The WASH complex controls actin dynamics on endosomes, and its functional mechanism is poorly defined. The WASH complex subunit Fam21 bears many copies of a novel motif that directly interacts with the retromer cargo-selective complex. Endosomal localization of FAM21 requires both the retromer and multivalency of the repeat elements.
Abstract
Wiskott–Aldrich syndrome protein (WASPs) control actin dynamics in cellular processes, including cell motility, receptor-mediated endocytosis, bacterial invasion, and vesicular trafficking. We demonstrated that WASH, a recently identified WASP family protein, colocalizes on endosomal subdomains with the cargo-selective complex (CSC) of the retromer, where it regulates retrograde sorting from endosomes in an actin-dependent manner. However, the mechanism of WASH recruitment to these retromer-enriched endosomal subdomains is unclear. Here we show that a component of the WASH regulatory complex (SHRC), FAM21, which contains 21 copies of a novel L-F-[D/E]3-10-L-F motif, directly interacts with the retromer CSC protein VPS35. Endosomal localization of FAM21 is VPS35 dependent and relies on multivalency of FAM21 repeat elements. Using a combination of pull-down assays and isothermal calorimetry, we demonstrate that individual repeats can bind CSC, and binding affinity varies among different FAM21 repeats. A high-affinity repeat can be converted into a low-affinity one by mutation of a hydrophobic residue within the motif. These in vitro data mirror the localization of FAM21 to retromer-coated vesicles in cells. We propose that multivalency enables FAM21 to sense the density of retromer on membranes, allowing coordination of SHRC recruitment, and consequent actin polymerization, with retromer sorting domain organization/maturation.
INTRODUCTION
Actin dynamics plays a central role in numerous cellular processes, such as migration, adhesion, and vesicle trafficking (Chhabra and Higgs, 2007; Firat-Karalar and Welch, 2011). Members of the Wiskott–Aldrich syndrome protein (WASP) family, including WASP, N-WASP, WAVE(1-3), WHAMM, JMY, and WASH, control actin dynamics by activating the ubiquitous Arp2/3 complex (Takenawa and Suetsugu, 2007; Padrick and Rosen, 2010; Rottner et al., 2010). A conserved C-terminal VCA domain, found in all WASP family proteins, binds and activates the Arp2/3 complex, whereas distinct N-termini allow individual members of the WASP family to integrate into unique protein complexes with different cellular functions.
WASH, a recently identified member of the WASP family, is found in all genetically characterized eukaryotic organisms except yeast and Arabidopsis (Linardopoulou et al., 2007; Veltman and Insall, 2010). WASH localizes to sorting domains on endosomes, where it is suggested to regulate epidermal growth factor receptor (EGFR) trafficking, as well as the recycling of transferrin receptors and α5β1 integrins to the cell surface (Derivery et al., 2009; Duleh and Welch, 2010; Zech et al., 2011). In addition, we recently demonstrated that WASH regulates actin-dependent retrograde sorting of cation-independent mannose 6-phosphate receptor (CI-MPR) to the trans-Golgi network (Gomez and Billadeau, 2009).
WASH exists in cells in a stable complex known as the WASH regulatory complex (SHRC), which includes four additional proteins: FAM21, strumpellin, SWIP, and CCDC53 (Derivery et al., 2009; Gomez and Billadeau, 2009; Jia et al., 2010). A combination of sequence, biochemical, and structural data has shown that the SHRC is homologous to the WAVE regulatory complex (WRC), a pentameric assembly containing the WASP protein WAVE. WASH, strumpellin, SWIP, and CCDC53 are predicted to be largely ordered in the SHRC, based on analogy to the WRC structure (Chen et al., 2010; Jia et al., 2010). In contrast, FAM21 integrates into the SHRC through only its N-terminal 220 residues. The C-terminal ∼1100 residues of FAM21, which are unrelated to WRC elements, are predicted to be mostly unstructured. This C-terminal tail of FAM21 plays a key role in controlling SHRC localization (Gomez and Billadeau, 2009). It is necessary for WASH targeting to endosomes and can bind endosomes independent of the remaining SHRC subunits (Gomez and Billadeau, 2009). Moreover, FAM21-dependent SHRC targeting is requisite for efficient CI-MPR sorting (Gomez and Billadeau, 2009). However, the mechanism by which FAM21 is recruited to endosomal membranes remains ill defined.
We recently showed that the SHRC colocalizes strongly with retromer-enriched endosomal subdomains (Gomez and Billadeau, 2009). The retromer is a conserved protein complex that controls the retrograde transport of a wide range of endosomal cargoes (Seaman et al., 1998). Retromer is required for lysosomal morphology and function, Wnt signaling, and transport of certain bacterial toxins (Bonifacino and Hurley, 2008; Burd, 2011; Cullen and Korswagen, 2011). Mammalian retromer consists of two loosely linked complexes. One is a trimeric assembly of VPS35, VPS29, and VPS26, known as the cargo-selective complex (CSC), responsible for recognizing membrane-bound cargo proteins; the other is formed by sorting nexin (SNX) proteins (SNX1/2/5/6), important for membrane deformation through their BAR domains (Bonifacino and Hurley, 2008; Wassmer et al., 2009; Burd, 2011; Cullen and Korswagen, 2011). Recent studies indicate an interaction between FAM21 and the CSC component VPS35, suggesting that SHRC recruitment to endosomes involves direct binding to retromer (Harbour et al., 2010, 2012). However, it remains unclear how SHRC recruitment could be coordinated with the assembly of retromer on membranes to ensure appropriate membrane dynamics and consequent endosomal trafficking.
Here we show that FAM21 contains 21 repeats of a novel L-F-[D/E]3-10-L-F motif (referred to hereafter as the LFa motif), which binds directly to the VPS35 subunit of the retromer CSC. Adjacent motifs can act cooperatively to bind multiple CSCs, although there is significant variability in the affinities of different motifs for retromer. Multivalency of FAM21 is requisite for endosomal localization of SHRC. Taken together, these observations lead to a model for SHRC recruitment to CSC-enriched endosomal subdomains in which multivalency enables FAM21 to bind membrane-associated CSC with high cooperativity and thus sense the organization and/or density of retromer on membranes. This mechanism would couple SHRC recruitment with maturation of retromer sorting domains.
