. Initial analyses were based on analysis of variance models with repeated measures to assess relative differences between stages of pregnancy and during the postpartum period within each cohort. ANOVA models were then used along with the Tukey-Kramer method of adjustment for multiple comparisons to compare differences in study variables between cohorts at each stage of pregnancy and postpartum. Multiple regression models were developed to examine potential relations between bone calcium kinetic variables and other measured variables [maternal age, race-ethnicity, prepregnancy BMI, calcium intake, 25(OH)D, 1,25(OH)2D, PTH, breastfeeding practice]. All independent variables were initially included in the multiple regression models, and those that were nonsignificant were sequentially removed by using backward elimination. Variables remaining in the model were significant at the P < 0.10 level. Significance was assessed at the P < 0.05 level and considered to be borderline if P values were between 0.05 and 0.10.
RESULTS
Prepregnancy height did not significantly differ between groups, and mean (±SD) values were 162.8 ± 5.1 cm, 159.6 ± 6.4 cm, and 164.1 ± 7.4 cm in the Baltimore, Brazil, and California cohorts, respectively. Prepregnancy weight also did not significantly differ between groups and averaged 62.8 ± 12.7 kg, 60.3 ± 7.2 kg, and 58.6 ± 9.5 kg in the Baltimore, Brazil, and California cohorts, respectively. Mean weight gain (and range in weight gain) over pregnancy was 16.8 ± 5.6 kg (8–28 kg) and 13.3 ± 4.0 kg (6.5–18.9 kg) in the California and Brazil cohorts, respectively. Total weight gain over pregnancy was not available for the Baltimore adolescents.
At entry into the study, the entire Baltimore cohort was nulliparous, non-Hispanic, and aged ≤18 y. Nearly all (87%) adolescents were African American, and they had calcium intakes similar to those of individuals in the California cohort (∼1200 mg/d). Adolescents were recruited into the study only if they were nulliparous to avoid additional challenges to their calcium stores, which may have been depleted if they had more than one child during their adolescent years. Parity was not used as an inclusion or exclusion criterion in the adult cohorts. The entire California cohort was white; 15% were Hispanic. The majority (77%) of women were nulliparous at recruitment into the study. The entire Brazil cohort was Hispanic, and most were of African ethnicity (60%), were multiparous (90%), and had a mean calcium intake of ∼450 mg/d. All of the adult women breastfed their infants in the postpartum period compared with only 53% of the adolescents.
Serum 25(OH)D did not change from pregnancy to postpartum and did not differ significantly between the Baltimore (latitude 39°N; 48.2 ± 24.2 nmol/L) and California (latitude 37°N; 55.8 ± 22.5 nmol/L) cohorts; serum 25(OH)D was not measured in the Brazil cohort (latitude 23°S) (Table 1). In the Baltimore cohort, 22% and 40% of teens had 25(OH)D concentrations <30 nmol/L at the third trimester and postpartum, respectively (the Institute of Medicine cutoff at which individuals are at an increased risk of deficiency) (43). In the California cohort, 25(OH)D concentrations <30 nmol/L were found in 15% of women both at the third trimester and postpartum. Concentrations of 25(OH)D in the Baltimore and California cohorts were not significantly associated with 1,25(OH)2D during pregnancy or lactation. Despite the small sample size and limited racial variability in the Baltimore cohort, serum 25(OH)D was significantly lower by −46 ± 10 (SE) nmol/L (P = 0.0001) in African American (n = 20) than in white (n = 3) teens at T3 and by −54 ± 14 (SE) nmol/L (P = 0.002) in African American (n = 13) than in white (n = 2) teens during the early postpartum period.
TABLE 1.
