Abstract
Functional neuroimaging has shown that multiple brain regions are active during volitional swallowing. Little is known, however, about which regions integrate motor execution and sensory feedback in the swallowing system. Although unilateral brain lesions in either hemisphere can produce swallowing deficits, some functional neuroimaging studies indicate that the left hemisphere has greater activation in certain sensory and motor-related swallowing regions. In this study, correlation coefficients were computed for five seed regions during volitional saliva swallowing to determine the functional relationships of these regions with the rest of the brain: the anterior and posterior insula, inferior frontal gyrus (BA44), primary sensory cortex (S1), and primary motor cortex (M1). A laterality index (LI) was derived that accounts for relative differences in total, positive connected voxels for the left/right hemisphere seeds. Clusters of significantly connected voxels were greater from the anterior and posterior insula than from the other three seed regions. Interactions of the insula with other brain regions were greater on the left than on the right during volitional swallowing. Group means showed laterality in the anterior insula (LI = 0.25) and the posterior insula (LI = 0.33). BA44 showed a lesser degree of difference in left versus right hemisphere interactions (LI = 0.12) while S1 did not show lateralization (LI = 0.02) and M1 showed some predominance of interactions in the right hemisphere (LI = −0.19). The greater connectivity from the left hemisphere insula to brain regions within and across hemispheres suggests that the insula is a primary integrative region for volitional swallowing in humans.
Keywords: Swallowing, Neuroimaging, Functional connectivity, Correlations, fMRI
Introduction
Swallowing is a complex behavior with dynamic neural coordination at the cerebral as well as brainstem levels. Functional magnetic resonance imaging (fMRI) studies have identified anatomic regions that are active during swallowing, including the primary sensory and motor cortex, supplementary motor area (SMA), cingulate cortex, insula, operculum, prefrontal and inferior frontal cortex, basal ganglia, thalamus, and cerebellum (Hamdy et al. 1999; Kern et al. 2001a, b; Martin et al. 2001a, 2004; Lowell et al. 2008; Soros et al. 2008; Malandraki et al. 2009). The majority of these studies have addressed the individual or modular activation of various brain regions during swallowing and related tasks, but have not determined the functional relationships and integrative importance of these regions within the swallowing neural network. Among these modular studies of swallowing, laterality of brain function has been a topic of interest due to its implications for the management of swallowing disorders, which are frequently related to unilateral or bilateral cerebral vascular accidents (CVA). Whereas several studies have addressed hemispheric differences in modular activation of brain regions during swallowing, determining the laterality of functionally interacting regions would yield new information about the neural networks involved during volitional swallowing.
Mosier and Bereznaya (2001) implemented a principal component analysis to determine whether functional connections between swallowing regions supported a hierarchical pathway organization involving serial components, or a multilevel organization involving parallel processing systems. Results indicated that multiple parallel pathways were involved in volitional swallowing, with network modules that included primary sensory and motor regions, motor planning regions, the inferior frontal gyrus (IFG), the insula and the cerebellum. Among these regions, anatomic lesion studies have indicated that the insula (Daniels et al. 1996; Daniels and Foundas 1997; Riecker et al. 2009), frontal operculum (Meadows 1973; Pender and Ferguson 2007) and primary sensorimotor cortex (Daniels et al. 1996) may be necessary for normal swallowing. Swallowing dysfunction occurs with isolated insular lesions (Daniels and Foundas 1997) and is frequently associated with damage to the sensorimotor cortex. Cortical regions just anterior to the primary motor cortex can evoke responses in swallowing musculature when transcranial magnetic stimulation is applied (Hamdy et al. 1996), and electrical stimulation of similar regions in early human neurosurgery studies produced a swallowing response (Penfield and Boldery 1937). The primary sensorimotor regions and the insula involve complex neural integration of sensory and motor information, with neural representation in the insula for multiple sensory components that include taste, temperature and proprioception (Francis et al. 1999; Craig 2003; Brooks et al. 2005).