RESULTS
FAM21 contains multiple copies of a novel leucine-phenylalanine-acidic motif
FAM21 can be divided into two distinct regions: a “head” domain consisting of the N-terminal ∼220 amino acids, which is responsible for incorporation into the SHRC, and a largely disordered “tail” containing the remaining ∼1100 amino acids (Figure 1A). The FAM21 tail harbors at least 21 copies of a novel motif, which contains 3–10 acidic residues flanked by two hydrophobic Leu–Phe residues (Figure 1A and Supplemental Figure S1). We term this the leucine-phenylalanine-acidic (LFa) motif. Leucine in the LFa motif is occasionally replaced with similar hydrophobic residues, such as isoleucine, valine, or methionine; some other residues, in particular serine, are frequently found within the acidic stretch. Enriched with hydrophobic and acidic residues, the LFa motif is reminiscent of sorting motifs that are recognized by adapter proteins and Golgi-localized, γ-ear-containing, Arf-binding (GGA) proteins (Bonifacino and Traub, 2003). However, FAM21 is not a cargo protein, and, to our knowledge, the LFa motif is distinct from known protein–protein interaction motifs.
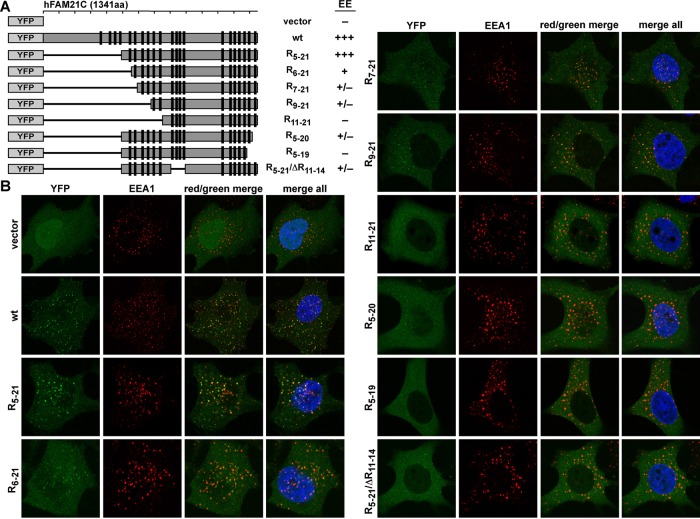
FIGURE 1:
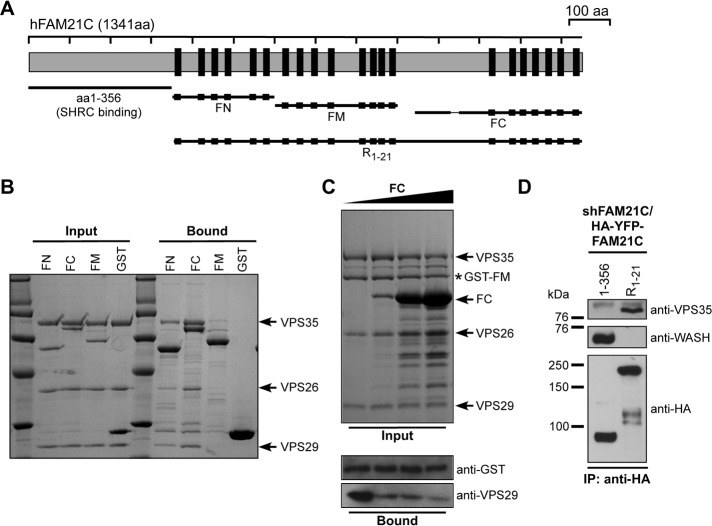
FAM21 directly interacts with retromer CSC. (A) Schematic representation of FAM21 constructs used. FAM21 contains 21 copies of the L-F-[D/E]3-10-L-F motif (LFa motif; black boxes). FN, FM, and FC contain repeats 1–6, 7–14, and 15–21, respectively. (B) GST-FAM21 pull-down of purified retromer CSC. Shown is a Coomassie blue–stained SDS–PAGE gel of input and bound samples for FN, FC, FM, and GST control. (C) GST-FM pull-down of retromer CSC with addition of increasing amounts of untagged FC protein. All input samples contained 1 nmol of GST-FM and 0.8 nmol of retromer CSC in the absence (lane 1) or presence of FC protein (lane 2, 1.5 nmol; lane 3, 10 nmol; lane 4, 20 nmol). Top, input samples separated by SDS–PAGE and stained with Coomassie blue. Bottom, diluted bound samples blotted against GST (loading control) and VPS29, respectively. (D) HeLa cells were transfected with shFAM21/HA-YFP-FAM21 rescue vectors expressing HA-YFP-FAM21 fusion proteins (amino acids 1–356 or R1-21), which were analyzed for association with VPS35 and WASH via immunoprecipitation.
FAM21 directly interacts with retromer CSC
We previously showed that the FAM21 tail was important for endosomal localization of SHRC (Gomez and Billadeau, 2009). To learn how FAM21 is targeted to endosomes, we took an unbiased approach to identify binding partners of the FAM21 C-terminus. We engineered a fragment encompassing LFa repeats 1–6 of FAM21 (FN) and another containing repeats 15–21 (FC; Figure 1A). The 19-residue CapZ-binding motif of FAM21 (Jia et al., 2010) was removed from FC. The two FAM21 fragments were expressed as glutathione S-transferase (GST) fusions, immobilized, and incubated with bovine brain extracts. Three different bands were specifically retained by FN or FC fragments but not by GST control (Supplemental Figure S2). Mass spectrometry analysis revealed that they corresponded to adaptor protein 2 α2 (AP2α2), WAFL/FKBP15, and the retromer CSC component VPS35. The interactions of FAM21 with AP2α2 and WAFL/FKBP15 have been reported (Schmid et al., 2006; Viklund et al., 2009; Harbour et al., 2010, 2012; Pan et al., 2010), and we focused on the interaction with VPS35.
In cells VPS35 is constitutively associated with VPS26 and VPS29 to form the CSC (Hierro et al., 2007). To determine whether the interaction between FAM21 and CSC was direct, we performed pull-down assays with purified recombinant proteins. Purified CSC was incubated with GST-FN, -FC, and -FM (a middle fragment of the FAM21 tail containing repeats 7–14). As shown in Figure 1B, all three GST-FAM21 fragments could efficiently pull down CSC. FC retained more CSC than FN and FM, and untagged FC could compete with FM for CSC binding (Figure 1C). These results demonstrate that different fragments of FAM21 can directly interact with a common site(s) on the CSC.