Calcium kinetic measurements during pregnancy and postpartum by study cohort1
| Variable and study cohort | Prepregnancy/first trimester | Third trimester | Postpartum |
| Age at entry in study (y) | |||
| BA | — | 16.5 ± 1.4 | — |
| CA | 29.5 ± 2.6a | — | — |
| BR | 30.4 ± 4.0a | — | — |
| BMI (kg/m2) | |||
| BA | 23.8 ± 2.9b,A | — | 26.4 ± 6.1b,B |
| CA | 22.5 ± 2.7a,A | — | 24.3 ± 3.7a,B |
| BR | 22.3 ± 2.7a,A | — | 24.6 ± 2.5a,B |
| Urinary calcium (mg/d) | |||
| BA | — | 257 ± 85a,B | 62 ± 33a,A |
| CA | 173 ± 88b,B | 249 ± 149a,C | 75 ± 49a,A |
| BR | 296 ± 136a,C | 216 ± 124a,B | 94 ± 44a,A |
| Fractional calcium absorption (%) | |||
| BA | — | 52.6 ± 15.2a,A | 29.6 ± 10.8a,B |
| CA | 32.5 ± 9.4a,A | 53.4 ± 11.6a,B | 35.5 ± 10.1a,A |
| BR | 70.9 ± 15.7b,A | 87.6 ± 13.4b,B | 67.5 ± 19.1b,A |
| Endogenous fecal calcium (mg/d) | |||
| BA | — | 114 ± 28a,A | 106 ± 25a,A |
| CA | 91 ± 11a,A | 113 ± 13a,A | 98 ± 12a,A |
| BR | 89 ± 13a,A | 105 ± 14 a,A | 94 ± 14a,A |
| 25(OH)D (nmol/L) | |||
| BA | — | 50.5 ± 22.2 a,A | 45.8 ± 26.2a,A |
| CA | 62.8 ± 17.5A | 52.0 ± 24.5a,A | 52.5 ± 25.5a,A |
| BR | — | — | — |
| 1,25(OH)2D (pmol/L) | |||
| BA | — | 252 ± 104a,B | 95 ± 22b,A |
| CA | 108 ± 35a,A | 230 ± 90a,B | 106 ± 41a,b,A |
| BR | 160 ± 60a,A | 225 ± 48a,B | 134 ± 44a,A |
| PTH (pmol/L) | |||
| BA | — | — | — |
| CA | 3.4 ± 1.4 | 2.6 ± 1.5 | 3.1 ± 1.9 |
| BR | 1.2 ± 0.6A | 1.3 ± 0.6A,B | 1.7 ± 0.6B |
| VO+ (mg/d) | |||
| BA | — | 1422 ± 364a,B | 1162 ± 353a,A |
| CA | 428 ± 141a,A | 929 ± 274b,C | 644 ± 83b,B |
| BR | 461 ± 170a,A | 1015 ± 305b,C | 737 ± 139a,B |
| TEP (mg) | |||
| BA | — | 5314 ± 1085b,B | 4705 ± 1170a,A |
| CA | 4531 ± 1914a,A | 5209 ± 1009a,b,B | 4470 ± 1026a,A |
| BR | 3918 ± 600a,A | 4296 ± 796a,A | 3943 ± 841a,A |
| VO+/TEP | |||
| BA | — | 0.27 ± 0.06a,A | 0.26 ± 0.08a,A |
| CA | 0.11 ± 0.04a,A | 0.19 ± 0.07b,C | 0.15 ± 0.03b,B |
| BR | 0.12 ± 0.05a,A | 0.24 ± 0.06a,b,B | 0.19 ± 0.04b,B |
| Breast-milk calcium (mg/d) | |||
| BA | 158 ± 69a | ||
| CA | — | — | 237 ± 85a |
| BR | — | — | — |
All values are means ± SDs. Values within a column with different superscript lowercase letters are significantly different, P < 0.05 (ANOVA followed by Tukey-Kramer test). Values within a row with different superscript uppercase letters are significantly different, P < 0.05 (repeated-measures ANOVA). BA, Baltimore (n = 23; the sample size for the BA cohort during the postpartum period was n = 15); BR, Brazil (n = 10); CA, California (n = 13); PTH, parathyroid hormone; TEP, total exchangeable pool; VO+, bone calcium deposition rate; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Serum 1,25(OH)2D increased across pregnancy in the California (P < 0.001) and Brazil (P < 0.02) adult groups and decreased significantly from the third trimester to the postpartum period in all 3 cohorts (P < 0.001) (Table 1). The 1,25(OH)2D concentrations between groups did not significantly differ during pregnancy despite the variable calcium intake, different analytic approaches, and younger age of the Baltimore cohort. At postpartum, the Brazil cohort exhibited higher 1,25(OH)2D concentrations compared with the Baltimore and California groups, but the difference was significant only in relation to the Baltimore group (P < 0.05).
