Abstract
Background: Putrescine, spermidine, and spermine are the polyamines required for human cell growth. The inhibition of ornithine decarboxylase (ODC), which is the rate-limiting enzyme of polyamine biosynthesis, decreases tumor growth and the development of colorectal adenomas. A database was developed to estimate dietary polyamine exposure and relate exposure to health outcomes.
Objective: We hypothesized that high polyamine intake would increase risk of colorectal adenoma and that the allelic variation at ODC G>A +316 would modify the association.
Design: Polyamine exposure was estimated in subjects pooled (n = 1164) from the control arms of 2 randomized trials for colorectal adenoma prevention [Wheat Bran Fiber low-fiber diet arm (n = 585) and Ursodeoxycholic Acid placebo arm (n = 579)] by using baseline food-frequency questionnaire data. All subjects had to have a diagnosis of colorectal adenoma to be eligible for the trial.
Results: A dietary intake of polyamines above the median amount in the study population was associated with 39% increased risk of colorectal adenoma at follow-up (adjusted OR: 1.39; 95% CI: 1.06, 1.83) in the pooled sample. In addition, younger participants (OR: 1.94; 95% CI: 1.23, 3.08), women (OR: 2.43; 95% CI: 1.48, 4.00), and ODC GG genotype carriers (OR: 1.59; 95% CI: 1.00, 2.53) had significantly increased odds of colorectal adenoma if they consumed above-median polyamine amounts.
Conclusions: This study showed a role for dietary polyamines in colorectal adenoma risk. Corroboration of these findings would confirm a previously unrecognized, modifiable dietary risk factor for colorectal adenoma.
INTRODUCTION
Polyamines (eg, putrescine, spermidine, and spermine) are small, cationic molecules that are necessary for cell growth and derived from intrinsic (ie, intracellular biosynthesis) and extrinsic (ie, diet, gut flora, sloughed cells, and digestion-related secretions) sources (Figure 1). The combination of polyamine sources results in submicromolar amounts of polyamines in sera (1–11). Polyamines move across the gastrointestinal epithelial barrier via apical transporters and accumulate in intestinal and colonic cells at near-millimolar concentrations. The transport of polyamines from intestinal epithelia to other tissues occurs via basolateral transporters (9).
FIGURE 1.
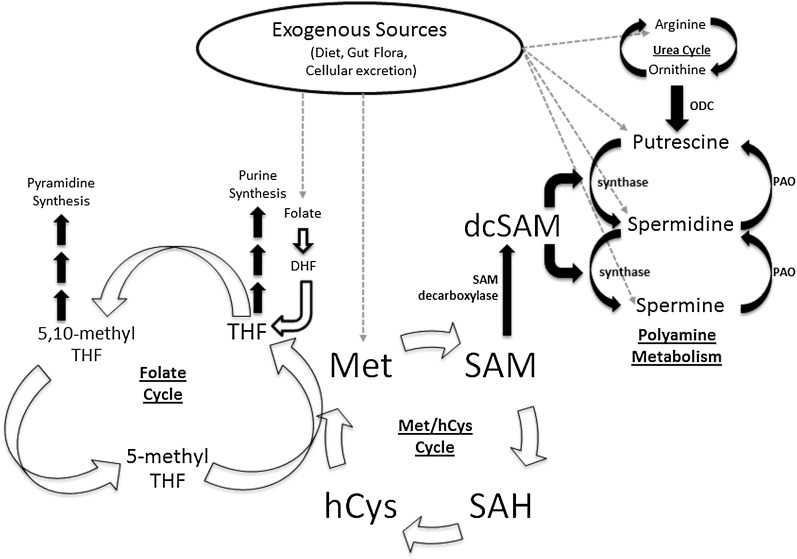
Overview of polyamine biosynthesis and dietary sources related to the polyamine pathway. Polyamines come from exogenous sources (mainly the diet), or they can be biosynthesized in the cell. Putrescine is synthesized after arginine is converted into ornithine via the urea cycle. Ornithine is then decarboxylated by the rate-limiting enzyme in polyamine synthesis, ODC. The addition of a propylamine group to putrescine gives rise to spermidine. This propylamine group is donated by dcSAM via spermidine synthase. Spermidine can be subsequently propylaminated by spermine synthase to make spermine. Spermine can be recycled back to spermidine and eventually putrescine via PAO. The polyamine cycle is closely tied to the Met cycle via SAM (20). SAM is derived from adding an adenosine group to Met and can be decarboxylated by SAM decarboxylase to form dcSAM, which is vital for polyamine biosynthesis. SAM can also be demethylated to SAH, hCys, and eventually recycled to Met. Met can also be derived from the diet. The conversion of hCys to Met is a required coreaction to the 5-methyl THF to THF. This is the link between folate and Met cycles. THF can go on to purine synthesis or be converted to 5,10-methyl THF for pyramidine synthesis. Like Met, THF can be derived from the diet. However, dietary folate must first be converted to DHF and then to THF. dcSAM, decarboxylated S-adenosylmethionine; DHF, dihydrofolate; hCys, homocysteine; Met, methionine; ODC, ornithine decarboxylase; PAO, polyamine oxidase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate.
Putrescine is the most abundant of the polyamines in humans with de novo synthesis dependent on arginine and ornithine (Figure 1). Ornithine is decarboxylated by the rate-limiting enzyme ornithine decarboxylase (ODC)4 (4, 10, 12, 13). Both spermine and spermidine are dependent on the methionine pool because decarboxylated S-adenosyl-methionine acts as the propylamine donor (4, 9, 14).