To confirm that the FAM21 tail is important for retromer binding in cells, we suppressed endogenous FAM21 and reexpressed the C-terminal tail containing the LFa motifs (R1-21) or the head domain involved in SHRC assembly (amino acids 1–356). Only FAM21 R1-21 was able to coimmunoprecipitate with endogenous VPS35, whereas the SHRC-interacting N-terminus did not (Figure 1D). Together, these data suggest that the FAM21 tail interacts with retromer.
Interaction between FAM21 and CSC is mediated by LFa motifs and VPS35
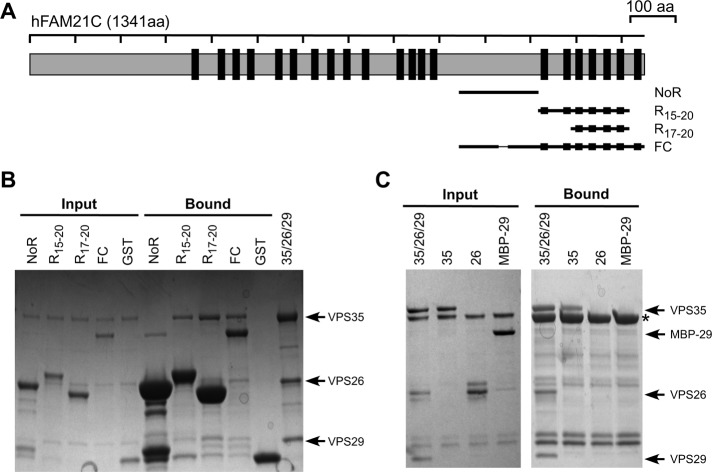
FN, FM, and FC can each bind the CSC. Yet these fragments have little similarity except for harboring multiple LFa motifs. Thus we examined whether the motifs could mediate interaction with CSC. We generated three additional FAM21 tail fragments: one is devoid of motifs (noR); the other two (R15-20 and R17-20) contain six and four repeats, respectively. Similar to the FC fragment, both R15-20 and R17-20 could effectively retain CSC. In contrast, the noR fragment failed to interact with CSC (Figure 2B). Thus CSC binding is likely mediated by the LFa motifs in the FAM21 tail.
FIGURE 2:
Interaction between FAM21 and CSC requires the FAM21 motif and VPS35. (A) Schematic representation of FAM21 constructs used. (B) GST pull-down of retromer CSC using GST-FC and its derived fragments. Shown is a Coomassie blue–stained SDS–PAGE gel of input and bound samples for one FC subfragment that does not contain any repeats (noR), one containing six repeats (R17-20), one containing four repeats (R15-20), FC, and GST control. (C) GST-FC pull-down of retromer CSC or individual VPS35, VPS26, and VPS29 proteins. Shown is Coomassie blue–stained gel of input and bound samples from GST pull-down assays and purified retromer proteins. Uncomplexed VPS26 has some degradation products. VPS29 is an MBP fusion. Fusion tags of VPS35, VPS26, and VPS29 were proteolytically removed in retromer CSC. Asterisk, GST-FC.
We next tested whether FAM21 could interact with an individual protein of the CSC or required the intact trimer. Individual VPS35, VPS26, and VPS29 proteins were used in pull-down assays with immobilized FC. Both VPS35 and the trimeric CSC, but not VPS26 or VPS29, were effectively retained by FC (Figure 2C). Therefore VPS35 is necessary and sufficient to interact with FAM21.
VPS35 regulates FAM21 and SHRC localization
We previously showed that FAM21 and SHRC colocalized with retromer and that suppression of FAM21 had no effect on the localization of retromer components (Gomez and Billadeau, 2009). To determine whether retromer CSC regulates the localization of FAM21, we suppressed VPS35 using RNA interference (RNAi) and examined localization of FAM21 (Supplemental Figure S3). Consistent with a previous report (Harbour et al., 2012), suppression of VPS35 resulted in significant dissociation of FAM21 from endosomal membranes and concomitant cytosolic dispersal of the protein. FAM21 localization could be rescued by reexpression of RNAi-resistant yellow fluorescent protein (YFP)–VPS35, which displayed strong colocalization with endogenous FAM21 (Supplemental Figure S3). Because FAM21 has the potential to target the entire SHRC to endosomes (Gomez and Billadeau, 2009), we conclude that FAM21 and SHRC localization to retromer-enriched endosomal subdomains is VPS35 dependent.
Both the quality and the quantity of LFa repeats are important for interaction with retromer CSC in vitro
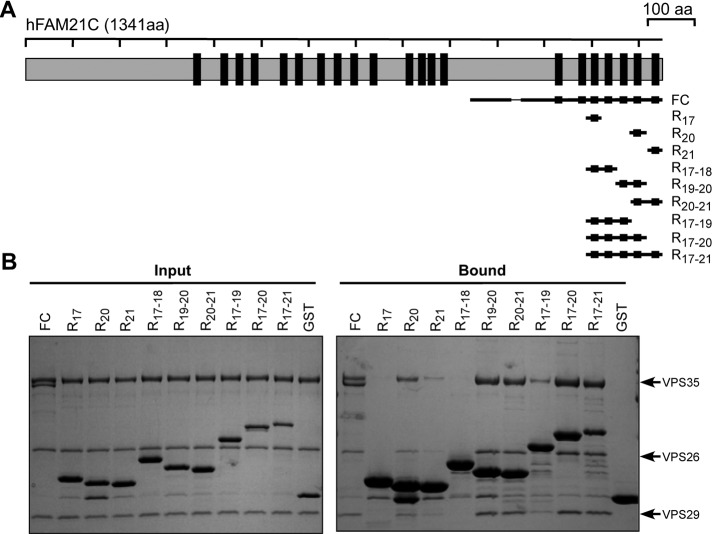
The FAM21 tail contains 21 LFa motifs, each with a distinct sequence (Supplemental Figure S1). Because the FC fragment demonstrated the most robust binding to VPS35, we used this region of FAM21 to better understand the LFa-CSC interaction. Specifically, we sought to learn which motifs could bind CSC most tightly, the stoichiometry of binding, and which residues of the LFa motifs contribute most significantly to binding. We tested several immobilized fragments derived from FC, consisting of one to seven repeats, for their ability to retain recombinant CSC (Figure 3, A and B). We used the same total repeat concentration in each assay (rather than the same protein concentration), to normalize for the different number of repeats in each fragment. The retromer-binding ability of these FAM21 fragments varied substantially, with fragments bearing repeats 19, 20, or 21 interacting strongly and other fragments only weakly (if at all). Two different single-repeat constructs—R20 and R21—were able to retain CSC, but R17 was not. Similarly, the double-repeat fragments R19-20 and R20-21 retained significantly more CSC than any of the single-repeat fragments, but fragment R17-18 failed to retain any protein. Moreover, several of the larger fragments—namely R17-20, R17-21, and FC—retained CSC to an extent similar to the double-repeat fragments R19-20 and R20-21, whereas the triple-repeat fragment R17-19 retained a comparable amount of CSC to single-repeat R20 (Figure 3B). Thus, in solution-phase interactions (see Discussion) the final three repeats (19–21) make the most important contributions to binding the FC fragment. We note that repeats 19–21 are three of the four LFa repeats within FAM21 that contain a hydrophobic residue at the sixth position of the repeat (Supplemental Figure S1C), suggesting that this might be a feature that contributes to increased affinity for VPS35 (see later discussion of Figure 6).
