Abstract
Spatial heterogeneity in light supply is common in nature. Many studies have examined the effects of heterogeneous light supply on growth, morphology, physiology and biomass allocation of clonal plants, but few have tested those effects on intraspecific competition. In a greenhouse experiment, we grew one (no competition) or nine ramets (with intraspecific competition) of a stoloniferous clonal plant, Duchesnea indica, in three homogeneous light conditions (high, medium and low light intensity) and two heterogeneous ones differing in patch size (large and small patch treatments). The total light in the two heterogeneous treatments was the same as that in the homogeneous medium light treatment. Both decreasing light intensity and intraspecific competition significantly decreased the growth (biomass, number of ramets and total stolon length) of D. indica. As compared with the homogeneous medium light treatment, the large patch treatment significantly increased the growth of D. indica without intraspecific competition. However, the growth of D. indica with competition did not differ among the homogeneous medium light, the large and the small patch treatments. Consequently, light heterogeneity significantly increased intraspecific competition intensity, as measured by the decreased log response ratio. These results suggest that spatial heterogeneity in light supply can alter intraspecific interactions of clonal plants.
Introduction
Spatial heterogeneity in light supply is common in natural habitats [1]–[3], and connected individuals (ramets) of clonal plants often grow across patches differing in light supply [4]–[6]. Although many studies have shown that spatial heterogeneity in light supply can affect the growth, morphology, physiology and/or biomass allocation of clonal plants [7]–[17], little is known about the effects of heterogeneous light supply on the interactions between clonal plants.
A few studies addressed the effects of spatial heterogeneity in soil nutrients on interactions between plants, and the results differed [18]–[22]. Soil nutrient heterogeneity increased intraspecific competition in Briza media [19] and interspecific competition between Festuca rubra and Anthoxanthum odoratum [20] and between F. ovina and B. media [19], but did not affect intraspecific competition in F. ovina [19] and interspecific competition between Achillea millefolium and six other species [21]. Spatial heterogeneity in soil nutrients was also found to change the relative abundance of species grown in mixtures [3], [22], [23]. So far, however, no study has tested the effects of spatial heterogeneity in light supply on intraspecific competition of clonal plants.
When a clone grows in environments with heterogeneous light supply consisting of low and high light patches, connected ramets growing in patches with different levels of light supply may exchange carbohydrates through clonal integration driven by source-sink relations [5], [7]–[11], [13], [17], [24], [25]. Consequently, performance of the ramets growing in low light patches may be greatly enhanced [4]–[10], [14], [18]. In some cases, such a support to the ramets in low light patches does not impose any costs on their connected ramets growing in high light patches, or the benefits of clonal integration to the ramets in low light patches are significantly larger than the costs to the ramets in high light patches [5], [11]. As a result, clonal integration greatly increases performance of the whole clone [5], [11]. Studies have shown that different clones of the same plant species may differ greatly in the ability of clonal integration under heterogeneous light supply [7]–[9], [13]. When a number of clones that differ in the ability of clonal integration grow in the same heterogeneous light environment, performance of clones may differ greatly due to the differences in the ability of clonal integration. In this case, light heterogeneity may change the intraspecific interactions of clonal plants.
Patch scale is an important element of spatial heterogeneity, and may have a substantial effect on performance of clonal plants [26]–[28]. For instance, Glechoma hederacea clones grown in heterogeneous conditions with the intermediate patch size (25 cm×25 cm) produced nearly four times as much biomass as those grown in heterogeneous environments with the smallest patch size (6.25 cm×6.25 cm) [28]. Therefore, clonal plants that respond to spatial heterogeneity in resource supply at one spatial scale may not do so at other scales [27]–[28]. This suggests that patch scale of light heterogeneity may also affect plant-plant interactions, i.e., light heterogeneity that affects intraspecific interactions at one scale may not at other scales. However, this hypothesis remains untested.
To address the effects of spatial heterogeneity in light supply on intraspecific competition, we conducted a greenhouse experiment with a stoloniferous herb Duchesnea indica. We grew one (no competition) or nine (with intraspecific competition) ramets of D. indica under three homogeneous light treatments (high, medium and low light intensity) and two heterogeneous light treatments differing in patch scale (large and small patch treatments). Specifically, we address the following questions: (1) Do light intensity in homogeneous conditions, light heterogeneity, and plant density (intraspecific competition) affect the growth of D. indica? We predicted that both decreasing light intensity and intraspecific interaction would decrease plant performance. We also predicted that light heterogeneity would increase plant performance when there was no competition because offspring ramets located in high light patches may support ramets located in low light patches at no or very low costs. (2) Does light intensity and heterogeneity affect intraspecific competition? We expected that both increasing light intensity and heterogeneity would increase intraspecific competition. (3) Does the scale of light heterogeneity matter? We predicted that effects of light heterogeneity on plant performance and intraspecific competition would depend on the patch scale, i.e., heterogeneity affect plant performance and intraspecific competition at one scale may not at another scale.