Serum PTH concentrations did not significantly differ across the 3 periods in the California cohort, and no significant correlations were noted between PTH and other variables measured in the California group. Among the Brazil women, PTH was significantly higher at postpartum compared with the first trimester (1.7 ± 0.6 compared with 1.2 ± 0.6 pmol/L; P < 0.02). Simple correlations between biochemical measures and maternal characteristics were examined in each physiologic state within each cohort and in the group as a whole. Of interest, among Brazil women, PTH was positively associated with dietary calcium at the first trimester (r = 0.779, P = 0.008) and postpartum (r = 0.733, P = 0.016) and negatively associated with 1,25(OH)2D at postpartum (r = −0.722, P = 0.018). Serum 1,25(OH)2D was negatively associated with dietary calcium intake at postpartum when all groups were combined (r = −0.325, P = 0.05).
Among the adult subjects at trimester 3, VO+ was not significantly different between California and Brazil women despite the variable calcium intake between cohorts (Table 1). In the Baltimore adolescents, VO+ was significantly higher (P < 0.05) than that observed among the California or Brazil cohorts. In all groups, VO+ was significantly higher at trimester 3 than postpartum (P < 0.05).
In the combined adult cohorts (California and Brazil), mean VO+ increased by 119% from prepregnancy/trimester 1 to trimester 3 (P < 0.01). Despite the differing physiologic stage at which the first study measure was obtained, the magnitude of increase in VO+ observed during trimester 3 was similar in the California and Brazil groups. Moreover, in the combined adult groups, the relative fraction of TEP used for bone deposition (VO+/TEP) increased by 86% during pregnancy (P < 0.05).
In the combined adult cohorts, VO+ decreased by 29% from trimester 3 to the postpartum period (P < 0.05), and similarly among the Baltimore teens VO+ decreased by 18% from the third trimester to the postpartum period (P < 0.008). In the adult women (California and Brazil), VO+/TEP values decreased by 21% from the third trimester to the postpartum period (P < 0.05), but this did not significantly differ from the third trimester to the postpartum period among the adolescent Baltimore cohort.
Multiple regression analysis of determinants of calcium kinetics
Potential relations between calcium kinetic variables and maternal variables within each cohort and in the group as a whole were examined (Table 2). In the Baltimore cohort at trimester 3, both age (across the age range of 13.5–18.3 y; P = 0.096) and 1,25(OH)2D (P = 0.059) exhibited nonsignificant trends for inverse associations with VO+. Similarly, VO+/TEP was inversely associated with 1,25(OH)2D (P = 0.003) and positively associated with dietary calcium (P = 0.004). During the postpartum period, VO+ was positively related to dietary calcium intake (P = 0.002) and 25(OH)D (P = 0.047), and it was higher among African Americans (P = 0.004), as was also seen with African ethnicity in the Brazil cohort. In addition, VO+ was negatively associated with breastfeeding (P = 0.002) (Table 2). In these adolescent women, the combined factors of dietary calcium, 25(OH)D, African ethnicity, prepregnancy BMI, and breastfeeding explained ∼84% of the variability of VO+ during the postpartum period. In the California cohort at trimester 3, there was a trend (P = 0.055) for an inverse relation between serum 25(OH)D and TEP. At postpartum, 1,25(OH)2D was positively related to TEP (P = 0.044), and there was a nonsignificant trend for a positive association between 25(OH)D and TEP (P = 0.096) (Table 2).
TABLE 2.