Increased polyamine synthesis is common in epithelial tumors, and the inhibition of polyamine biosynthesis has reduced tumor growth in experimental models (3, 5–7, 13). Hixson et al (15) documented that colorectal cancers display approximately double the amount of putrescine (0.17 ± 0.17 nmol/mg protein) and spermidine (0.52 ± 0.37 nmol/mg protein) than do normal rectal mucosa. In addition, the synthesis of polyamines via ODC differs between individuals as a result of G>A genetic variation at the +316 position in intron 1 of the ODC gene (rs2302615) wherein the carriage of a G allele has been associated with increased risk of colorectal adenoma (16–18). Although synthesis has been most widely studied in tumorigenesis, animal studies suggested that colonic mucosa growth is dependent on exogenous polyamines. For colon tumorigenesis, feeding to mice that have a mutation in one copy of their APC gene a diet high in putrescine reduced the efficacy of sulindac, which is a nonselective, nonsteroidal antiinflammatory drug shown to prevent tumorigenesis in this model of human carcinogenesis (19). However, despite experimental evidence, the assessment of polyamines in the human diet and exposure-related cancer risk has been largely unstudied. Zoumas-Morse et al (11) developed a dietary polyamine content database for polyamine exposure by using data from 165 subjects in whom the average exposure was 159.1 μmol putrescine/d, 54.7 μmol spermidine/d, and 35.7 μmol spermine/d. This polyamine database was designed for the estimation of individual- and population-level polyamine exposures from foods to relate exposure to health outcomes (eg, colorectal neoplasia).
Therefore, to test hypotheses about the effect of dietary polyamine exposure on colorectal neoplasia risk, we applied the polyamine-content database to food-frequency data collected from participants in control arms of 2 large, completed randomized chemoprevention trials with colorectal adenoma as an endpoint. In addition, we explored the relation between the high-risk ODC +316 genotype, dietary polyamines, and colorectal adenoma incidence.
SUBJECTS AND METHODS
Study population
Patients who had a colorectal adenoma detected at a recent qualifying examination were recruited to participate in 1 of 2 clinical trials to test the effect of an agent to prevent metachronous colorectal adenomas (ie, the development of a colorectal adenoma during follow-up). Patients were excluded if they had familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, or a strong family history of colorectal cancer (ie, in ≥2 first-degree relatives). Briefly, the Wheat Bran Fiber (WBF) Trial (recruitment of study participants began in September 1990 and was completed in July 1995) was a randomized, double-blinded, controlled trial that tested the effect of a high-fiber compared with low-fiber cereal supplement on the prevention of metachronous colorectal adenoma (20). The Ursodeoxycholic Acid (UDCA) Trial (recruitment of study participants began on 11 November 1995 and was completed on 17 December 1999) was a randomized, double-blinded, placebo-controlled trial that tested the effect of UDCA on the prevention of metachronous colorectal adenoma (21). The current study included only participants from the following 2 control arms: the WBF low-fiber diet arm (n = 585) and the UDCA placebo arm (n = 579). Participants in treatment arms were excluded to eliminate potential confounding by interventions. In addition, only participants who successfully completed the trial (ie, had a follow-up colonoscopy) were included in the analysis (95.9% completion rate in the WBF low-fiber arm; 93.9% completion rate in the UDCA placebo arm). Participants who did not complete the trial were lost to follow-up, withdrew from the trial, or died. Participants in the study had a mean follow-up time to physician-determined colonoscopy of 3.5 ± 1.3 y (3.8 ± 1.4 y in the WBF low-fiber arm; 3.2 ± 1.2 y in the UDCA placebo arm). In addition, the study evaluated adenoma endpoints including the characterization of adenomas. Advanced adenoma was defined as an adenoma that included one or more of the following characteristics: 1) ≥1 cm, 2) villous histology, 3) high-grade dysplasia, or 4) ≥3 adenomas detected. The Institutional Review Board at the University of Arizona approved both original studies and ancillary studies of dietary factors. All subjects provided written informed consent before enrollment.
Dietary measurement of polyamines and other nutrients
Dietary intakes were measured at the time of study enrollment in both study populations by using 1 of 2 versions of the validated Arizona food-frequency questionnaire (AFFQ) (22, 23). The AFFQ is a semiquantitative multi-item questionnaire that is a modified version of the Block National Cancer Institute Health Habits and History Questionnaire food-frequency component (24). The AFFQ was used to ask respondents to report the frequency of foodstuff consumption over the previous 12-mo period. In addition, participants were queried about their usual portion size (ie, small, medium, or large). The polyamine database, which was originally developed for use with the Fred Hutchinson Cancer Research Center food-frequency questionnaire, served as the basis for the development of AFFQ-specific measures (11). AFFQs administered before mandatory folate fortification in the United States (1998) derived dietary folate intake by using prefortification values, whereas AFFQs administered after folate fortification derived dietary folate intake from postfortification amounts. All WBF and 11% of UDCA participants used an earlier version of the AFFQ (113 items), whereas the other 89% of UDCA participants used an updated version (153 items).
Polyamine values for AFFQ food items that were not listed in the original polyamine database developed by Zoumas-Morse et al (11) were imputed by using a previously described methodology by the same research group at the University of California, San Diego. Polyamine values for individual food items were merged into the proprietary Metabolize software (version 3.1; The University of Arizona) for the AFFQ by using established protocols and were reported as nanomoles per serving. Average daily intakes of putrescine, spermidine, and spermine were calculated; the sum of these 3 measures yielded the total polyamine intake.
ODC genotypes
As noted, polyamine synthesis is dependent on the activity of the ODC enzyme. ODC contains a single-nucleotide polymorphism of G (major allele) or A (minor allele) at the +316 nucleotide position (rs2302615) that influences the binding efficiency of the transcriptional repressor Mad and the inducer Myc (18). A portion of participants in each trial donated blood from which DNA was extracted, as previously described (23). Briefly, samples were genotyped for rs2302615 (25). The WBF low-fiber group had 168 GG participants (54.7%) and 139 GA/AA participants (45.3%) compared with 251 GG participants (54.2%) and 212 GA/AA participants (45.8%) in the UDCA placebo arm. Both control arms were individually, and in combination, in Hardy-Weinberg equilibrium (data not shown).