FIGURE 3;
Different FAM21 repeats show different efficiencies of CSC binding. (A) Schematic representation of FAM21 constructs used. (B) GST pull-down of retromer CSC using FC and its derived fragments. Shown is Coomassie blue–stained gel of input and bound samples from GST pull-down assays. Each input sample, except for GST control, contained 700 pmol of total LFa motifs and 800 pmol of retromer CSC.
FIGURE 6:
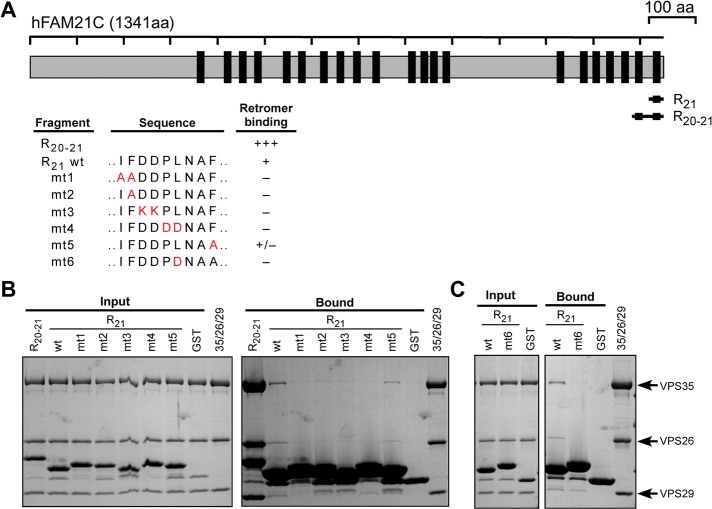
All three elements of the FAM21 motif contribute to binding the CSC. (A) Schematic representation of FAM21 constructs used and degree of retromer binding determined by GST pull-down. (B) GST pull-down of retromer CSC using FAM21 R21 and its mutants 1–5. FAM21 R20-21 is used as a positive control and GST as a negative control. Shown is Coomassie blue–stained gel of input and bound samples from GST pull-down assays. (C) GST pull-down of retromer CSC using FAM21 R21 and mutant 6. Shown is Coomassie blue–stained gel of input and bound samples from GST pull-down assays.
Next we used isothermal titration calorimetry (ITC) to quantify the interaction between FAM21 and CSC (Table 1 and Supplemental Figure S4). Different FAM21-derived fragments were titrated into CSC. ITC experiments showed that R21 binds CSC with Kd of 16.6 μM and ∼1:1 stoichiometry. Moreover, titrations of the double-repeat fragments R19-20 and R20-21, when fitted to a model that assumes identical repeats within a fragment, showed that these fragments can bind to CSC in a ∼2:1 manner (1/N = 2.56 and 2.04 for R19-20 and R20-21, respectively). These data yielded apparent Kd values of 6.8 and 3.2 μM for each site in R19-20 and R20-21, respectively (although these values should be viewed as approximate, as described in Materials and Methods). The data for the FC fragment can also be fitted to a single Kd of 2.4 μM for each site and stoichiometry of 2.38:1 (1/N = 2.38; stoichiometry for a system with seven potential binding sites should be viewed as highly approximate), supporting the notion that in solution the interactions between FC and retromer CSC are mediated primarily by the last three repeats, which exhibit high affinity for CSC. Due to the stoichiometry of the interaction, it is likely better to titrate CSC into Fam21 fragments; however, the high concentration of CSC required (at least 200 μM) makes such an experiment unfeasible.
TABLE 1:
Activities of FAM21 tail constructs.
| Name | Sequence | Relative binding to retromer CSC | Stoichiometry (N) by ITC | Kd (μM) by ITC | Endosomal localization |
|---|---|---|---|---|---|
| FN | 356–600 | ++ | 0.32 ± 0.23 | 11.5 ± 3.8 | |
| FC | 938–1341∆1029–1047 | +++ | 0.42 ± 0.04 | 2.4 ± 0.6 | |
| FC* | 938–1341 | – | |||
| FM | 601–900 | + | |||
| R17 | 1186–1221 | – | |||
| R20 | 1280–1317 | + | |||
| R21 | 1309–1341 | + | 0.82 ± 0.24 | 16.6 ± 4.3 | |
| R17-18 | 1186–1254 | – | |||
| R19-20 | 1250–1317 | +++ | 0.39 ± 0.12 | 6.8 ± 1.4 | |
| R20-21 | 1278–1341 | +++ | 0.49 ± 0.02 | 2.5–16.6a | |
| R17-19 | 1186–1285 | + | |||
| R17-20 | 1186–1317 | +++ | |||
| R17-21 | 1186–1341 | +++ | |||
| R15-20 | 1121–1317 | +++ | |||
| No R | 938–1120 | – | |||
| R5-21 | 496–1341 | +++ | |||
| R6-21 | 549–1341 | + | |||
| R7-21 | 594–1341 | +/– | |||
| R9-21 | 675–1341 | +/– | |||
| R11-21 | 743–1341 | – | |||
| R5-20 | 496–1309 | +/– | |||
| R5-19 | 496–1275 | – | |||
| R1-19 | 357–1275 | + | |||
| R5-21/ΔR11-14 | 496–1341/Δ803–888 | +/– | |||
| Fusion FC*/ R11-21 | 938–1341/743–1341 fusion | +++ |
1/N is the number of retromer CSCs bound to each Fam21 fragment.
aSee Materials and Methods for explanation of range.