Materials and Methods
Plant species and experimental material
Duchesnea indica Focke is a perennial rosette herb belonging to the Rosaceae family, and distributed mainly in Asia [29]. This species occurs in many regions in China. It produces red fleshy fruits and compound leaves usually consisting of a slender petiole and three leaflets. This species produce long stolons with rooted ramets on its nodes [8], [30]. Interconnected ramets are often located in heterogeneous light environments [31].
In March 2011, more than 420 similar-sized ramets of D. indica were collected from a stock population in a greenhouse at Forestry Science Co, Ltd. of Beijing Forestry University. The exact genotypic information of these ramets was not known, but they were originally propagated from a number of seedlings established from seeds collected in the wild (Yu-Bao Sun, personal communications). Therefore, the ramets most likely belong to a number of different genotypes. For the experiments, 420 ramets were used and all stolons (if any) were removed. All the ramets were standardized by removing all the leaves except the youngest three or four and by cutting the roots to 5 cm long. Then, 20 ramets were randomly selected and dry mass was determined after drying at 70°C for 48 h to obtain the pre-planting biomass value (0.062±0.013 g, mean ± SE, n = 20).
Experimental design
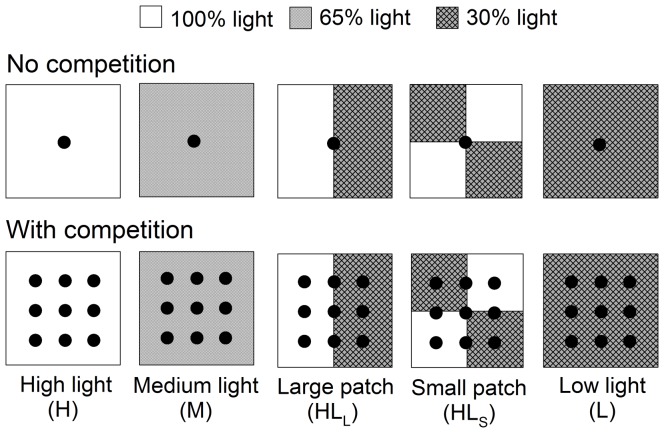
The experiment was conducted in a heated glasshouse at the Institute of Botany, the Chinese Academy of Sciences in Beijing. There were ten treatments, i.e., two competition treatments (with or without intraspecific competition) fully crossed with three homogeneous light treatments (H, M and L for high, medium and low light intensity, respectively) and two heterogeneous light treatments differing in patch size (HLL and HLS for large and small patch treatments, respectively). In the competition-free treatments, one ramet of D. indica was planted in the center of each plastic container (34 cm long×34 cm wide), whereas in the competition treatments, nine ramets were planted within each container (Fig. 1). Ramets in the containers received full light in the glasshouse in the homogeneous high light treatment (H), and 65% and 30% of full light in the homogeneous medium (M) and low (L) light treatment, respectively. The treatments of M and L were applied by covering the containers with two types of black, neutral shading net without changing the red light to far red light ratio. In the large patch treatment (HLL) each container was divided into two large patches and in the small patch treatment (HLS) each divided into four small patches (Fig. 1). In HLL, half of the containers (34 cm×17 cm in size) received full light, whereas the other half was covered using the shading net applied in L that allows 30% of the full light to pass through. In HLS, the shading net covering the container was divided into four 17 cm×17 cm patches, and two of them were removed (and the edges were fixed with wires) so that 100% of full light could pass through and the other two were not so that 30% of full light could pass through. Therefore, the total amount of light received by plants in the two patchy treatments was the same as that in M.
Figure 1. Experimental design.
The experiment consisted of three homogeneous treatments (High light – the plants received full light in the greenhouse, coded as “H”; Medium light – the plants received 65% of full light, coded as “M”; Low light – the plants received 30% of full light, coded as “L”) and two heterogeneous treatments (Large patch, coded as “HLL” – the whole container was divided into two large patches; one patch received full light and the other 30% of full light; Small patch, coded as “HLS” – the whole container was divided into four small patches; two patches received full light and the other two 30% of full light), fully crossed with two treatments of competition (No competition – one plant per container; With competition – nine plants per container). The light received by the plants in the two patchy treatments was the same as that in the homogeneous medium light treatment.