Statistical analysis
Participant and adenoma characteristics were compared between WBF and UDCA trials by using 2-sample t tests for continuous variables and chi-square tests for categorical variables. For measurements of dietary intakes, variables were log transformed, and mean amounts estimated from trial populations were compared by using 2-sample t tests. In the case of supplemental folic acid, values were compared by using a Wilcoxon's rank-sum test because many participants reported zero intake, and the amounts of intake were ordinal-like rather than continuous. Correlations between measurements of dietary intake were calculated by using Pearson's correlation coefficient for the pooled population. The association between the total polyamine intake (ie, the sum of spermidine, spermine, and putrescine) and colorectal adenoma development was tested by using unconditional logistic regression and treating polyamine intake as a binary (above or below the median of 289.2 μmol/d), categorical (quartiles), or continuous variable. The binary version was similar to the intake cutoff of ∼233 μmol polyamine/d used by Raj et al (26). ORs and 95% CIs were calculated for the effect of baseline polyamine exposure on adenoma risk at a follow-up colonoscopy, which occurred, on average, 3.5 y after baseline. Multivariate models were adjusted for age, sex, trial (WBF or UDCA), total energy intake (continuous and log transformed), total folate intake (food plus supplements; continuous and log transformed), and follow-up time (continuous). Separate stratified analyses were performed on the basis of sex, age (<65 compared with ≥65 y), and folate. Potential interactions between polyamine intake and sex, age, and arginine, methionine, and folate intakes on colorectal adenoma development were tested by using likelihood-ratio tests. All statistical analyses were performed with Stata 12.1 (StataCorp), and all statistical tests were 2 sided.
RESULTS
Baseline characteristics of both control groups of WBF and UDCA trials
The combined study population (n = 1164) predominantly consisted of non-Hispanic whites (94.9%) and men (65.8%) (Table 1). Participants were followed for an average of 3.5 y, and nearly one-half of participants (48.1%) had a colorectal adenoma at a follow-up colonoscopy. No significant differences were noted between the 2 trials with respect to sex, race, aspirin use, and proportion of participants with advanced colorectal adenoma at study entry. In contrast, WBF patients, compared with UDCA participants, were slightly younger (65.5 compared with 66.5 y, respectively; P = 0.049), had lower BMI (in kg/m2; 27.6 compared with 28.2, respectively; P = 0.013), and were much less likely to report the use of supplemental folic acid (35.7% compared with 54.2%, respectively; P < 0.001). In addition, WBF patients, compared with UDCA participants, were less likely to report a personal history of any colorectal polyp before a qualifying colonoscopy (39.3% compared with 45.5%, respectively; P = 0.044) or a family history of colorectal cancer (16.8% compared with 29.5%, respectively; P < 0.001). Also, the qualifying colorectal adenoma of WBF patients was less likely than that of UDCA participants to be located in the proximal region of the colon (48.4% compared with 54.3%, respectively; P = 0.045), and WBF patients were more likely than UDCA participants to develop any adenoma at follow-up (52.3% compared with 43.9%, respectively; P = 0.004).
TABLE 1.
Patient and adenoma characteristics of control groups by trial (WBF Trial low-fiber arm and UDCA Trial placebo arm)1
| Characteristic | Combined trials (n = 1164) | WBF Trial (n = 585) | UDCA Trial (n = 579) |
| Age (y) | 66.0 ± 8.623 | 65.5 ± 8.9 | 66.5 ± 8.3 |
| BMI (kg/m2)4 | 27.9 ± 4.63 | 27.6 ± 4.4 | 28.2 ± 4.8 |
| Sex, M [n (%)] | 766 (65.8) | 385 (65.8) | 381 (65.8) |
| White race [n (%)] | 1096 (94.9) | 561 (95.9) | 535 (93.9) |
| Aspirin use [n (%)]5 | 317 (27.2) | 156 (26.7) | 161 (27.8) |
| Supplemental folic acid use [n (%)]6 | 523 (44.9)3 | 209 (35.7) | 314 (54.2) |
| Family history of colorectal cancer [n (%)]47 | 263 (23.4)3 | 92 (16.8) | 171 (29.5) |
| Baseline advanced adenoma status [n (%)]48 | 597 (51.5) | 300 (51.7) | 297 (51.3) |
| Baseline proximal adenoma [n (%)]4 | 580 (51.4)3 | 266 (48.4) | 314 (54.3) |
| Any adenoma at follow-up [n (%)] | 560 (48.1)3 | 306 (52.3) | 254 (43.9) |
UDCA, Ursodeoxycholic Acid; WBF, Wheat Bran Fiber.
Mean ± SD (all such values).
Significant difference between the 2 trials by using t tests for continuous variables and chi-square tests for categorical variables, P < 0.05.
Data are missing for BMI (0 subjects in the WBF group and 7 subjects in the UDCA group), family history (38 subjects in the WBF group and 0 subjects in the UDCA group), baseline advanced status (5 subjects in the WBF group and 0 subjects in the UDCA group), and baseline proximal adenoma (35 subjects in the WBF group and 1 subject in the UDCA group).
Regular use of aspirin within the month before enrollment.
Any supplement that contained any amount of folic acid.
A parent, sibling, or child with a history of colorectal cancer.
Any adenoma ≥1 cm, villous histology, high-grade dysplasia, or ≥3 adenomas at qualifying colonoscopy.
Baseline dietary intakes of polyamines, arginine, methionine, and folate
Mean daily dietary intakes of selected nutrients were compared between the 2 trials. No significant differences were noted for total energy, protein, arginine, methionine, total fiber, total polyamines, putrescine, spermine, or spermidine (Table 2). However, WBF participants, compared with UDCA participants, consumed more total fat (67.5 compared with 60.7 g/d, respectively; P < 0.001) and saturated fat (22.3 compared with 19.6 g/d, respectively; P < 0.001). Overall, putrescine was the primary contributor of polyamines in the diet and accounted for 72.3% of the total polyamine intake in both trials combined.