Multiple regression analysis of calcium kinetic measures1
| Prepregnancy/first trimester | Third trimester | Postpartum | |
| VO+ (mg/d) | |||
| BA | — | R2 = 0.25 1,25(OH)2D [0.059]* Age [0.096]* | R2 = 0.84 Calcium [0.002] Breastfeeding [0.002]* Ethnicity [0.004] Prepregnancy BMI [0.035]* 25(OH)D [0.047] |
| CA | R2 = 0.59 Calcium [0.016] | None | None |
| BR | R2 = 0.59 Calcium [0.019]Prepregnancy BMI [0.06] | R2 = 0.96 Ethnicity [0.074]* Prepregnancy BMI [0.062] 1,25(OH)2D [0.008]* PTH [0.003] | R2 = 0.99 Ethnicity [0.001] Prepregnancy BMI [0.016] 1,25(OH)2D [0.038]* Calcium [0.0001] PTH [0.0004]* |
| Combined groups | — | R2 = 0.37 Age [<0.0001]* 1,25(OH)2D [0.029]* | R2 = 0.69 Ethnicity [<0.0001] Calcium [0.0003] Breastfeeding [0.0012]* |
| TEP (mg) | |||
| BA | — | R2 = 0.16 Prepregnancy BMI [0.064] | R2 = 0.46 Ethnicity [0.022]* 1,25(OH)2D [0.090]* |
| CA | None | R2 = 0.39 25(OH)D [0.055]* | R2 = 0.58 1,25(OH)2D [0.044] 25(OH)D [0.096] |
| BR | R2 = 0.59 1,25(OH)2D [0.048] Prepregnancy BMI [0.063]* Ethnicity [0.089] | R2 = 0.62 Ethnicity [0.021]* Prepregnancy BMI [0.055] | R2 = 0.55 Prepregnancy BMI [0.023] Ethnicity [0.079]* |
| Combined groups | — | R2 = 0.24 Age [0.010]* Ethnicity [0.056]* Prepregnancy BMI [0.027] | R2 = 0.25 Calcium [0.032] Breastfeeding [0.036]* |
| VO+/TEP | |||
| BA | — | R2 = 0.591,25(OH)2D [0.003]*Calcium [0.004]Prepregnancy BMI [0.002]* | R2 = 0.31 Ethnicity [0.048] |
| CA | R2 = 0.36 Calcium [0.089] | R2 = 0.45 25(OH)D [0.035] | None |
| BR | None | R2 = 0.64 1,25(OH)2D [0.043]* PTH [0.052] | R2 = 0.62 Ethnicity [0.015] Prepregnancy BMI [0.020]* |
| Combined groups | — | R2 = 0.32 Ethnicity [0.002] 1,25(OH)2D [0.018]* Prepregnancy BMI [0.060]* | R2 = 0.42 Ethnicity [<0.0001] |
P values are shown in brackets. Each bone calcium kinetic measurement (dependent variable) was initially related to the following maternal independent variables: age, ethnicity (African compared with non-African), breastfeeding (yes or no), prepregnancy BMI, serum 1,25(OH)2D, serum 25(OH)D, serum PTH, and dietary calcium intake. Independent variables were sequentially removed by backward elimination based on P values. Variables left in the model were significant at the P < 0.10 level. *Denotes a significant inverse association between variables. BA, Baltimore (n = 23; the sample size for the BA cohort during the postpartum period was n = 15); BR, Brazil (n = 10); CA, California (n = 13); PTH, parathyroid hormone; TEP, total exchangeable pool; VO+, bone calcium deposition rate; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
In the Brazil women at the first trimester, significant positive relations between dietary calcium and VO+, and between 1,25(OH)2D and TEP, were observed. There was a nonsignificant trend for African ethnicity to be positively associated with TEP (P = 0.089). By the third trimester, VO+ was significantly negatively associated with 1,25(OH)2D (P = 0.008) and positively associated with PTH (P = 0.003). African ethnicity was significantly negatively associated with TEP at trimester 3 (P = 0.021). At postpartum, VO+ was significantly positively related to dietary calcium (P = 0.0001) and African ethnicity (P = 0.001) and negatively related to PTH (P < 0.001) and 1,25(OH)2D (P = 0.038) (Table 2). In these adult women, the combined factors of 1,25(OH)2D, PTH, African ethnicity, and prepregnancy BMI explained ∼96% of the variability of VO+ at the third trimester, and with the addition of dietary calcium explained ∼99% of the variability of VO+ at postpartum.
Pooled analysis of all trimester 3 cohorts found VO+ to be significantly higher among adolescents (1422 ± 364 mg/d; n = 23) compared with adults (966 ± 287 mg/d; n = 23) (P < 0.001) (Table 2). In the pooled data at trimester 3, serum 1,25(OH)2D was significantly inversely associated with both VO+ (P < 0.03) and with VO+/TEP (P < 0.02).
A pooled analysis at postpartum showed that serum 1,25(OH)2D was no longer significantly associated with VO+ or VO+/TEP; instead, VO+ was significantly positively associated with dietary calcium intake (P < 0.0001). African ethnicity was significantly positively related and breastfeeding was significantly negatively related to VO+ at postpartum (P < 0.001).