The growth substrate in each container was a 25-cm-deep, 1∶1 (v∶v) mixture of washed river sand and commercial peat (Screening: 0–10 mm; NPK fertilizer, Mg and micro nutrients are added). There were eight replicates in each treatment.
The experiment lasted from 4 March to 31 May 2011. During the experiment the mean temperature in the greenhouse was set to 25°C and the relative humidity to 65%. The light intensity in the greenhouse was about 60% of the outside, and no additional artificial light was provided. Tap water was supplied regularly to ensure there was sufficient water for plants to grow. The containers were randomly placed within a small area of about 25 m2 and all containers were rotated horizontally for 180° to avoid potential effects of positions.
Harvest and measurements
During the experiment each initial (parent) ramet produced a number of offspring ramets that were confined within the containers and allowed to root. At harvest, parent ramets and offspring ramets were harvested separately. For the two heterogeneous treatments, we harvested offspring ramets located in the high light patches and those located in the low light patches separately. We counted number of all ramets (parent plus offspring ramets) and measured stolon length. Then, parent ramets and offspring ramets were oven-dried at 70°C for 48 h, and weighed.
Data analysis
Before analysis, values of all variables in the competition treatments (i.e., with nine ramets per container) were divided by nine so that the values were scaled to the level of per initial plant. To measure the intensity of intraspecific interactions, we calculated the log response ratio [32] as: LnRR = ln(B+/B0), where LnRR is the log response ratio, B+ is biomass per initial plant with competition and B0 is the mean biomass per initial plant without competition across the eight replicates. Values of LnRR are symmetrical around zero, with negative values indicating competition and positive values indicating facilitation [33].
Two-way ANOVAs were used to test the effects of competition (with and without), light conditions (H, M, L, HLS and HLL) and their interactions on biomass, number of ramets and stolon length per initial plant per container. When significant effects were found, Duncan multiple comparison tests were conducted to examine for differences between the ten treatments [34]. We used one-way ANOVA followed by Duncan tests to compare the means of LnRR among the five light treatments.
To test the effects of competition and light heterogeneity on the growth measures of the plants in the high light patches, we used two-way ANOVAs. In these analyses, the growth measures of the plants in the high light patches in the heterogeneous treatments (HLL and HLS) and 50% of the value of each growth measure of the plants in the homogeneous high light treatments (H) were used. We used 50% of the values in H because the area with high light in H was two times of that in the HLL or HLS. Similarly, we tested the effects of competition and light heterogeneity on the growth measures of the plants in the low light patches. In these analyses, the growth measures of the plants in the low light patches in HLL and HLS, and 50% of the value of each growth measure of the plants in L were used. If significant effects were detected, then Duncan multiple comparison tests were used to compare the means between the treatments.
All analyses were conducted with SPSS 17.0 software (SPSS, Chicago, IL, USA). Prior to ANOVAs, all data were checked for normality and homoscedasticity. The differences were considered to be significant if P<0.05.