TABLE 2.
Daily dietary intakes in control groups of select nutrients at baseline by trial (WBF Trial low-fiber arm and UDCA Trial placebo arm)1
| Nutrients | Combined trials (n = 1164) | WBF Trial (n = 585) | UDCA Trial (n = 579) |
| Total energy (kcal) | 1914.2 ± 721.8 | 1896.5 ± 678.0 | 1932.0 ± 763.6 |
| Total protein (g) | 72.0 ± 27.9 | 71.0 ± 25.8 | 73.0 ± 29.7 |
| Arginine (g) | 3.31 ± 1.34 | 3.34 ± 1.30 | 3.27 ± 1.37 |
| Methionine (g) | 1.40 ± 0.57 | 1.42 ± 0.55 | 1.39 ± 0.59 |
| Total fat (g) | 64.1 ± 29.82 | 67.5 ± 29.5 | 60.7 ± 29.8 |
| Saturated fat (g) | 20.9 ± 10.82 | 22.3 ± 10.9 | 19.6 ± 10.5 |
| Total fiber (g) | 21.9 ± 10.1 | 22.0 ± 10.0 | 21.7 ± 10.2 |
| Total polyamines (μmol)3 | 332.5 ± 195.8 | 331.7 ± 199.5 | 333.4 ± 192.2 |
| Putrescine (μmol) | 238.6 ± 173.7 | 240.3 ± 179.9 | 236.9 ± 167.4 |
| Spermidine (μmol) | 62.0 ± 27.2 | 59.8 ± 24.0 | 64.2 ± 29.8 |
| Spermine (μmol) | 31.9 ± 14.5 | 31.6 ± 13.7 | 32.3 ± 15.2 |
| Folate, food only (μg)4 | 396.7 ± 204.72 | 326.8 ± 139.1 | 467.4 ± 234.0 |
| Folic acid, supplements only (μg)5 | 196.9 ± 245.12 | 155.2 ± 230.5 | 239.0 ± 252.4 |
| Total folate (μg)6 | 593.6 ± 336.12 | 481.9 ± 265.9 | 706.4 ± 361.2 |
All values are means ± SDs. UDCA, Ursodeoxycholic Acid; WBF, Wheat Bran Fiber.
Significant difference in intake between the 2 trials (P < 0.05) by using a Wilcoxon's rank-sum test (folic acid supplements) or t tests (all other variables, first log transformed).
Sum of putrescine, spermidine, and spermine in foods and beverages.
Folate that originated from foods and beverages but not dietary supplements (ie, multivitamins).
Folic acid that originated from dietary supplements only.
Sum of food-only and supplement-only folate.
Consistent with secular trends, all 3 measures of folate and folic acid intake (food, supplements, and total) were lower in WBF than in UDCA patients (all P < 0.001; Table 2). A majority of participants in both trials (64.0%) showed total folate intakes greater than the Recommended Dietary Allowance of 400 μg/d, although the proportion was significantly higher in UDCA than in WBF participants (77.4% compared with 50.8%, respectively; P < 0.001).
Dietary sources of polyamines
Grapefruit juice, orange juice, bananas, specific cheeses (ie, cheddar, Monterey Jack, Swiss, and cream cheeses), oranges, and tangerines contributed ∼50% of total dietary polyamines in both trials; grapefruit juice was the single highest dietary contributor. Our findings were slightly different from those of Zoumas-Morse et al (11) because they showed that the major contributors of polyamines to the diets of their participants (n = 165) were citrus juice (putrescine), green peas (spermidine), and ground meat (spermine). Because of the limited exploration of dietary polyamines and adenoma risk in previous studies, and because each food or beverage item contained multiple nutrients, we examined correlations of nutrients. Specifically, we looked at folate (27–29), arginine (30), and methionine (31), which have previously been shown to modify adenoma risk. In our pooled population, arginine, methionine, and food-derived folate intakes were strongly correlated with total and individual polyamines (Table 3).
TABLE 3.
Dietary constituent correlations in the control arms from both trials (Wheat Bran Fiber Trial low-fiber arm and Ursodeoxycholic Acid Trial placebo arm; n = 1164)1
| Nutrient | Total polyamines2 | Putrescine | Spermidine | Spermine | Folate, food only3 | Arginine |
| Total polyamines2 | ||||||
| Putrescine | 0.977 | |||||
| Spermidine | 0.757 | 0.622 | ||||
| Spermine | 0.533 | 0.376 | 0.849 | |||
| Folate, food only3 | 0.605 | 0.511 | 0.746 | 0.619 | ||
| Arginine | 0.534 | 0.394 | 0.804 | 0.887 | 0.626 | |
| Methionine | 0.485 | 0.353 | 0.747 | 0.837 | 0.597 | 0.975 |
Pearson correlation coefficients are provided for each log-transformed variable (all P < 0.001).
Sum of putrescine, spermidine, and spermine in foods and beverages.
Folate that originated from foods and beverages but not dietary supplements (ie, multivitamins).
Association between baseline polyamine intake and risk of adenoma at follow-up
With both trials combined (Table 4), participants in the top 2 quartiles of polyamine intake had nonsignificantly higher odds of colorectal adenoma at follow-up compared with those of participants in the lowest quartile after adjustment for age, sex, trial, energy, total folate intake, and follow-up time. When treated as a continuous variable, we observed a nonsignificant increase for colorectal adenoma development (OR: 1.04; 95% CI: 0.80, 1.36). Because colorectal adenoma risk differs by age and sex (32, 33), we conducted stratified analyses to examine the effect of polyamine intake in different subgroups. For women, risk of colorectal adenoma development showed a significant positive trend with increasing population-based (P-trend = 0.005) and sex-specific quartiles (P-trend = 0.037) of polyamine exposure. However, there was no significant association between adenoma in women and polyamine exposure treated as a continuous variable (OR: 1.17; 95% CI: 0.75, 1.82). In men, we saw no main effect of polyamine exposure, regardless of the modeling strategy.