DISCUSSION
The heterogeneity of the 3 pregnant and postpartum cohorts studied provided an opportunity to assess common relations while also identifying notable differences in determinants of VO+ across pregnancy and the early postpartum period. In the adult cohorts studied, 1,25(OH)2D increased significantly by 40–50% from early/prepregnancy to the third trimester. In all pregnant cohorts, 1,25(OH)2D concentrations during the third trimester were significantly higher than those observed during the early postpartum period. Comparable pregnancy-related increases in 1,25(OH)2D over the course of gestation and similar concentrations during the third trimester have been noted in several other studies in adult pregnant women (13–17). At present, functional outcomes associated with these increases remain poorly characterized.
Although recent longitudinal human studies have noted elevated concentrations of 1,25(OH)2D during pregnancy (17, 20), few data on determinants of these increases and their relation to VO+ are available. Synthesis of 1,25(OH)2D is known to increase in response to suboptimal calcium intakes. Both the adolescents and Brazil women had physiologic challenges that may have affected calcium homeostasis across gestation and lactation. The Brazil women went through pregnancy with calcium intakes of 450 mg/d; <5% of US pregnant women have similarly low calcium intakes (43). The Baltimore teens ingested more than twice the calcium but needed to provide 20–30 g Ca for fetal skeletal mineralization before the age at which peak bone mass is achieved. Despite the highly variable calcium intakes, ages studied, and variable analytic methods used, mean 1,25(OH)2D concentrations during pregnancy did not significantly differ between cohorts and in the group as a whole at late pregnancy, and there was no significant relation between dietary calcium and 1,25(OH)2D. The mean concentrations of 1,25(OH)2D observed in these women were comparable to those recently reported in pregnant women receiving 400 IU vitamin D3 (17). In our study population, serum 1,25(OH)2D was negatively associated with dietary calcium intake in the group as a whole only during the postpartum period.
The systemic and cellular effects of elevated 1,25(OH)2D concentrations on calcium homeostasis during pregnancy have yet to be fully elucidated as a function of age, race, and habitual calcium intake. Recent data by Hollis et al (17) found that supplementation with vitamin D3 resulted in significant increases in 1,25(OH)2D throughout pregnancy, but no data on maternal or fetal skeletal outcomes were reported. Among our group as a whole, elevated 1,25(OH)2D during late pregnancy was associated with significantly lower VO+ and a significantly lower fraction of TEP being used for VO+. However, without concurrent data on bone calcium resorption, we were unable to determine whether the change in VO+ was associated with net differences in maternal or fetal calcium retention. In this study population, we previously reported a significantly positive association between 1,25(OH)2D and calcium absorption in both the California (25) and Brazil women (31), but similar serum 1,25(OH)2D concentrations among the Baltimore adolescents were not significantly associated with calcium absorption in this age group (30). In our group as a whole, elevated 1,25(OH)2D was associated with significantly increased calcium absorption only during the postpartum period. We did not have information on vitamin D binding protein in all women and were therefore unable to assess relative contributions of free compared with bound 1,25(OH)2D to observed outcomes. Genetic polymorphisms in genes involved in the vitamin D pathway are also known to affect 25(OH)D among African Americans (44) and others (45); their potential impact on variable responses to the physiologic challenges of pregnancy and lactation merits further study.
Dietary calcium intake had differential effects on VO+ between late pregnancy and lactation. During the third trimester, 1,25(OH)2D was a significant determinant of VO+, and dietary calcium intake at the third trimester was not significantly related to VO+, maternal 1,25(OH)2D, or PTH. A similar lack of a relation between dietary calcium intake and either 1,25(OH)2D (20) or bone turnover markers at either mid- or late gestation (46) has recently been reported in a cohort of 171 pregnant adolescents. In contrast, in each of our study groups at postpartum and in the entire cohort, dietary calcium intake was significantly positively associated with increased VO+. Differences in study designs and oral tracer administration between sites precluded us from analyzing bone calcium resorption and the degree to which the measured changes in bone calcium deposition were associated with skeletal calcium retention. Dietary calcium intake appears to have a differential impact on skeletal turnover between late pregnancy and early lactation. More data on these associations are needed as a function of habitual diet, race, and vitamin D status. Recent Gambian data showed that calcium supplementation of women with habitually low calcium intakes did not improve skeletal calcium retention across pregnancy but was instead associated with a significant decrease in postpartum bone mass at the hip (47).