Results
Effects of competition and light intensity at whole plant (container) level
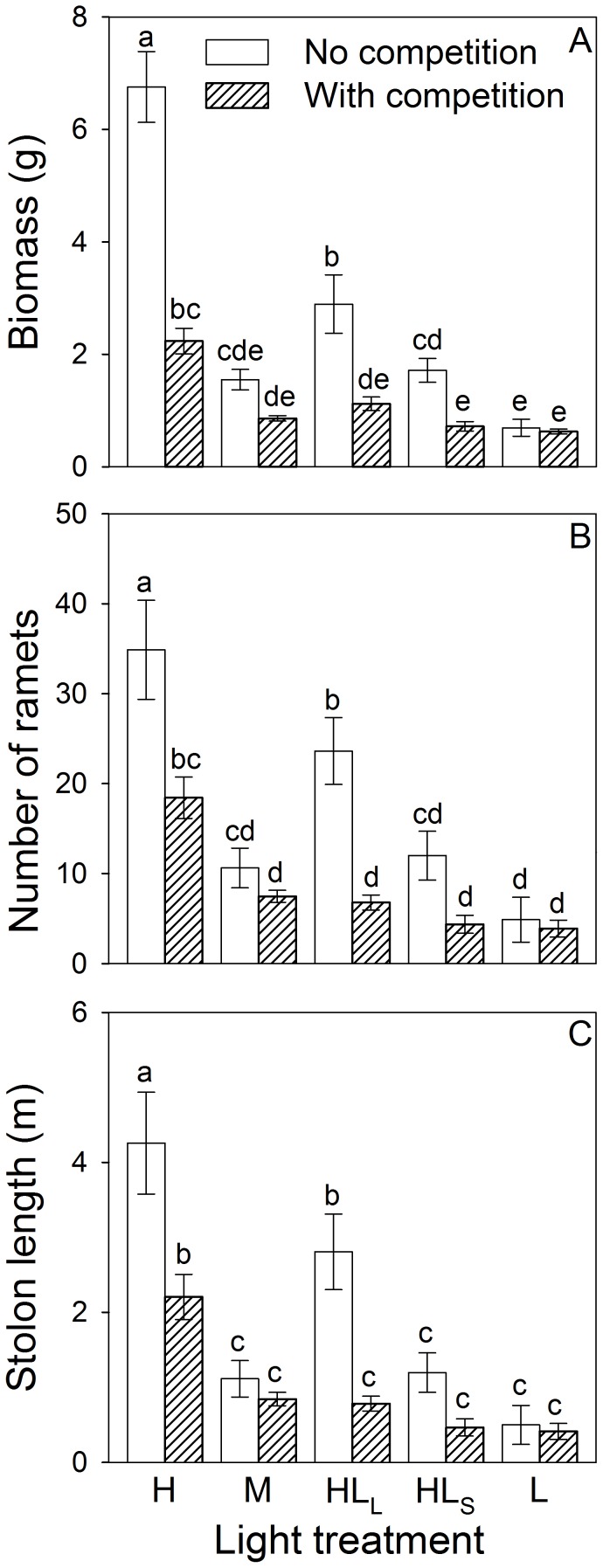
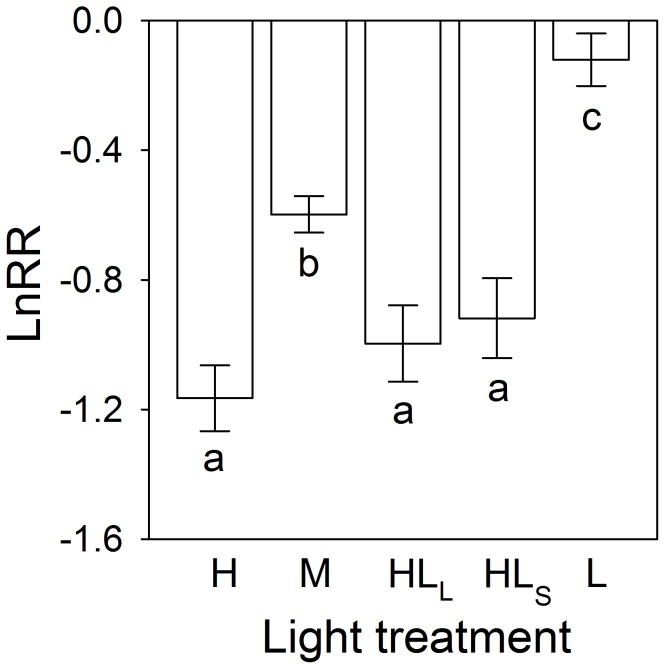
Under homogeneous treatments, decreasing light intensity significantly decreased biomass, number of ramets and total stolon length of D. indica (Fig. 2; Table 1). The presence of neighbors significantly decreased these three growth measures in the high light treatments, but not in the medium or low light conditions (Fig. 2). Decreasing light intensity significantly decreased competition intensity as measured by the increased log response ratio of biomass (Fig. 3).
Figure 2. Effects of competition and light treatment on the whole clone of Duchesnea indica.
Mean values (+SE) of biomass (A), number of ramets (B) and total stolon length (C) are given. Bars sharing the same letters are not different at P = 0.05. Treatment codes are in Figure 1.
Table 1. ANOVAs for effects of competition and light treatments (intensity and heterogeneity) on the growth measures of Duchesnea indica at whole plant level.
| Effect | Biomass | No. of ramets | Stolon length | ||||
| DF | F | P | F | P | F | P | |
| Competition (C) | 1,70 | 76.9 | <0.001 | 28.4 | <0.001 | 25.6 | <0.001 |
| Light (L) | 4,70 | 53.5 | <0.001 | 21.4 | <0.001 | 23.4 | <0.001 |
| C×L | 4,70 | 18.2 | <0.001 | 3.8 | 0.007 | 4.3 | 0.004 |
Figure 3. Effects of light treatment on competition intensity of Duchesnea indica.