TABLE 4.
Effect of polyamine intake on colorectal adenoma at follow-up colonoscopy in control arms from both trials (Wheat Bran Fiber Trial low-fiber arm and Ursodeoxycholic Acid Trial placebo arm) stratified by sex and quartile of polyamine exposure1
| Polyamine intake | All | Women | Men |
| Regular quartiles2 | |||
| 1 | 1.00 | 1.00 | 1.00 |
| 2 | 0.96 (0.67, 1.36) | 0.62 (0.33, 1.15) | 1.11 (0.71, 1.73) |
| 3 | 1.41 (0.97, 2.06) | 1.65 (0.87, 3.13) | 1.30 (0.81, 2.07) |
| 4 | 1.28 (0.85, 1.92) | 2.29 (1.13, 4.66)3 | 1.03 (0.63, 1.70) |
| P-trend | 0.082 | 0.0053 | 0.829 |
| Sex-specific quartiles4 | |||
| 1 | 1.00 | 1.00 | 1.00 |
| 2 | 0.95 (0.67, 1.35) | 0.85 (0.45,1.61) | 0.96 (0.63, 1.48) |
| 3 | 1.29 (0.89, 1.88) | 1.46 (0.75, 2.84) | 1.22 (0.77, 1.92) |
| 4 | 1.21 (0.81, 1.82) | 1.85 (0.90, 3.80) | 1.00 (0.31, 1.64) |
| P-trend | 0.169 | 0.0373 | 0.772 |
| Continuous | 1.04 (0.80, 1.36) | 1.17 (0.75, 1.82) | 0.97 (0.70, 1.36) |
All values are ORs; 95% CIs in parentheses. Values were calculated by using unconditional logistic regression adjusted for age, sex, trial, energy and total folate intakes, and follow-up time.
Polyamine intake quartile ranges for both sexes pooled [quartiles 1 (35 to ≤194 μmol/d), 2 (>194 to ≤289 μmol/d), 3 (>289 to ≤412 μmol/d), and 4 (>412 to 1295 μmol/d)].
P < 0.05.
Polyamine intake quartile ranges for each sex separately [quartiles 1 (women: 35–177 μmol/d; men: 40 to ≤207 μmol/d), 2 (women: 187–262 μmol/d; men: >207 to 297 μmol/d), 3 (women: 266–396 μmol/d; men: 298 to ≤425 μmol/d), and 4 (women: 398–1183 μmol/d; men: >425 to 1295 μmol/d).
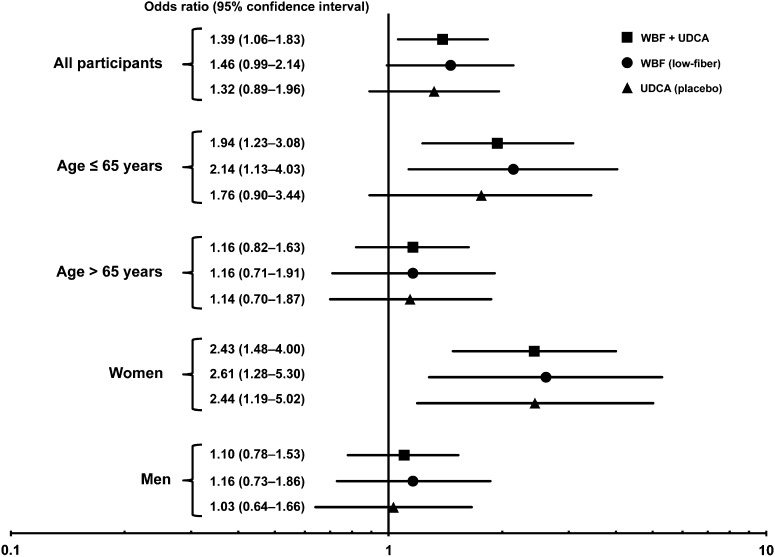
On the basis of the apparent threshold effect in the total population near the median of polyamine exposure (289 μmol/d), we subsequently used this value as the cutoff for low compared with high polyamine intakes to assess the consistency of the main effect across sex, age, and study (Figure 2). We showed that women with polyamine intakes above the population median had significantly increased odds of colorectal adenoma in the pooled sample (OR: 2.43; 95% CI: 1.48, 4.00). There was no association for men, and a test for polyamine-by-sex interaction was significant (P = 0.010). When stratified by age, subjects ≤65 y old showed significantly increased odds of colorectal adenoma with higher polyamine consumption (OR: 1.94; 95% CI: 1.23, 3.08); nonetheless, there was no association in older (>65 y of age) participants (test for polyamine-by-age interaction, P = 0.129). Stratification by both age and sex simultaneously revealed that high-polyamine consumers who were women, regardless of age, had significantly higher odds of adenoma (age ≤65 y, OR: 2.46; 95% CI: 1.09, 5.56; age >65 y, OR: 2.38; 95% CI: 1.26, 4.47). In men, only younger participants with higher polyamine intakes had increased risk (OR, 1.76; 95% CI: 1.01, 3.07); there was no significant association in older men (OR: 0.83; 95% CI: 0.54, 1.27; test for polyamine-by-age interaction in men, P = 0.096). All associations were similar in magnitude and direction in each of the individual trials.
FIGURE 2.
Forest plot of ORs for associations between total polyamine intake (high compared with low) and adenoma development in the WBF (low-fiber arm) and UDCA (placebo arm) chemoprevention trials. Each sample was also stratified by sex or age. Logistic regression models were adjusted for age, sex, study, energy and total folate intakes, and follow-up time. P values of interactions with respect to odds of metachronous adenoma were as follows: polyamine-by-sex, P = 0.010; polyamine-by-age, P = 0.129; and polyamine-by-sex-by-age, P = 0.38. UDCA, Ursodeoxycholic Acid; WBF, Wheat Bran Fiber.