Finally, ITC data on the FN fragment, which contains six repeats predicted to have low affinity for CSC, could be fitted to a single Kd of 11.5 μM for each site and stoichiometry of ∼3:1. This affinity is lower than that of the FC fragment, in agreement with our pull-down results (Figure 1B). Taken together, our biochemical data indicate that the LFa motif is the minimal CSC-binding element but that different motifs have quite different affinities for CSC. As described in the Discussion, it is likely that when retromers are bound to membranes, avidity effects involving both high-affinity and low-affinity sites in the FAM21 tail will afford high overall affinity of the SHRC for membranes.
Both the quality and the quantity of FAM21 repeats are important for its endosomal localization
We next determined how the number and quality of LFa repeats collectively contribute to endosomal localization of FAM21. We used a suppression/reexpression system to deplete endogenous FAM21 and reexpress YFP-tagged FAM21 truncation mutants (Figures 4A and 5A). As we showed previously (Gomez and Billadeau, 2009), wild-type YFP-FAM21 had a punctate localization juxtaposed with the early endosome marker EEA1 (Figure 4B). Similarly, FAM21 retained robust endosomal localization when up to five repeats were deleted from the N-terminus of FAM21(R5-21). However, when we made additional N-terminal deletions (R6-21, R7-21, R9-21), the colocalization of FAM21 mutants with EEA1 diminished appreciably, although not completely. Finally, FAM21 was redistributed from endosome membranes to the cytosol when 10 repeats were deleted from the N-terminus (R11-21). Consistent with the importance of R20 and R21 in binding retromer in vitro, we found that FAM21 became cytosolic with deletion of both C-terminal repeats (R5-19), and deletion of only R21 substantially diminished FAM21 localization (R5-20). Last, deletion of R11-14 in the context of R5-21(R5-19/ΔR11-14) also resulted in considerably diminished endosomal localization. These experiments suggest that the endosomal localization of FAM21 requires a combination of multiple repeats of high and low affinity. Therefore both the number of FAM21 repeats (which will likely afford avidity when retromer is at membranes; see later discussion) and the exact repeat sequence (which determines the affinity of any particular repeat) collectively contribute to the FAM21 distribution between cytosol and endosomal membranes.
FIGURE 4:
Number of the FAM21 repeat dictates its endosomal localization. (A) Schematic representation of YFP-fused FAM21 truncation mutants and degree of early endosomal (EE) localization. (B) HeLa cells were transfected with control vector (YFP only) or various shFAM21/YFP-FAM21 dual suppression/rescue vectors. YFP-FAM21 fusion proteins (green) were analyzed for localization with EEA1 (red) via immunofluorescence.
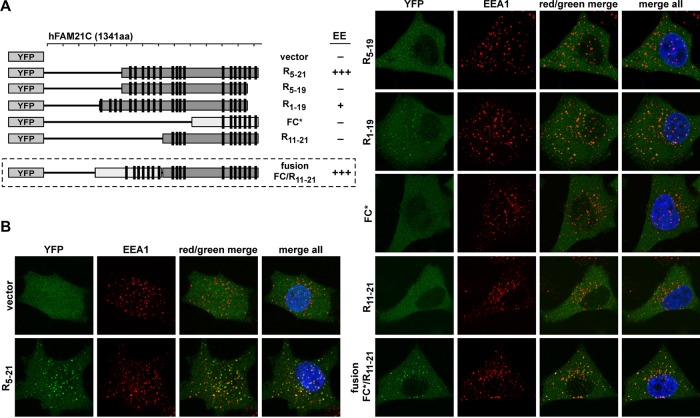
FIGURE 5:
Quality of FAM21 repeat also dictates endosomal localization. (A) Schematic representation of YFP-fused FAM21 truncation mutants and degree of early endosomal (EE) localization. (B) HeLa cells were transfected with control vector (YFP only) or various shFAM21/YFP-FAM21 dual suppression/rescue vectors and were analyzed as in Figure 4. (FC* contains the CapZ-binding motif.)
Because R5-21 but not R5-19 can localize to early endosomes (Figures 4 and 5), we tested whether addition of the four N-terminal repeat motifs onto R5-19 (R1-19) would affect localization. Importantly, R1-19 partially rescued endosomal localization (Figure 5, A and B). Thus the addition of lower-affinity repeats to the N-terminus can partially rescue FAM21 localization, confirming that a combination of low-affinity interactions can support endosomal targeting. Interestingly, although FC can efficiently bind retromer CSC in vitro, a YFP-tagged FAM21-FC* (asterisk indicates that it contains the CAPZ-binding motif) construct was unable to localize to endosomal membranes, suggesting that the high-affinity binding repeat motifs found in this region are insufficient to cause membrane targeting (Figure 5, A and B). To test whether the number of repeats could affect subcellular localization, we created a fusion protein of FC* and R11-21 fragments of FAM21, which each individually does not localize to endomembranes. Significantly, this fusion protein (FC*/R11-21) relocalized to EEA1+ endosomes, consistent with the idea that both the number and the strength of retromer CSC-binding interactions contribute to FAM21 targeting to endosomes (Figure 5, A and B). Thus, varying the LFa motif number and sequence can shift the distribution of FAM21 between endosomes and cytosol.
Mutagenesis of the FAM21 repeat sequence
The LFa motif comprises three elements: two Leu–Phe or Ile–Phe dipeptides and one acidic stretch. We mutated each of the three elements in the context of the R21 repeat and tested the interaction with CSC (Figure 6, A and B). As shown in Figure 3, wild-type R21 retained CSC on the solid support. However, when the first IF dipeptide motif was mutated to AA (mt1) the interaction with CSC was abolished; the single F-to-A mutant (mt2) behaved similarly. In contrast, mutation of F to A (mt5) in the second dipeptide element only weakly affected binding. Changing the central residues from DD to KK (mt3) or PL to DD (mt4) also dramatically decreased CSC binding (Figure 6B). Last, a single L-to-D mutation (mt6) of the hydrophobic residue at position 6 resulted in a significant loss of binding (Figure 6, A and C). Similar conclusions are supported by mutagenesis of double-repeat FAM21 fragments (Supplemental Figure S5, A–E). Taken together, these data demonstrate that the LF/IF dipeptide motifs, as well as the acidic charged region, are critical to CSC interaction and support the notion that the hydrophobic residue at position 6, which is found in R19-R21, may contribute to increased affinity for VPS35.