In both adult groups of our study, and in the adolescents, VO+ was significantly lower during the postpartum period than during pregnancy, but this decrease was less pronounced in the adolescents. In the postpartum period, adolescents had significantly higher VO+ than did adult California women with similar calcium intakes, but VO+ was not significantly higher than that observed among the Brazil women postpartum. In the postpartum group as a whole, VO+ was significantly inversely associated with dietary calcium intake. Previous kinetic data on VO+ among lactating women consuming low- (n = 4) or high- (n = 4) calcium diets found that VO+ tended to be lower in the group with a low calcium intake, but this difference was not significant (P = 0.12) (48). In the present study, all of the adult women were breastfeeding so the impact of lactation alone on VO+ could not be assessed among these cohorts. However, teens who breastfed partially or exclusively had significantly lower VO+ when compared with those who formula fed their infants.
Both higher bone calcium retention and increased VO+ have been noted in both African American children and adults in comparison with other ethnic groups (49). A positive effect of African ethnicity on VO+ was evident during the postpartum period but not during pregnancy in our study group. At postpartum, African ethnicity was associated with significantly higher VO+, independent of age, calcium intake, and breastfeeding status. Racial differences in VO+ were less evident during pregnancy, which may suggest that during gestation physiologic mechanisms of calcium and bone homeostasis prevail over genetic/lifestyle factors. Whether the racial differences noted at postpartum translate into differences in bone mass across lactation in women of African ethnicity is not known.
The TEP represents the readily exchangeable fraction of the miscible calcium pool. As expected, TEP was significantly higher during pregnancy than during the postpartum period in the California and Brazil groups. It was also significantly higher among the adolescents (Baltimore) compared with the adult Brazil women at the third trimester. In all pregnant women combined, the size of this exchangeable calcium compartment was not significantly affected by 1,25(OH)2D or calcium intake. There were notable differences in VO+ as a fraction of TEP. During pregnancy in the group as a whole, a significant inverse association was evident between 1,25(OH)2D and VO+/TEP, which suggested that less miscible calcium was being used for VO+ in those with elevated 1,25(OH)2D.
During pregnancy, VO+ reflects calcium deposition into maternal and/or fetal bone; relative partitioning between the two cannot be determined. Another study limitation is the lack of standard reference materials to allow for standardization of biochemical measures between sites. Standard reference materials are now available for 25(OH)D but not for calcitriol or PTH. Despite these analytic limitations, remarkably similar trends and associations with 1,25(OH)2D were noted between sites.
In summary, higher serum 1,25(OH)2D concentrations were associated with lower VO+ during pregnancy; additional work is needed to identify determinants and functional maternal or fetal outcomes associated with 1,25(OH)2D concentrations across pregnancy. During pregnancy, VO+ was largely independent of calcium intake but was influenced by maternal age. In contrast, during the early postpartum period, higher dietary calcium intakes were significantly associated with higher VO+, and VO+ varied as a function of ethnicity and lactation. These findings highlight the need for future research that assesses the determinants of whole-body calcium dynamics and their impact on net skeletal balance across the reproductive period.
Acknowledgments
We thank the study participants for volunteering to participate in these longitudinal studies and the nursing staff at each of the clinical centers for their support of the work. We also thank Stephanie Atkinson for helpful feedback and advice on the interpretation of these data.
The authors’ responsibilities were as follows—KOO, CMD, LDR, SAA, and JCK: designed the research and performed the experiments; and GG and CMD: were responsible for the statistical analyses. All of the authors assisted in the interpretation of the data and in the writing of the manuscript. None of the authors had any conflicts of interest to disclose.