Polyamine intake amounts by sex and age
When adjusted for energy intakes, women had a significantly higher mean polyamine intake than did men (200.2 ± 102.1 compared with 166.8 ± 83.0 μmol/1000 kcal, respectively; P < 0.001), and individuals aged ≤65 y had a significantly lower mean intake than did older subjects (158.7 ± 82.0 compared with 191.7 ± 94.9 μmol/1000 kcal, respectively; P < 0.001). Thus, although we did not see a significant 3-way interaction (P = 0.38) between sex, age, and polyamine intake on odds of colorectal adenoma, there were clear sex- and age-specific differences in the consumption of polyamine-rich foods.
Effect of arginine, methionine, and folate on risk of adenoma at follow-up
As mentioned, de novo synthesis of polyamines is dependent on arginine (putrescine) and methionine (spermine and spermidine) availability (30, 31). To explore the potential contribution of de novo synthesis of polyamines on adenoma risk, we assessed whether dietary intakes of arginine or methionine increased odds of adenoma development. We observed no association between arginine or methionine intake and colorectal adenoma risk in the pooled sample (P = 0.42 and 0.66, respectively). In addition, exploratory analyses conducted in the total population and by sex did not support an interactive effect of arginine or methionine amounts with risk of adenoma (data not shown). In contrast, analyses of polyamine intake stratified by folate amounts showed a significant interaction for polyamine (continuous) by folate and polyamine (quartiles) by folate in women only (P = 0.029 and 0.044, respectively). Exploratory stratified analyses suggested that high folate amounts may partially modify risk of adenoma associated with a high polyamine intake (see Table 1 under “Supplemental data” in the online issue). However, small numbers yielded unstable estimates. Polyamine-associated risk of adenoma in women with high folate intakes was limited to women with the highest polyamine consumption (P-trend = 0.043), but risk in women with low folate intakes was inconsistent across polyamine quartiles with no clear trend. When polyamine intake was treated as a continuous variable and stratified by folate amounts, the interaction was significant for both men and women. The apparent risk-reducing effect of folate was also present when we stratified at the recommended daily allowance of 400 μg folate/d (data not shown).
Potential effect modification of ODC genotype on the association between polyamine intake and risk of adenoma at follow-up
Although we have shown that dietary polyamine exposure above the median increased odds of colorectal adenoma at follow-up by 39% overall (Figure 2), it is unknown whether this risk was modified by the ODC +316 genotype. Although we detected no main effect of the GG genotype on risk of colorectal adenoma in the pooled control arms (data not shown), GG carriers (ie, the high-risk genotype) with a high polyamine consumption had a significant 59% increased odds of a metachronous adenoma compared with those of GG carriers with a low polyamine intake (Table 5). In contrast, we detected no effect of polyamine intake on metachronous colorectal adenoma at follow-up in carriers of the AA/AG genotypes, which previously have been associated with lower risk of colorectal adenoma at follow-up (test for polyamine-by-genotype interaction, P = 0.171). We lacked sufficient numbers of participants to explore the effect of polyamine intake on adenoma development in subgroups defined by sex, age, and ODC genotype simultaneously.
TABLE 5.
Main effect of polyamine intake on colorectal adenoma at follow-up colonoscopy in control arms from both trials (Wheat Bran Fiber Trial low-fiber arm and Ursodeoxycholic Acid Trial placebo arm) stratified by ODC genotype (rs2302615)
| ODC genotype and polyamine intake | n/total (%) adenoma | OR (95% CI)1 |
| GG | ||
| Low | 95/210 (45.2) | 1.00 |
| High | 110/209 (52.6) | 1.59 (1.00, 2.53)2 |
| AA/AG | ||
| Low | 82/170 (48.2) | 1.00 |
| High | 77/181 (42.5) | 0.80 (0.49, 1.32) |
Calculated by using unconditional logistic regression adjusted for age, sex, trial, energy and total folate intakes, and follow-up time.
P < 0.05.
DISCUSSION
To our knowledge, this study provided the first evidence to support an association between high dietary polyamine intake and risk of colorectal adenoma. In addition, we observed that the relation between polyamine consumption and adenoma risk was modified by sex and ODC genotype.
Little is known about dietary exposure to polyamines, but our data suggested that there is a distribution of exposure that varies by sex and age. Although we could not definitively explain the apparent interaction of polyamine exposure with age or sex, our data suggested that being a woman or being older was associated with a higher polyamine consumption, after adjustment for energy intake, as follows: older women had greater intake than all other age-by-sex groups; younger women had approximately the same intake as older men; older men had lower intake than all other age-by-sex groups. Although these data supported a relation between polyamines and adenoma risk, we did not find consistent evidence of a linear relation between polyamine intake and risk of metachronous colorectal adenoma. In the total population, our results suggested a threshold effect of polyamine intake for increased risk of colorectal adenoma near the median intake of polyamines that was significant in women and not present in men. When adjusted for energy intake, women had significantly higher exposure amounts than did men to total polyamines from their diets. The median amount of polyamine intake in the population approximated the exposure from two 8-oz glasses of processed orange juice per day, which was one of the primary sources of exposure in our population. For women, risk of colorectal adenoma development showed a positive trend with increasing quartiles of polyamine intake; however, when treated as a continuous exposure, the association between polyamine intake and adenoma risk in women was not significant, which suggested that the association may be nonlinear. Future studies are needed to examine the dose-response relation in women, and possibly in younger men, as well as to explore the potential effect modification caused by folate. Our data suggested that this amount of exposure could be modified to potentially reduce risk of adenoma in select subgroups of the population.