The competition intensity was measured by the log response ratio (LnRR) of biomass of the whole clone. Bars are mean values (+SE). Bars sharing the same letters are not different at P = 0.05. Treatment codes are in Figure 1.
Effects of competition and light heterogeneity at whole plant (container) level
Without competition all three growth measures of D. indica in the large patch treatment (HLL) were significantly greater than those in the homogeneous medium light treatment (M). With competition, however, these growth measures did not differ significantly between M and HLL (Fig. 2; Table 1). No matter whether there was competition or not, none of the three growth measures differed significantly between M and the small patch treatment (HLS; Fig. 2). Log response ratio was negative and significantly larger in M than in the two heterogeneous treatments (HLL and HLS; Fig. 3), and it did not differ significantly between HLL and HLS (Fig. 3).
Effects of competition and light heterogeneity at patch level
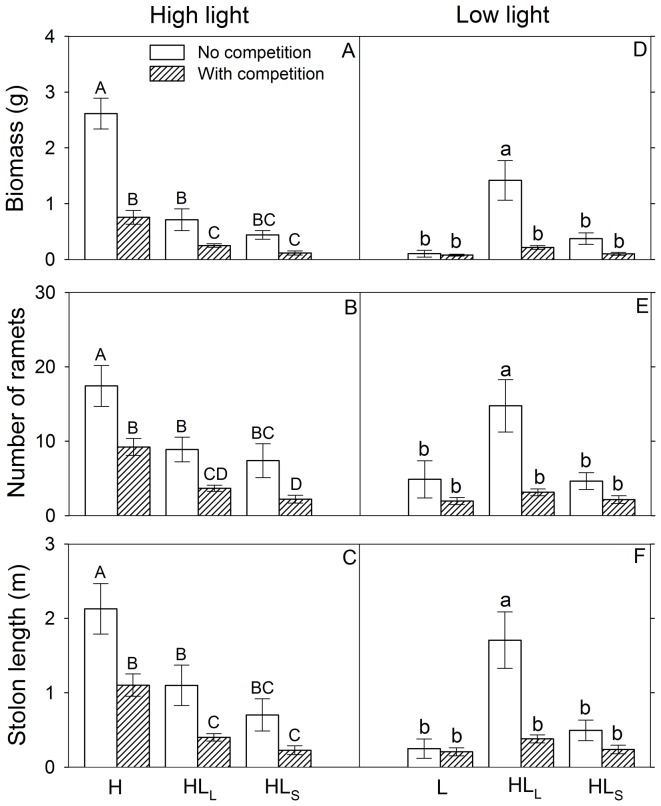
No matter whether there was competition or not, all three growth measures in the high light patches were significantly smaller in HLL or HLS than in the comparable area (half a container) of H (Fig. 4A, B and C, Table 2). Without competition all three growth measures in the low light patches were markedly larger in HLL than in L or HLS, whereas with competition none of these growth measures differed significantly among L, HLL and HLS (Fig. 4D, E and F). Competition greatly decreased all growth measures in HLL, but did not significantly affect the growth in L or HLS (Fig. 4D, E and F).
Figure 4. Effects of competition and light heterogeneity on growth measures of Duchesnea indica.
Bars in the left panels (A–C) are mean values (+SE) of three growth measures in the high light patches and bars in the right panels (D–F) are those in the low light patches. Bars sharing the same letters are not different at P = 0.05. Bars are mean values (+SE). Treatment codes are in Figure 1.
Table 2. ANOVAs for effects of competition and light heterogeneity on the growth measures of Duchesnea indica in the high (A) and low light patches (B).