DISCUSSION
The SHRC can be directly localized to endosomal membranes through an interaction with retromer (Gomez and Billadeau, 2009; Harbour et al., 2010, 2012). Here we showed that this interaction is mediated by the LFa motifs within the C-terminal tail of FAM21, which directly bind the VPS35 subunit of the retromer CSC. FAM21 harbors >20 copies of this novel motif, which consists of 3–10 acidic residues flanked by two hydrophobic dipeptides. Both acidic and hydrophobic residues within the motif are important for interaction with the CSC. Individual LFa motifs bound CSC with varying affinities. Multiple repeats are required for the proper localization of FAM21 to endosomes, and localization can be mediated by various combinations of high- and low-affinity motifs.
WASH complex directly interacts with retromer
Retromer mediates both endosome-to–trans-Golgi network (TGN) and endosome-to-plasma membrane trafficking (Cullen and Korswagen, 2011; Temkin et al., 2011). The direct interaction between retromer and FAM21 suggests that the SHRC is likely important for both trafficking processes as well. Indeed, WASH depletion compromises the transport of CI-MPR, a membrane cargo that is transported to TGN in a retromer-dependent manner (Gomez and Billadeau, 2009). A more recent study implicates the WASH complex in the trafficking of α5β1 integrins through the endosomal pathway to the leading edge of migrating cells (Zech et al., 2011). Moreover, the β2 adrenergic receptor (β2AR) localizes to distinct endosomal subdomains that are enriched with retromer as well as the WASH complex, Arp2/3, and actin (Puthenveedu et al., 2010; Temkin et al., 2011). Interestingly, β2AR recycling depends on retromer and actin dynamics (Puthenveedu et al., 2010; Temkin et al., 2011). An attractive idea is that the β2AR, as well as α5β1 integrins, is recognized in a sequence-specific manner by the retromer and ultimately trafficked out of the endosome by FAM21-mediated SHRC recruitment by VPS35. In addition, the SHRC might control actin cytoskeletal dynamics on specific endosomal regions, which could be important in organizing/stabilizing these subdomains or concentrating recycling cargoes for efficient trafficking (Puthenveedu et al., 2010).
Identifying which regions of the sorting/recycling endosome where the SHRC and retromer are functioning will be important for fully elucidating their cellular activities. It remains possible that some retromer-dependent cargoes may be trafficked independent of the SHRC, whereas others are SHRC dependent. This might result from the fact that the classical retromer complex is believed to consist of retromer-associated SNXs for membrane tubulation and the cargo recognition complex. We previously showed that WASH puncta were strongly coincident with YFP-VPS29, whereas SNX1/2 puncta were instead more often adjacent to WASH puncta (Gomez and Billadeau, 2009). Consistent with this, we do not observe endogenous SNX1/2 staining in cells strongly superimposed with endogenous VPS35 staining. Instead, they more often exhibit adjacent staining (unpublished data). This suggests that although the CSC and SNXs functionally cooperate, they are likely not always complexed during the sorting process. Thus, depleting SNXs, CSC, or SHRC could result in differential effects on sorting. However, our data presented here suggest that depletion of SHRC should phenocopy depletion of retromer CSC.
The relationship between WASH and retromer during trafficking of cargo proteins from endosomes may be analogous to that of N-WASP and clathrin during endocytic processes. During clathrin-mediated endocytosis, N-WASP is recruited transiently to clathrin-coated pits (Benesch et al., 2005). There, N-WASP controls actin dynamics, which is important for both membrane invagination and vesicle scission (Merrifield et al., 2005; Yarar et al., 2005). Similarly, we previously showed that during retromer-mediated trafficking events, SHRC is recruited to retromer-coated structures, where it generates Arp2/3-dependent F-actin required for retromer-dependent sorting events (Gomez and Billadeau, 2009). Actin polymerization on these structures, promoted by WASH, could provide a mechanism to concentrate retromer cargoes and/or generate the force for tubulation and scission of retromer-coated tubules. The recruitment of N-WASP and WASH to membranes is also analogous, despite differences in detailed mechanism. No direct interaction has been observed between N-WASP and clathrin. instead, N-WASP is recruited to clathrin-coated pits indirectly via multiple SH3-containing adaptor proteins. These adaptors, which are believed to form arrays on membranes (Frost et al., 2008), can engage the multiple proline-rich motifs in N-WASP. WASH is directly linked with retromer-containing structures via binding to FAM21. Unlike other WASP-family members, WASH does not have a large proline-rich region to bind multiple SH3 domains. Yet, in the context of the SHRC, the FAM21 tail can play an analogous role, with multiple LFa motifs engaging arrays of retromer on membranes. In both mechanisms, the recruitment of a WASP family member to membrane surfaces at high density will result in increased affinity for the Arp2/3 complex and thus enhanced actin assembly (Padrick et al., 2008, 2011; Padrick and Rosen, 2010). The direct, multivalent, low-affinity interaction between FAM21 and retromer could provide an important mechanism that regulates the maturation of retromer-coated endosomal regions for membrane tubulation, scission, and trafficking.
Maturation of retromer-coated tubules
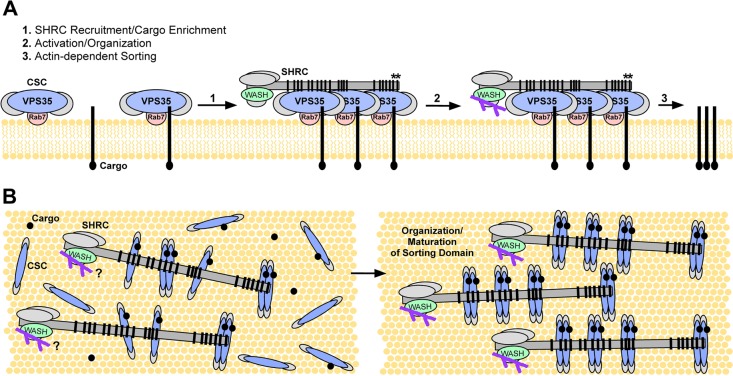
Formation of a coated vesicle can be regarded as a three-step process (Pucadyil and Schmid, 2009): assembly of the coat protein complex on the membrane through contacts with membrane-bound cargoes and GTPases (e.g.; Rab7-GTP; Rojas et al., 2008; Seaman et al., 2009; Figure 7A), formation of a coated bud, and scission of the coated bud. Little is known about how the maturation of retromer-coated tubules is regulated. Our study demonstrates that FAM21 binds multiple retromer molecules, each with low affinity, through its highly multivalent tail. Similarly, when retromer is arrayed on a membrane surface it functions essentially in multivalent manner. Thus the retromer–FAM21 interaction can be viewed as that between two multivalent entities. In such systems, affinity can increase sharply with valency due to increasing avidity effects (Hogg and Winzor, 1985) and also with density of the membrane-bound component (Yang et al., 2003). Consequently, the recruitment of FAM21, and thus the SHRC, should be sensitive to the number of repeat motifs, as we observe. In addition, recruitment should be sensitive to the density of retromer CSC on endosomal membranes (Figure 7, A and B). The low LFa motif–retromer affinity suggests that SHRC will not bind appreciably to retromer in solution or when retromer is only sparsely arrayed on membranes. Only when retromer accumulates on membranes to high density will the SHRC be recruited. The nature of the FAM21 tail and its interactions with retromer could thus provide a mechanism to coordinate actin polymerization with assembly and maturation of the retromer coat. Such coordination is likely needed for proper maturation of the retromer subdomain, vesicle scission, and endocytic trafficking.