A polymorphic nucleotide at the +316 position (rs2302615) of the ODC gene influences polyamine content in rectal tissue (GG carriers have higher rectal polyamine amounts) and relative baseline risk of colorectal adenoma (16, 25, 34, 35). To assess a gene-by-diet interaction, we investigated the relation between this polymorphism and dietary polyamine intake on colorectal adenoma risk by stratifying by ODC genotype. Participants with higher polyamine intakes and the GG genotype had significantly higher odds of colorectal adenoma compared with participants with the same genotype but lower polyamine intakes. We observed no effect of polyamine exposure on colorectal adenoma risk in carriers of any A allele. These findings suggested that a gene (ODC)-by-diet (polyamine exposure) interaction may influence risk of colorectal adenomas. Although the exact mechanism of this relation is unknown, it is possible that certain ODC genotypes are able to reset polyamine homeostatic pathways and, thereby, affect the polyamine-transport processes. However, future studies are required to identify this exact relation and to inform on specific recommendations regarding polyamine intake in individuals at risk of adenomas on the basis of the individual genotype of ODC.
Although our findings were supported by studies in commonly used mouse models of colorectal carcinogenesis, in which a high dietary polyamine intake increased the degree of polyp dysplasia (19), there have been limited studies in humans that related polyamine exposure from dietary intake and colorectal adenoma development. With the use of the same database as was used in the current study, Raj et al (26) reported a positive correlation between polyamine intake and the rectal mucosa polyamine content. In addition, this group showed that the efficacy of targeted inhibition of polyamine synthesis was modified by dietary polyamine amounts. Previous studies have demonstrated a 70% reduction in risk of colorectal adenoma on treatment with difluoromethylornithine (DFMO) and sulindac (36, 37). However, Raj et al (26) showed that, in individuals in the upper quartile of polyamine intake, there was no reduction in risk with DFMO and sulindac. Seventy-six percent of subjects in the DFMO and sulindac trial (36, 37) were men with a mean age of ∼60 y, and the upper quartile of polyamine intake was equivalent to the median intake of our population (26).
Although our study has a number of strengths, including the replication of results across the 2 cohorts and a strong biological basis, it was a secondary analysis and should be considered hypothesis generating. In addition, inherent to any food-frequency questionnaire analysis of dietary intake is the recall bias of subjects, inaccuracy of portion sizes, and limited food options on the food-frequency questionnaire or in the polyamine database itself. Also, we showed that the polyamine content in food was positively correlated with other nutrients that modify adenoma risk (ie, folate, methionine, and arginine). Intakes of these other nutrients could have confounded our findings, despite our attempts to control for statistically relevant factors. Specifically, arginine (30), which is the precursor of ornithine and, therefore, putrescine, has proneoplastic effects on the colon, whereas methionine, which is an important methyl donor, has antineoplastic effects (31). The correlation between folate and folic acid and polyamine intake is also of great interest because, in our previous study, supplemental folic acid was shown to be protective against adenoma in individuals with low plasma folate, whereas increased risk was observed in subjects with replete plasma folate who were exposed to high folic acid amounts (28). Our results for polyamine exposure and adenoma risk by folate suggested that higher folate amounts may act to partially mitigate the neoplastic effect of polyamines in the colorectum and shift the tumorigenic dose of polyamines higher. If corroborated, the manipulation of the joint effects of folate and polyamine exposure may offer a dietary approach for risk reduction for colorectal neoplasia.
An important limitation when pooling study populations is the inherent differences in secular dietary trends across time as described for differences in WBF and UDCA because of the mandatory folate fortification that took place after the WBF trial (28). Therefore, to address dietary and other differences between trial populations, we conducted all of our analyses in each study separately. The magnitude and direction of the effects shown in both the pooled sample and individual control arms of the 2 trials were similar and, therefore, provided consistency to our findings across more than one study. Last, because no study, to our knowledge, has measured dietary polyamine exposure in a representative US population, and our sample included only participants with a history of adenoma, our findings may not be generalizable to the US population at large.
In conclusion, although our findings describe the first human evidence, to our knowledge, that links polyamine intake to risk of colorectal neoplasia, future studies are needed to confirm these findings and determine the thresholds at which dietary polyamine intake may protect against or promote disease. This effort should include gaining a better understanding of polyamine exposure in the general population by age and sex and a consideration of genetic background as a modifier of risk. The development of an accurate biomarker of dietary polyamine exposure at systemic (urinary) or tissue-specific levels would improve the accuracy of exposure measurements beyond food-frequency questionnaire estimates. In addition, biomarkers would establish the reliability of the existing database for the estimation of polyamine content in common diet items. Because of the abundance of polyamines in the food supply and the strong preclinical data that relate polyamine exposure to tumor growth, our findings support the need for additional investigation of dietary polyamines in human health.
Supplementary Material
Acknowledgments
We thank Christine Zoumas-Morse, Vern Hartz, and Kim Nicolini for their aid in this project.