Lateralization of swallowing function has implications for the effects of neurological damage and the potential for recovery through contralateral hemisphere compensation (Hamdy et al. 1998; Hamdy and Rothwell 1998). At the group level, neuroimaging studies have generally not shown an overall hemispheric predominance during swallowing across multiple brain regions (Hamdy et al. 1999; Martin et al. 2001a). Data suggest that individuals may show an overall hemisphere predominance (Hamdy et al. 1996, 1997) that gets averaged out at the group level. Hemispheric lateralization of specific brain regions that are important in swallowing has been reported at the group level, but the findings are not always consistent. Volume and strength of activation in the insula has shown right hemisphere predominance in some studies (Hamdy et al. 1999; Kern et al. 2001a; Martin et al. 2001a) and left hemisphere predominance in other studies (Dziewas et al. 2003; Watanabe et al. 2004). Greater activity of the left hemisphere in the IFG/operculum and sensorimotor cortex is evidenced in some magnetoencephalography (MEG) and fMRI studies (Dziewas et al. 2003; Watanabe et al. 2004; Martin et al. 2007), although this lateralization can vary by swallowing task (Mosier et al. 1999b; Martin et al. 2001a).
Understanding more about the integration among swallowing regions for each hemisphere may help explain the occurrence of dysphagia with unilateral CVA, and may guide the application of rehabilitative techniques to regions that can maximally communicate with other areas of the brain. For example, rather than assessing laterality of activation for individual regions, an alternative approach is to determine the degree of lateralized connectivity that a given region shows throughout the brain. By studying differences in functional connectivity of left versus right hemisphere regions, relative communication of each to areas throughout the brain can be assessed. Degree of brain-wide communication of regions will fundamentally impact the resulting disruption after a CVA and the potential role of the region in recovery, which may require cross-communication with regions in the unaffected hemisphere (Hamdy et al. 1998; Hamdy and Rothwell 1998).
The purpose of this study was to determine the functional interactions of several brain regions that are critical to the cortical control of swallowing, specifically the insula, primary sensorimotor cortex, and IFG, and to determine whether laterality of functional interactions is evidenced for each region during swallowing. Multiple analysis methods can be implemented to study functional interactions between brain regions (Horwitz 2003), but several of these methods require prior knowledge of anatomical connections between regions and directionality of interactions (McIntosh et al. 1994; Marrelec et al. 2006a, b). Validity of these analyses is dependent on accurate modeling (Friston 1994). Knowledge of cortical anatomical pathways and their directionality in human swallowing is limited to inferences based on animal models (Sumi 1969; Car 1973; Jean and Car 1979; Miller 1986) or early clinical stimulation studies (Penfield and Boldery 1937). The study of simple correlations (i.e., functional connectivity) between brain regions does not require anatomical modeling, but these correlations can be influenced by multiple factors such as stimulus-related inputs, common neural inputs or common synaptic connections (Friston 1994; Bokde et al. 2001). To reduce the influence of stimulus-based inputs, we studied interactions of specified regions through correlations of the blood oxygen level dependent (BOLD) signal residuals that remained after all task, head motion and other modeled effects were removed.
Materials and methods
Participants
Fourteen healthy adults (7 males, 7 females, mean age 52 ± 10.4 years) participated in this study. All participants were native English speakers and had no history of neurological, swallowing, or psychiatric disorders. All participants provided written informed consent prior to participating in the study, which was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Tasks and equipment
Functional magnetic resonance imaging (fMRI) data were used to determine the functional connectivity of specific regions to the rest of the brain during swallowing. Data for this functional connectivity analysis were collected for a previously published study that addressed the univariate analysis of swallowing and related tasks (Lowell et al. 2008). The present study determined the functional interactions of regions during volitional, saliva swallowing, which was shown to be statistically equivalent to water swallowing in a prior study that addressed functional connectivity (Mosier and Bereznaya 2001). Additional tasks that were included in the original study paradigm were oral-sensory (air-pulse) stimulation, covert swallowing and breath-holding. During air-pulse stimulation, participants received a series of air pulses directed to the right posterior oral area. Compressed air was transmitted through tubing inserted into a dental impression that was fit to the right posterior dentition. During covert swallowing, participants imagined what they feel and do during a saliva swallow, without actually completing a swallow. Before the scanning session, participants were trained to assure correct task performance. Swallowing and other tasks were visually cued with black and white images displayed via projector/screen using Eprime stimulus presentation software (Psychology Software Tools, Inc., Pittsburgh, PA).