FIGURE 7:
Model of SHRC interaction with retromer on membranes. (A) We propose that the CSC of the retromer is recruited to the endosomal membrane through mechanisms involving GTP-bound Rab 7 and cargo proteins that are destined to enter the retromer-mediated sorting pathway. When retromer accumulates to high density on the endomembrane, the SHRC is then recruited through cumulative binding of higher-affinity (*) and lower-affinity FAM21 repeats with VPS35 proteins, thus facilitating the cargo enrichment process and promoting subsequent F-actin–mediated maturation of the retromer subdomain, vesicle scission, and trafficking. (B) Bird's-eye view of the process discussed in A after initial recruitment of the SHRC via FAM21-mediated interactions with VPS35.
MATERIALS AND METHODS
Antibodies and plasmids
Reagents were from Sigma-Aldrich (St. Louis, MO) unless specified. Anti-HA Affinity Matrix and anti–hemagglutinin (HA)-horseradish peroxidase were from Roche (Indianapolis, IN). We used monoclonal anti-EEA1 (BD Transduction Laboratories, Lexington, KY) and fluorescently conjugated secondary reagents from Molecular Probes (Invitrogen, Carlsbad, CA). Anti-FAM21 and anti-WASH were as described (Gomez and Billadeau, 2009). Anti-VPS35 was generated by immunizing rabbits with GST fusion protein containing the AA461 end of hVPS35 (Cocalico Biologicals, Reamstown, PA). The dual shFAM21/HA-YFP-FAM21 rescue vector system was as described previously (Gomez and Billadeau, 2009). We also generated a shVPS35/HA-YFP-VPS35 rescue vector system using shVPS35 (CAGAGCAGATTAACAAACA) and VPS35 cDNA made short hairpin RNA resistant via standard mutagenesis (CtGAaCAaATcAAtAAACA).
Cell culture, transfection, immunoprecipitation, and GST pull-down assays
HeLa cells were grown in RPMI 1640 medium with 5% fetal bovine serum, 5% fetal calf serum, and 4 mM l-glutamine and were transfected using electroporation as described (Gomez and Billadeau, 2009). For immunoprecipitations, cells were lysed in NP-40 lysis buffer (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.9, 50 mM NaCl, 1 mM EDTA, 0.5 mM CaCl2, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 5 μg/ml aprotinin), and 500–1000 μg was prepared and analyzed by immunoblot as described (Gomez and Billadeau, 2009). GST pull-down experiments were performed by mixing 700 pmol of GST or different GST-FAM21 proteins, 800 pmol of purified retromer CSC, and 30 μl of glutathione Sepharose 4B resin in 1 ml of pull-down buffer (PB; 20 mM Tris, pH 8.0, 50 mM NaCl, 5% [wt/vol] glycerol, 5 mM β-mercaptoethanol [βME]). After gentle mixing at 4°C for 20 min, the resin was washed four times with 1 ml of PB, followed by elution with reduced glutathione. Eluted proteins were resolved by SDS–PAGE and visualized with Coomassie blue.
Immunofluorescence
HeLa cells were grown directly on coverslips, fixed in 4% paraformaldehyde, and prepared for immunofluorescence as described (Gomez and Billadeau, 2009). Images were obtained with an LSM-710 laser scanning confocal microscope (Carl Zeiss, Jena, Germany).
Protein purification
DNA encoding full-length human VPS35 was inserted in a modified pGEX vector, resulting in a N-terminal GST tag followed by a tobacco etch virus (TEV) protease site. Full-length VPS29 and residues 9–327 of VPS26A were expressed as N-terminal TEV-cleavable hexahistidine (His6)-tagged fusions. VPS35 was transformed into Escherichia coli BL21(DE3)-T1R (Sigma-Aldrich) and expressed at 16°C overnight. Both VPS26 and VPS29 were expressed at 20°C overnight. Cell pellets from 1 l of VPS35, 1 l of VPS29, and 2 l of VPS26 were mixed and colysed by high-pressure homogenization in buffer containing 20 mM Tris, pH 8.0, 200 mM NaCl, 1 mM EDTA, and 5 mM βME. VPS35 is the limiting component in this procedure. The lysate was cleared by ultracentrifugation and subjected to glutathione–Sepharose 4B affinity (GE Healthcare, Piscataway, NJ) chromatography. The bound proteins were eluted with reduced glutathione and cleaved with TEV protease. The cleaved proteins were further purified by Source Q ion exchange (GE Healthcare) and gel filtration (Superdex 200; GE Healthcare) chromatographies, resulting in the 1:1:1: trimeric retromer CSC.
For purification of individual protein within the CSC, GST-VPS35 was purified similarly, and the GST fusion was cleaved and removed by Source Q ion exchange chromatography. VPS26 was purified with nickel-nitriloacetic acid (Ni-NTA) agarose resin (Qiagen, Valencia, CA). Isolated VPS26 had more severe degradations than complexed protein. The degradations probably occurred on the C-terminus of VPS26, as they were able to bind to Ni-NTA agarose resin. VPS29 was expressed as a maltose-binding protein (MBP) fusion and purified using amylose resin (New England BioLabs, Ipswich, MA).