The authors’ responsibilities were as follows—AJV: designed the research, analyzed data, wrote the manuscript, obtained funding, and had primary responsibility for the final content of the manuscript; BCW: analyzed data, conducted statistical analyses, and wrote the manuscript; EWG: designed the research, obtained funding, and wrote the manuscript; CAT: designed the research, analyzed data, and wrote the manuscript; CLR: provided essential database access and wrote the manuscript; and PAT: designed the research, analyzed data, obtained funding, and wrote the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AFFQ, Arizona food-frequency questionnaire; DFMO, difluoromethylornithine; ODC, ornithine decarboxylase; UDCA, Ursodeoxycholic Acid; WBF, Wheat Bran Fiber
REFERENCES
- 1.Basuroy UK, Gerner EW. Emerging concepts in targeting the polyamine metabolic pathway in epithelial cancer chemoprevention and chemotherapy. J Biochem 2006;139:27–33. [DOI] [PubMed] [Google Scholar]
- 2.Carper SW, Tome ME, Fuller DJ, Chen JR, Harari PM, Gerner EW. Polyamine catabolism in rodent and human cells in culture. Biochem J 1991;280:289–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerner EW. Cancer chemoprevention locks onto a new polyamine metabolic target. Cancer Prev Res (Phila) 2010;3:125–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004;4:781–92 [DOI] [PubMed] [Google Scholar]
- 5.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res 2009;15:758–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerner EW, Meyskens FL, Jr, Goldschmid S, Lance P, Pelot D. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids 2007;33:189–95 [DOI] [PubMed] [Google Scholar]
- 7.Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol 2001;13:1021–5 [DOI] [PubMed] [Google Scholar]
- 8.Uemura T, Gerner EW. Polyamine transport systems in mammalian cells and tissues. Methods Mol Biol 2011;720:339–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura T, Stringer DE, Blohm-Mangone KA, Gerner EW. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2010;299:G517–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J 2003;376:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoumas-Morse C, Rock CL, Quintana EL, Neuhouser ML, Gerner EW, Meyskens FL., Jr Development of a polyamine database for assessing dietary intake. J Am Diet Assoc 2007;107:1024–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tome ME, Gerner EW. Cellular eukaryotic initiation factor 5A content as a mediator of polyamine effects on growth and apoptosis. Biol Signals 1997;6:150–6 [DOI] [PubMed] [Google Scholar]
- 13.Wallace HM. The polyamines: past, present and future. Essays Biochem 2009;46:1–9 [DOI] [PubMed] [Google Scholar]
- 14.Shantz LM, Pegg AE. Translational regulation of ornithine decarboxylase and other enzymes of the polyamine pathway. Int J Biochem Cell Biol 1999;31:107–22 [DOI] [PubMed] [Google Scholar]
- 15.Hixson LJ, Garewal HS, McGee DL, Sloan D, Fennerty MB, Sampliner RE, Gerner EW. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev 1993;2:369–74 [PubMed] [Google Scholar]
- 16.Hubner RA, Muir KR, Liu JF, Logan RF, Grainge MJ, Houlston RS. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res 2008;14:2303–9 [DOI] [PubMed] [Google Scholar]
- 17.Zell JA, McLaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL. Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. J Natl Cancer Inst 2010;102:1513–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zell JA, Ziogas A, Ignatenko N, Honda J, Qu N, Bobbs AS, Neuhausen SL, Gerner EW, Anton-Culver H. Associations of a polymorphism in the ornithine decarboxylase gene with colorectal cancer survival. Clin Cancer Res 2009;15:6208–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignatenko NA, Besselsen DG, Roy UK, Stringer DE, Blohm-Mangone KA, Padilla-Torres JL, Guillen RJ, Gerner EW. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer 2006;56:172–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med 2000;342:1156–62 [DOI] [PubMed] [Google Scholar]
- 21.Alberts DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 2005;97:846–53 [DOI] [PubMed] [Google Scholar]
- 22.Martínez ME, Marshall JR, Graver E, Whitacre RC, Woolf K, Ritenbaugh C, Alberts DS. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol Biomarkers Prev 1999;8:941–6 [PubMed] [Google Scholar]
- 23.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials 2002;23:728–56 [DOI] [PubMed] [Google Scholar]
- 24.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69 [DOI] [PubMed] [Google Scholar]
- 25.Martinez ME, O'Brien TG, Fultz KE, Babbar N, Yerushalmi H, Qu N, Guo Y, Boorman D, Einspahr J, Alberts DS, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci USA 2003;100:7859–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj KP, Zell JA, Rock CL, McLAren CE, Zoumas-Morse C, Gerner EW, Meyskens FL. Role of dietary polyamines in a phase III clinical trial of DFMO and sulindac for prevention of metachronous colorectal adenomas: A potential target for colon cancer chemoprevention. ASCO: Gastroentestinal Cancers Symposium 2010 (abstr 279)
- 27.Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr 2011;93:817–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez ME, Giovannucci E, Jiang R, Henning SM, Jacobs ET, Thompson P, Smith-Warner SA, Alberts DS. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer 2006;119:1440–6 [DOI] [PubMed] [Google Scholar]
- 29.Martínez ME, Thompson P, Jacobs ET, Giovannucci E, Jiang R, Klimecki W, Alberts DS. Dietary factors and biomarkers involved in the methylenetetrahydrofolate reductase genotype-colorectal adenoma pathway. Gastroenterology 2006;131:1706–16 [DOI] [PubMed] [Google Scholar]
- 30.Zell JA, Ignatenko NA, Yerushalmi HF, Ziogas A, Besselsen DG, Gerner EW, Anton-Culver H. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer 2007;120:459–68 [DOI] [PubMed] [Google Scholar]
- 31.de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr 2008;138:2372–8. [DOI] [PubMed] [Google Scholar]
- 32.Kolligs FT, Crispin A, Munte A, Wagner A, Mansmann U, Goke B. Risk of advanced colorectal neoplasia according to age and sex. PLoS ONE 2011;6:e20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006;355:1863–72 [DOI] [PubMed] [Google Scholar]
- 34.Barry EL, Baron JA, Bhat S, Grau MV, Burke CA, Sandler RS, Ahnen DJ, Haile RW, O'Brien TG. Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention. J Natl Cancer Inst 2006;98:1494–500 [DOI] [PubMed] [Google Scholar]
- 35.Zell JA, Mclaren CE, Gerner EW, Meyskens FL. Ornithine decarboxylase (Odc)-1 gene polymorphism effects on baseline tissue polyamine levels and adenoma recurrence in a randomized phase III adenoma prevention trial of DFMO + sulindac versus placebo. ASCO: Annual Meeting 2008 (abstr 1502)
- 36.Meyskens FL, Jr, Gerner EW, Emerson S, Pelot D, Durbin T, Doyle K, Lagerberg W. Effect of alpha-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst 1998;90:1212–8 [DOI] [PubMed] [Google Scholar]
- 37.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.