For the volitional swallowing task, participants swallowed their own saliva once every 10 s (for three swallows per block) while minimizing any head motion. This swallowing interval has been successfully implemented by other researchers (Suzuki et al. 2003) and allows sufficient recovery time between swallows (Kleinjan and Logemann 2002). Swallowing was continuously monitored using an MRI-compatible pneumatic belt placed on the neck at the thyro-hyoid level and adjusted to provide a maximum change during a swallow. This allowed verification of the cued swallow responses, as well as the determination of uncued swallows (which were added into the regression model to account for their effects). An additional pneumatic belt was placed over the abdomen to record abdominal movement during respiration for verification of breath-holding. The resulting signals were displayed and recorded using a PowerLab 16/30 data acquisition system (ADInstruments, Inc., Colorado Springs, CO).
Functional image acquisition
Using a mixed design, three discrete swallowing trials were elicited every 10 s within a 30-s block, with an additional rest period of 10 s imposed after the third trial and prior to the next 30-s block. Thus, there was a 20-s gap between the last trial in one block/condition and the first trial in the next block/condition, sufficiently separating each condition. Each slow epoch trial was synchronized with continuous scanning and full brain coverage every 2 s. Each trial lasted for 3 s followed by 7 s of rest/fixation. Scanning onsets were synchronized to occur at 0, 2, 4, 6, and 8 s after trial onset. With a TR of 2.0 s, we sufficiently sampled the data to capture the form of the hemodynamic response for each of the three trials within a block. Three blocks of the volitional swallowing task were randomly presented among other tasks in each run. A total of 45 swallowing trials were collected, with 9 trials in each of 5 runs.
Images were acquired in a 3.0-T scanner (Signa, General Electric Medical Systems, Milwaukee, WI). Continuous scanning acquisition was used to collect echo-planar images during each scanning run using: TR = 2.0 s, TE = 30 ms, flip angle = 90°, FOV = 240 mm, 35 sagittal slices, and slice thickness = 4 mm(no gap), with 240 volumes acquired per run. Four initial scans were included at the start of each run to allow the signal to reach a steady state, which were then discarded from the data analysis. A high-resolution, T1-weighted structural scan for anatomic localization and co-registration was also collected using the following parameters: MPRAGE, TE = 3.0 ms, TI = 450 ms, flip angle = 10°, bandwidth = 31.25, FOV = 240 mm, slices = 128, and slice thickness = 1.3 mm (no gap).
Functional image analysis for correlations
To investigate functional interactions, we determined the correlation coefficients between designated seed regions and all other brain regions, with seed regions derived from cortical sensory and motor regions that are consistently represented in swallowing. All image processing and analysis was performed using AFNI (Cox 1996) software. Multiple head motion parameters were implemented in the regression model to minimize false-positive activation, and a cluster threshold was used to correct for multiple comparisons (Soltysik and Hyde 2006). Preprocessing steps included correction of slice acquisition timing, motion correction (three translation and three rotation parameters), spatial smoothing with a 4-mm full-width at half max Gaussian filter, and scaling by the voxel-wise mean across time to percent signal change. Uncued swallows throughout each functional run were entered as a separate regressor of no interest to control for their effects during other conditions. Hemodynamic response function (HRF) effects due to uncued swallows were therefore minimized from the results. Initial group univariate analysis of functional data was performed to generate the seed coordinates for the later correlation analysis. Response amplitude was estimated as a regression coefficient for each condition in a multiple linear regression model. The regressor corresponding to each condition was created using a fixed-shape, gamma-variate hemodynamic response function (HRF) convolved with the durations (3-s boxcars) of all trials of that condition. Using this model, the idealized curves clearly showed three separate response peaks within each 30-s stimulus block of three trials, along with the expected overlap of the HRF tails. Each participant’s amplitude coefficients were transformed to standard space (Talairach and Tournoux 1988), and group activation maps were generated using a mixed effects analysis of variance (ANOVA).