| Effect | Biomass | No. of ramets | Stolon length | ||||
| DF | F | P | F | P | F | P | |
| (A) High light | |||||||
| Competition (C) | 1,42 | 50.7 | <0.001 | 20.0 | <0.001 | 18.2 | <0.001 |
| Heterogeneity (H) | 2,42 | 50.0 | <0.001 | 14.5 | <0.001 | 16.3 | <0.001 |
| C×H | 2,42 | 15.6 | <0.001 | 0.5 | 0.593 | 0.9 | 0.426 |
| (B) Low light | |||||||
| Competition (C) | 1,42 | 15.8 | <0.001 | 14.1 | 0.001 | 13.9 | 0.001 |
| Heterogeneity (H) | 2,42 | 12.4 | <0.001 | 6.0 | 0.005 | 12.1 | <0.001 |
| C×H | 2,42 | 9.1 | 0.001 | 3.9 | 0.029 | 7.5 | 0.002 |
Discussion
Decreasing light intensity significantly decreased intraspecific competition intensity of D. indica. Many studies have shown that competition becomes more intense when resource supply is higher [19], [35], [36]. The likely reason is that under high resource conditions (e.g., high light conditions in the present study) plants will grow vigorously so that they strongly compete for light, nutrients and/or water, but under very low resource conditions, plants grow so weakly that they do not need to compete for such resources [37]. This explanation is supported by the fact that under high light conditions the presence of neighbors significantly decreased the growth of D. indica but under low light conditions it did not.
Studies generally show positive effects of spatial heterogeneity in resource supply on the growth of single clones [8], [11], [38]–[43]. For instance, clones of G. hederacea grown in heterogeneous conditions in soil nutrients produced over 1.5 times more biomass than those in homogeneous conditions [39], and clones of Potentilla reptans grown in reciprocally or coincidentally patchy conditions both accumulated 70% more biomass than those grown in homogeneous conditions [11]. The underlying mechanism is very likely that in heterogeneous conditions the concentration of ramets, roots or leaves in resource-rich patches allows clones to highly efficiently uptake resources and such resources are re-distributed within the clones through physiological integration to increase the growth of the whole clones [11], [39].
We also found that D. indica without intraspecific competition grew more when light availability was spatially heterogeneous than when it was homogeneous (Fig. 2). During the experiment, the parent ramets of D. indica planted at the borders between high and low light patches produced many offspring ramets that were located either in high light patches or in low light ones (P Wang personal observation). We found that the growth of D. indica in the high or low light patches of the heterogeneous treatments differed greatly from that in the corresponding areas of the homogeneous treatments (Fig. 4). These results suggest that clonal integration (most likely for carbohydrates) was likely to occur among interconnected parent ramets in the patch borders and offspring ramets located in the high or low light patches in the heterogeneous light treatments [5], [44]. Such clonal integration may have markedly increased the growth of the plants in the low light patches, and further led to the increased growth of the whole plant in the large patch treatment without competition (Fig. 2).
However, when there was strong intraspecific competition, the growth of D. indica could not benefit from spatial heterogeneity in light supply, which has not been reported before. The reason might be that, when growing in heterogeneous conditions, all clones will concentrate their leaves or offspring ramets in the high light patches [4], [11], [39], [45]. This may result in great costs for intraspecific competition [37], and thus plants of D. indica with intraspecific neighbors could not benefit from light heterogeneity.
Light heterogeneity significantly increased the intraspecific competition intensity of D. indica. One explanation is that the ability of physiological integration and thus the ability to selectively place offspring ramets differ greatly among the genotypes of D. indica. When plants with different ability of physiological integration and/or morphological plasticity are grown in the same heterogeneous environment, the beneficial effects of heterogeneity on the growth will differ among plants [7], [16]. As a result, heterogeneity will change the intraspecific competition intensity. Another explanation is that when growing in heterogeneous conditions all plants will concentrate their leaves in the high light patches, which greatly increases the intensity of intraspecific competition [19], [37], [46]–[48].
Our results suggest for the first time that spatial heterogeneity in light supply can change intraspecific interactions of clonal plants. Therefore, spatial heterogeneity in light supply may be of great importance in regulating population structure and dynamics of clonal plants [11], [19], [46], [47], [49].
Acknowledgments
We thank Da-Yong Zhou, Bi-Cheng Dong, Jian Zhou, Qian Zhang, Hui-Feng Lin, Na Zhao and Peng-Cheng Shi for assistance with measurements and harvest.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Fundamental Research Funds for the Central Universities (Grant JC2011-4) and the National Science Foundation of China (Grant 31070371). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baldocchi D, Collineau S. Caldwell MM, Pearcy RW, editors. The physical nature of solar radiation in heterogeneous canopies: spatial and temporal attributes. 1994. pp. 21–71. Exploitation of environmental heterogeneity by plants: ecophysiological processes above and below ground. San Diego: Academic Press.