Footnotes
Abbreviations used: PTH; parathyroid hormone; TEP, total exchangeable pool; VO+, rate of bone calcium deposition; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Sparks JW. Human intrauterine growth and nutrient accretion. Semin Perinatol 1984;8:74–93 [PubMed] [Google Scholar]
- 2.Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab 2001;86:2344–8 [DOI] [PubMed] [Google Scholar]
- 3.Schanler RJ, Oh W. Composition of breast milk obtained from mothers of premature infants as compared to breast milk obtained from donors. J Pediatr 1980;96:679–81 [DOI] [PubMed] [Google Scholar]
- 4.Abrams SA, O'Brien KO, Liang LK, Stuff JE. Differences in calcium absorption and kinetics between black and white girls aged 5-16 years. J Bone Miner Res 1995;10:829–33 [DOI] [PubMed] [Google Scholar]
- 5.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, Smith DL, Peacock M, Weaver CM. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab 2003;88:1043–7 [DOI] [PubMed] [Google Scholar]
- 6.Bezerra FF, Laboissiere FP, King JC, Donangelo CM. Pregnancy and lactation affect markers of calcium and bone metabolism differently in adolescent and adult women with low calcium intakes. J Nutr 2002;132:2183–7 [DOI] [PubMed] [Google Scholar]
- 7.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res 2000;15:557–63 [DOI] [PubMed] [Google Scholar]
- 8.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res 2000;15:129–37 [DOI] [PubMed] [Google Scholar]
- 9.Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet Gynecol 2000;96:189–93 [DOI] [PubMed] [Google Scholar]
- 10.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond) 1998;94:405–12 [DOI] [PubMed] [Google Scholar]
- 11.Prentice A. Calcium in pregnancy and lactation. Annu Rev Nutr 2000;20:249–72 [DOI] [PubMed] [Google Scholar]
- 12.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med 1997;337:523–8 [DOI] [PubMed] [Google Scholar]
- 13.Ardawi MS, Nasrat HA, BA'Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997;137:402–9 [DOI] [PubMed] [Google Scholar]
- 14.Uemura H, Yasui T, Kiyokawa M, Kuwahara A, Ikawa H, Matsuzaki T, Maegawa M, Furumoto H, Irahara M. Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J Endocrinol 2002;174:353–9 [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest 1979;63:342–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddow JE, Neveux LM, Palomaki GE, Lambert-Messerlian G, Canick JA, Grenache DG, Lu J. The relationship between PTH and 25-hydroxy vitamin D early in pregnancy. Clin Endocrinol (Oxf) 2011;75:309–14 [DOI] [PubMed] [Google Scholar]
- 17.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seely EW, Brown EM, DeMaggio DM, Weldon DK, Graves SW. A prospective study of calciotropic hormones in pregnancy and post partum: reciprocal changes in serum intact parathyroid hormone and 1,25-dihydroxyvitamin D. Am J Obstet Gynecol 1997;176:214–7 [DOI] [PubMed] [Google Scholar]
- 19.Okonofua F, Menon RK, Houlder S, Thomas M, Robinson D, O'Brien S, Dandona P. Calcium, vitamin D and parathyroid hormone relationships in pregnant Caucasian and Asian women and their neonates. Ann Clin Biochem 1987;24:22–8 [DOI] [PubMed] [Google Scholar]
- 20.Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O'Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with PTH and calcitriol in pregnant adolescents. J Bone Miner Res 2011;Sep 28 (Epub ahead of print; doi: 10.1022/jbmr.526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr 2011;31:89–115 [DOI] [PubMed] [Google Scholar]
- 22.Chantry CJ, Auinger P, Byrd RS. Lactation among adolescent mothers and subsequent bone mineral density. Arch Pediatr Adolesc Med 2004;158:650–6 [DOI] [PubMed] [Google Scholar]
- 23.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr 1995;61:514–23 [DOI] [PubMed] [Google Scholar]
- 24.Kent GN, Price RI, Gutteridge DH, Rosman KJ, Smith M, Allen JR, Hickling CJ, Blakeman SL. The efficiency of intestinal calcium absorption is increased in late pregnancy but not in established lactation. Calcif Tissue Int 1991;48:293–5 [DOI] [PubMed] [Google Scholar]
- 25.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 1998;67:693–701 [DOI] [PubMed] [Google Scholar]