For generating the seed regions, five bilateral cortical regions of interest (ROIs) were selected to represent sensory, motor, or mixed sensory–motor regions whose importance has been demonstrated in swallowing (Hamdy et al. 1999; Kern et al. 2001b; Martin et al. 2001a, 2007; Suzuki et al. 2003; Lowell et al. 2008; Malandraki et al. 2009): the primary sensory (S1) and primary motor (M1) cortex, anterior and posterior insula, and IFG. ROIs were generated from neuroanatomical atlas plug-ins available in AFNI. Specific, constituent portions of large regions of interest were used to allow comparisons of seed regions across hemispheres. For S1, the cytoarchitectonic area 3b was chosen based on its highest maximum voxel activation in the univariate analysis relative to areas 1, 2 and 3a, and due to its importance in processing tactile sensation (Kandel et al. 2000). For M1, area 4p was chosen based on its highest maximum voxel activation in the univariate analysis relative to area 4a, and due to its previously demonstrated role in laryngeal control (Simonyan et al. 2009). For the IFG, Brodmann’s area (BA) 44 was selected based on its involvement in multiple univariate studies of swallowing (Hamdy et al. 1999; Mosier et al. 1999b; Martin et al. 2001a, 2007). The cytoarchitectonic areas 3b, 4p and BA44 were extracted using maximum probability maps (Amunts et al. 1999, 2004; Eickhoff et al. 2006a, b) and all other regions were extracted from macro-label maps (Eickhoff et al. 2005). The insula was subdivided into anterior and posterior portions for increased specificity. The overall mask for the insula was divided by placing all voxels anterior to the y-coordinate zero point into the anterior insula mask, and all voxels posterior to y = zero into the posterior insula mask.
Seed coordinates were then determined for each region from the maximum t statistic voxel based on the group activation maps (corrected P < 0.05) from the univariate group analysis. See Table 1 for a list of all seed coordinates. Because maximally activated voxel clusters often spanned multiple atlas regions, seed coordinates for some neighboring ROIs such as the anterior and posterior insula were close to each other. Each participant’s time series was converted to standardized space (Talairach and Tournoux 1988) so that subsequent correlation analyses were performed with voxel correspondence to the group activation maps. The following steps were implemented to produce the seed time series for our correlation analyses. For each participant, a concatenated voxel-wise time series was first generated, representing the residuals after all modeled effects (tasks, 6 motion parameters, uncued swallows) were removed from the time series. Next, a seed region with a 4-mm radius was designated for each apriori ROI around the seed coordinates specified in Table 1. The data from the residuals were extracted at each ROI and then spatially averaged within the ROI. A task-specific seed time series was then generated that represented the mean signal for all voxels within the sphere. Each time series was limited to the TRs with a modeled BOLD response for the given task, with a separate time series generated for each of the four tasks. TRs that spanned each full 30-s stimulus block for the task plus the duration of the expected HRF were included, with all other TRs that were not relevant to the task removed. The remaining seed time series therefore represented the task-specific signal at the seed.
Table 1.
Seed coordinates for each region of interest, given in Talairach and Tournoux, RAI coordinate system
| Seed region | Volitional swallowing seed coordinates
|
||
|---|---|---|---|
| x | y | z | |
| Left area 3b | −50 | −11 | 27 |
| Right area 3b | 53 | −11 | 27 |
| Left area 4p | −50 | −11 | 33 |
| Right area 4p | 44 | −14 | 33 |
| Left BA44 | −53 | 2 | 18 |
| Right BA44 | 56 | 2 | 18 |
| Left anterior insula | −29 | 5 | 12 |
| Right anterior insula | 47 | 5 | 6 |
| Left posterior insula | −32 | −5 | 12 |
| Right posterior insula | 47 | −2 | 6 |
Voxel-wise analysis of the task-specific, partial correlations was performed for each participant between the task-specific signal at the seed and the rest of the brain while controlling for all other modeled effects. The multiple linear regression equation for each participant and task included the task-specific seed time series as the main regressor of interest. To account for all sources of signal variation, main task effects, the six head motion parameters and uncued swallow effects were all included in the overall regression model. By removing all modeled effects and examining the correlations of the remaining task-specific signal fluctuations, correlations better reflect the signal that may be related to common neural inputs or connections rather than the variance of the model-related regressors. From the regression analysis, task-specific correlation coefficients were thus generated for each participant as the dependent variables. The variable of interest was the correlations of the volitional swallowing seed time series against the whole brain time series for that task. Correlation coefficients were then normalized to Z scores by applying Fisher’s r-to-z′ transformation.