- 2.Hutchings MJ, Wijesinghe DK. Hutchings MJ, John EA, Stewart AJA, editors. The effects of heterogeneous nutrient supply on plant performance: a survey of responses, with special reference to clonal herbs. 2000. pp. 91–110. The ecological consequences of environmental heterogeneity. London: Blackwell.
- 3.Maestre FT, Reynolds JF. Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology. 2007;88:501–511. doi: 10.1890/06-0421. [DOI] [PubMed] [Google Scholar]
- 4.Dong M. Morphological plasticity of the clonal herb Lamiastrum galeobdolon (L.) Ehrend. & Polatschek in response to partial shading. New Phytologist. 1993;124:291–300. doi: 10.1111/j.1469-8137.1993.tb03819.x. [DOI] [PubMed] [Google Scholar]
- 5.Stuefer JF, During HJ, de Kroon H. High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environments. Journal of Ecology. 1994;82:511–518. [Google Scholar]
- 6.Tomasko DA, Dawes CJ. Evidence for physiological integration between shaded and unshaded short shoots of Thalassia testudinum. Marine Ecology-Progress Series. 1989;54:299–305. [Google Scholar]
- 7.Alpert P, Holzapfel C, Slominski C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. Journal of Ecology. 2003;91:27–35. [Google Scholar]
- 8.Chen J-S, Lei N-F, Yu D, Dong M. Differential effects of clonal integration on performance in the stoloniferous herb Duchesnea indica, as growing at two sites with different altitude. Plant Ecology. 2006;183:147–156. [Google Scholar]
- 9.Chen J-S, Yu D, Liu Q, Dong M. Clonal integration of the stoloniferous herb Fragaria vesca from different altitudes in Southwest China. Flora. 2004;199:342–350. [Google Scholar]
- 10.Guo W, Song Y-B, Yu F-H. Heterogeneous light supply affects growth and biomass allocation of the understory fern Diplopterygium glaucum at high patch contrast. Plos One. 2011;6:e27998. doi: 10.1371/journal.pone.0027998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W-M, Alpert P, Yu F-H, Zhang L-L, Dong M. Reciprocal and coincident patchiness of multiple resources differentially affect benefits of clonal integration in two perennial plants. Journal of Ecology. 2011;99:1202–1210. [Google Scholar]
- 12.Janecek S, Kantorova J, Bartos M, Klimesova J. Integration in the clonal plant Eriophorum angustifolium: an experiment with a three-member-clonal system in a patchy environment. Evolutionary Ecology. 2008;22:325–336. [Google Scholar]
- 13.Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- 14.Roiloa SR, Retuerto R. Responses of the clonal Fragaria vesca to microtopographic heterogeneity under different water and light conditions. Environmental and Experimental Botany. 2007;61:1–9. [Google Scholar]
- 15.Saitoh T, Seiwa K, Nishiwaki A. Importance of physiological integration of dwarf bamboo to persistence in forest understory: a field experiment. Journal of Ecology. 2002;90:78–85. [Google Scholar]
- 16.Alpert P. Effects of clonal integration on plant plasticity in Fragaria chiloensis. Plant Ecology. 1999;141:99–106. [Google Scholar]
- 17.Hartnett DC, Bazzaz FA. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology. 1983;64:779–788. [Google Scholar]
- 18.Bliss KM, Jones RH, Mitchell RJ, Mou PP. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytologist. 2002;154:409–417. doi: 10.1046/j.1469-8137.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- 19.Day KJ, John EA, Hutchings MJ. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Functional Ecology. 2003;17:454–463. [Google Scholar]
- 20.Fransen B, de Kroon H, Berendse F. Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology. 2001;82:2534–2546. [Google Scholar]
- 21.Rajaniemi TK. Root foraging traits and competitive ability in heterogeneous soils. Oecologia. 2007;153:145–152. doi: 10.1007/s00442-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 22.van der Waal C, Kool A, Meijer SS, Kohi E, Heitkönig IMA, et al. Large herbivores may alter vegetation structure of semi-arid savannas through soil nutrient mediation. Oecologia. 2011;165:1095–1107. doi: 10.1007/s00442-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijesinghe DK, John EA, Hutchings MJ. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology. 2005;93:99–112. [Google Scholar]
- 24.Chu Y, Yu F-H, Dong M. Clonal plasticity in response to reciprocal patchiness of light and nutrients in the stoloniferous herb Glechoma longituba L. Journal of Integrative Plant Biology. 2006;48:400–408. [Google Scholar]