- 26.O'Brien KO, Donangelo CM, Zapata CL, Abrams SA, Spencer EM, King JC. Bone calcium turnover during pregnancy and lactation in women with low calcium diets is associated with calcium intake and circulating insulin-like growth factor 1 concentrations. Am J Clin Nutr 2006;83:317–23 [DOI] [PubMed] [Google Scholar]
- 27.Chang SC, O'Brien KO, Nathanson MS, Mancini J, Witter FR. Characteristics and risk factors for adverse birth outcomes in pregnant black adolescents. J Pediatr 2003;143:250–7 [DOI] [PubMed] [Google Scholar]
- 28.Chang SC, O'Brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. Am J Clin Nutr 2003;77:1248–54 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen JN, O'Brien KO, Witter FR, Chang SC, Mancini J, Nathanson MS, Caulfield LE. High gestational weight gain does not improve birth weight in a cohort of African American adolescents. Am J Clin Nutr 2006;84:183–9 [DOI] [PubMed] [Google Scholar]
- 30.O'Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr 2003;78:1188–93 [DOI] [PubMed] [Google Scholar]
- 31.Vargas Zapata CL, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. Am J Clin Nutr 2004;80:417–22 [DOI] [PubMed] [Google Scholar]
- 32.O'Brien KO, Abrams SA. Effects of development on techniques for calcium stable isotope studies in children. Biol Mass Spectrom 1994;23:357–61 [DOI] [PubMed] [Google Scholar]
- 33.Brazilian Institute of Geography and Statistics. National Study of Family Expenses, Food Composition Tables Brazil: Brazilian Institute of Geography and Statistics, 1996. (in Portuguese) [Google Scholar]
- 34.Góes HC, Torres AG, Donangelo CM, Trugo NM. Nutrient composition of banked human milk in Brazil and influence of processing on zinc distribution in milk fractions. Nutrition 2002;18:590–4 [DOI] [PubMed] [Google Scholar]
- 35.Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB. Determination of fractional absorption of dietary calcium in humans. J Nutr 1994;124:674–82 [DOI] [PubMed] [Google Scholar]
- 36.Berman M, Weiss M. SAAM manual. Washington, DC: US Department of Health, Education and Welfare, 1978. [USDHEW Publication (NIH) 78-180.]
- 37.Abrams SA, Esteban NV, Vieira NE, Sidbury JB, Specker BL, Yergey AL. Developmental changes in calcium kinetics in children assessed using stable isotopes. J Bone Miner Res 1992;7:287–93 [DOI] [PubMed] [Google Scholar]
- 38.Yergey AL, Vieira NE, Abrams SA, Marini J, Goans RE. Use of stable isotopic tracers in studies of whole body calcium metabolism. Connect Tissue Res 1995;31:291–3 [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP, Skillman TG. Secretion and excretion of calcium by the human gastrointestinal tract. J Lab Clin Med 1964;64:29–41 [PubMed] [Google Scholar]
- 40.Abrams SA, Sidbury JB, Muenzer J, Esteban NV, Vieira NE, Yergey AL. Stable isotopic measurement of endogenous fecal calcium excretion in children. J Pediatr Gastroenterol Nutr 1991;12:469–73 [DOI] [PubMed] [Google Scholar]
- 41.Wastney ME, Ng J, Smith D, Martin BR, Peacock M, Weaver CM. Differences in calcium kinetics between adolescent girls and young women. Am J Physiol 1996;271:R208–16 [DOI] [PubMed] [Google Scholar]
- 42.Heaney RP, Skillman TG. Calcium metabolism in normal human pregnancy. J Clin Endocrinol Metab 1971;33:661–70 [DOI] [PubMed] [Google Scholar]
- 43.Institute of Medicine Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011 [PubMed] [Google Scholar]
- 44.Signorello LB, Shi J, Cai Q, Zheng W, Williams SM, Long J, Cohen SS, Li G, Hollis BW, Smith JR, et al. Common variation in vitamin D pathway genes predicts circulating 25-hydroxyvitamin D levels among African Americans. PLoS ONE 2011;6:e28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol 2010;121:471–7 [DOI] [PubMed] [Google Scholar]
- 46.Essley BV, McNanley T, Cooper EM, McIntyre AW, Witter F, Harris LZ. Osteoprotegerin (OPG) differs by race and is related to infant birth weight z-score in pregnant adolescents. J Dev Orig Health Dis 2011;2:272–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr 2010;92:450–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Specker BL, Vieira NE, O'Brien KO, Ho ML, Heubi JE, Abrams SA, Yergey AL. Calcium kinetics in lactating women with low and high calcium intakes. Am J Clin Nutr 1994;59:593–9 [DOI] [PubMed] [Google Scholar]
- 49.Walker MD, Novotny R, Bilezikian JP, Weaver CM. Race and diet interactions in the acquisition, maintenance, and loss of bone. J Nutr 2008;138:1256S–60S [DOI] [PubMed] [Google Scholar]