FAM21 proteins were expressed with an N-terminal GST fusion and purified by glutathione–Sepharose 4B affinity and ion exchange chromatographies. FN and FC also have a C-terminal His6 tag and were purified by glutathione–Sepharose 4B affinity, Ni-NTA agarose affinity, and gel filtration (Superdex 200) chromatographies. For ITC experiments, R19-20, R20-21, and R21 were expressed with an N-terminal, TEV-cleavable MBP tag and a C-terminal, noncleavable His6 tag. A tryptophan residue was inserted between FAM21 and the His6 tag to facilitate the determination of protein concentration. The proteins were first purified by Ni-NTA agarose affinity resin and eluted with imidazole. The eluted proteins were cleaved with TEV during dialysis (in order to remove imidazole), and MBP was removed by loading back to Ni-NTA resin. The proteins were finally purified by gel filtration (Superdex 75; GE Healthcare) chromatography.
Identification of FAM21-binding proteins from bovine brain extract
Preparation of bovine brain extract was described previously (Jia et al., 2010). Extract containing ∼75 mg of total protein was incubated at 4°C for 1 h with 0.15 mg of purified GST-FN or -FC proteins and glutathione–Sepharose 4B resin (100 μl) in 20 ml of pull-down buffer (PB2; 20 mM Tris, pH 8.0, 50 mM NaCl, 1 mM MgCl2, 2 mM ethylene glycol tetraacetic acid, 0.1% Triton X-100). The resin was washed four times with 1 ml of PB2 after incubation and eluted with reduced glutathione. Protein bands were excised from SDS–PAGE gels, digested with trypsin, and identified by nano–liquid chromatography–tandem mass spectrometry methods at the Protein Chemistry Core Research Facility of UT Southwestern Medical Center or the Protein Chemistry and Proteomics Shared Resource of Mayo Clinic.
Isothermal titration calorimetry
All microcalorimetry experiments were carried out using a VP-ITC MicroCalorimeter (MicroCal, Northampton, MA) at 20°C. Proteins were dialyzed extensively or purified by gel filtration chromatography in the ITC buffer (20 mM Tris, pH 8.0, 100 mM NaCl and 5 mM βME). FAM21 proteins (100–380 μM) were injected into the sample cell containing retromer CSC (10–16 μM). Each experiment involved one injection of 2 μl followed by 35 injections of 8 μl, with 240 s between injections. Data were analyzed with the Origin 7.0 software package (OriginLab, Northampton, MA) by fitting the “one-set-of-sites” model to the binding isotherm, yielding a single Kd value for all binding events. We also fitted the data according to two other models for the double-repeat fragment R20-21: two Kd values for two sites, both fitted; and two Kd values for two sites, one fixed to 16.6 μM (the value for R21 alone). The one-set-of-sites model yielded a single Kd value of 3.2 μM and stoichiometry of 0.49, suggesting two retromer binding sites. The second model yielded Kd values of 2.5 and 8.8 μM but was not a statistically better fit of the data according to an F test. The third model yielded a second Kd value of 3 μM but was also not a better fit than the one-set-of-sites model. From these data we conclude that there are likely two binding sites in the R20-21 construct, with Kd values in the range 2.5–16.6 μM. More specific statements about the affinities (e.g., regarding cooperativity in solution binding) are not warranted by the data. Titrations of CSC into Fam21 fragments, which could in principle give better data, were not possible due to the limited solubility of retromer CSC.
Supplementary Material
Acknowledgments
We thank members of the Rosen and Billadeau laboratories and Ryan Potts for helpful discussions. This work was supported by the Mayo Foundation, the Howard Hughes Medical Institute, National Institutes of Health Grants R01-AI065474 (D.D.B.) and R01-GM056322 (M.K.R), Welch Foundation Grant I-1544 (M.K.R.), Allergic Diseases Training Grant NIH-T32-AI07047 (T.S.G.), and a Cancer Research Institute Fellowship (D.J). D.D.B. is a Leukemia and Lymphoma Society Scholar.
Abbreviations used:
- EEA1
early endosome antigen 1
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- ITC
isothermal calorimetry
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-12-1059) on April 18, 2012.
REFERENCES
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Burd CG. Physiology and pathology of endosome-to-Golgi retrograde sorting. Traffic. 2011;12:948–955. doi: 10.1111/j.1600-0854.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, Umetani J, Billadeau DD, Otwinowski Z, Rosen MK. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2011;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 2010;67:193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011;23:4–13. doi: 10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci. 2010;123:3703–3717. doi: 10.1242/jcs.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended “tail” of Fam21 binding to the retromer protein VPS35. Biochem J. 2012;442:209–220. doi: 10.1042/BJ20111761. [DOI] [PubMed] [Google Scholar]
- Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg PJ, Winzor DJ. Effects of ligand multivalency in binding studies: a general counterpart of the Scatchard analysis. Biochim Biophys Acta. 1985;843:159–163. doi: 10.1016/0304-4165(85)90134-5. [DOI] [PubMed] [Google Scholar]
- Jia D, Gomez TS, Metlagel Z, Umetani J, Otwinowski Z, Rosen MK, Billadeau DD. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci USA. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, Trask BJ. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 2007;3:e237. doi: 10.1371/journal.pgen.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Padrick SB, et al. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Doolittle LK, Brautigam CA, King DS, Rosen MK. Arp2/3 complex is bound and activated by two WASP proteins. Proc Natl Acad Sci USA. 2011;108:E472–E479. doi: 10.1073/pnas.1100236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–735. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YF, Viklund IM, Tsai HH, Pettersson S, Maruyama IN. The ulcerative colitis marker protein WAFL interacts with accessory proteins in endocytosis. Int J Biol Sci. 2010;6:163–171. doi: 10.7150/ijbs.6.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Hanisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–661. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak-Chew SY, Mills IG, Benmerah A, McMahon HT. Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–2382. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DM, Insall RH. WASP family proteins: their evolution and its physiological implications. Mol Biol Cell. 2010;21:2880–2893. doi: 10.1091/mbc.E10-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viklund IM, et al. WAFL, a new protein involved in regulation of early endocytic transport at the intersection of actin and microtubule dynamics. Exp Cell Res. 2009;315:1040–1052. doi: 10.1016/j.yexcr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Wassmer T, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Baryshnikova OK, Mao H, Holden MA, Cremer PS. Investigations of bivalent antibody binding on fluid-supported phospholipid membranes: the effect of hapten density. J Am Chem Soc. 2003;125:4779–4784. doi: 10.1021/ja029469f. [DOI] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech T, Calaminus SD, Caswell P, Spence HJ, Carnell M, Insall RH, Norman J, Machesky LM. The Arp2/3 activator WASH regulates alpha5beta1-integrin-mediated invasive migration. J Cell Sci. 2011;124:3753–3759. doi: 10.1242/jcs.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.