- 25.Xu C-Y Schooler SS, van Klinken RD. Effects of clonal integration and light availability on the growth and physiology of two invasive herbs. Journal of Ecology. 2010;98:833–844. [Google Scholar]
- 26.Stuefer JF. Potential and limitations of current concepts regarding the response of clonal plants to environmental heterogeneity. Vegetatio. 1996;127:55–70. [Google Scholar]
- 27.Wijesinghe DK, Hutchings MJ. The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. Journal of Ecology. 1999;87:860–872. [Google Scholar]
- 28.Wijesinghe D, Hutchings M. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: an experimental study with Glechoma hederacea. Journal of Ecology. 1997;85:17–28. [Google Scholar]
- 29.Naruhashi N, Sugimoto M. The floral biology of Duchesnea (Rosaceae). Plant Species Biology. 1996;11:173–184. [Google Scholar]
- 30.Anon. Invonographia Cormophytorum Sinicorum (Tomus?). Science press, Beijing. 1994. 278
- 31.Dong M, S-M Zhang, Y-F Chen. Clonal plasticity in response to nutrient availability in the stoloniferous herb Duchesnea indica. Acta Botanica Sinica. 2000;42:518–522. [Google Scholar]
- 32.Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Qaten A. Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology. 1999;80:1119–1131. [Google Scholar]
- 33.James J, Richards J. Influence of temporal heterogeneity in nitrogen supply on competitive interactions in a desert shrub community. Oecologia. 2007;152:721–727. doi: 10.1007/s00442-007-0685-3. [DOI] [PubMed] [Google Scholar]
- 34.Zar JH. Upper Saddle River, NJ: Prentice Hall. 1999. Biostatistical analysis, 4th edn.
- 35.Hodge A, Robinson D, Griffiths B, Fitter A. Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant, Cell and Environment. 1999;22:811–820. [Google Scholar]
- 36.Robinson D, Hodge A, Griffiths BS, Fitter AH. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society of London Series B: Biological Sciences. 1999;266:431–435. [Google Scholar]
- 37.Kleunen M, Fischer M, Schmid B. Effects of intraspecific competition on size variation and reproductive allocation in a clonal plant. Oikos. 2001;94:515–524. [Google Scholar]
- 38.Alpert P, Mooney H. Resource sharing among ramets in the clonal herb, Fragaria chiloensis. Oecologia. 1986;70:227–233. doi: 10.1007/BF00379244. [DOI] [PubMed] [Google Scholar]
- 39.Birch CPD, Hutchings MJ. Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology. 1994;82:653–664. [Google Scholar]
- 40.Du J, Yu F-H, Alpert P, Dong M. Arbuscular mycorrhizal fungi reduce effects of physiological integration in Trifolium repens. Annals of Botany. 2009;104:335–344. doi: 10.1093/aob/mcp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman D, Alpert P. Reciprocal transport between ramets increases growth of Fragaria chiloensis when light and nitrogen occur in separate patches but only if patches are rich. Oecologia. 1991;86:76–80. doi: 10.1007/BF00317392. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Li Y, During HJ, Li L. Do clonal plants show greater division of labour morphologically and physiologically at higher patch contrasts? Plos One. 2011;6:e25401. doi: 10.1371/journal.pone.0025401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Dong B-C, Alpert P, Li H-L, Zhang M-X, et al. Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Annals of Botany. 2012;109:813–818. doi: 10.1093/aob/mcr314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wijesinghe DK, Hutchings MJ. Consequences of patchy distribution of light for the growth of the clonal herb Glechoma hederacea. Oikos. 1996;77:137–145. [Google Scholar]
- 45.Dong M. Morphological responses to local light conditions in clonal herbs from contrasting habitats, and their modification due to physiological integration. Oecologia. 1995;101:282–288. doi: 10.1007/BF00328813. [DOI] [PubMed] [Google Scholar]
- 46.Day KJ, Hutchings MJ, John EA. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. Journal of Ecology. 2003;91:541–553. [Google Scholar]
- 47.Day KJ, Hutchings MJ, John EA. The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. Journal of Ecology. 2003;91:305–315. [Google Scholar]
- 48.Littschwager J, Lauerer M, Blagodatskaya E, Kuzyakov Y. Nitrogen uptake and utilisation as a competition factor between invasive Duchesnea indica and native Fragaria vesca. Plant and Soil. 2010;331:105–114. [Google Scholar]
- 49.Hutchings MJ, John EA, Wijesinghe DK. Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology. 2003;84:2322–2334. [Google Scholar]