Next, group maps of the Z scores were generated using mixed effects ANOVA. To correct for multiple comparisons, cluster thresholding was applied to all data based on a Monte Carlo simulation that produced a corrected family-wise P value of < 0.05 at an individual voxel threshold of P = 0.0001. To determine lateralization of connectivity for specific seed regions, a laterality index (LI) was computed based on comparisons of total volume of significantly activated voxels in each hemisphere (Simonyan et al. 2009; Gaillard et al. 2011; Niskanen et al. 2012). The LI formula that we used accounts for connectivity both within and across hemispheres (Liu et al. 2009). The relative differences in total, positive connected voxels that exceeded the corrected, cluster threshold were computed for each left/right hemisphere seed region and its corresponding left/right hemisphere target, using the following formula in which LL is the left seed, left hemisphere connectivity, LR is the left seed, right hemisphere connectivity, RL is the right seed, left hemispheres connectivity, and RR is the is the right seed, right hemisphere connectivity:
LIs were computed to address the lateralization of S1, M1, BA44 and the anterior and posterior insula during volitional swallowing. In addition, contrasts were performed with Z score seed maps to determine any statistically significant differences between hemispheres.
Results
All results are provided as Z score transformations of correlations that showed significant connectivity after cluster thresholding was applied (corrected P < 0.05). Functional connections across multiple brain regions were demonstrated for all seed regions and were predominantly positive correlations. Of the total volume of significant correlations for each seed region during volitional swallowing, positive correlations represented 98.0 % for the insula as the seed region, 97.8 % for the primary motor cortex as the seed region, 92.9 % for the primary somatosensory cortex as the seed region, and 97.4 % for the BA44 as the seed region. Therefore, lateralization analyses focused on positive correlations to represent consistent types of interactions across regions.
Group lateralization of connectivity
To determine the extent of lateralized connectivity during swallowing, LIs were computed for the significantly correlated voxels that survived after cluster thresholding (corrected P < 0.05) in the left and right hemisphere for each ROI, with positive LI values reflecting a left hemisphere asymmetry and negative LI values representing a right hemisphere asymmetry. Researchers have defined hemispheric dominance by different levels of the LI, such as > 0.1 or < −0.1 (Niskanen et al. 2012), ≥0.2 or ≤ −0.2 (Gaillard et al. 2011), or > 0.3 or < −0.3 (Liu et al. 2009). In the current study, we consider LI values of > 0.3 or < −0.3 to show definitive lateralization.
Anterior insula
The LI for the anterior insula during volitional swallowing was 0.25. Volume of connectivity for the anterior insula was large and spanned both hemispheres. When considering the left and right hemisphere seeds, bilateral regions that were significantly connected to the anterior insula included the cerebellum, thalamus and basal ganglia regions, claustrum, lingual gyrus and cuneus/precuneus and other occipital lobe regions, insula, inferior, middle and superior frontal gyrus, multiple portions of the temporal and parietal lobes, primary sensorimotor cortex, secondary somatosensory cortex, cingulate gyrus (all portions), and the SMA. Connectivity for the left anterior insula as seed was predominantly symmetrical in the left and right hemispheres, whereas connectivity for the right anterior insula as seed showed greater volume in the ipsilateral hemisphere.
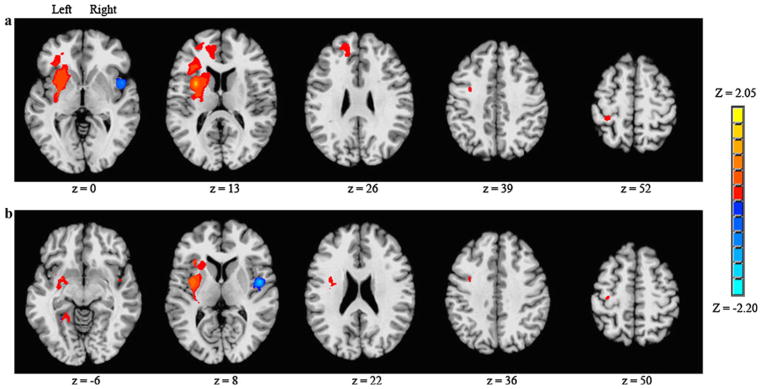
A contrast was performed to determine significant differences in brain connectivity for the anterior insula, left versus right hemisphere seeds (Fig. 1a). Surviving voxels for the left > right contrast (positive, red-tone voxels) showed substantially more brain regions and greater volume of connected voxels (20,250 mm3) than the right > left contrast (negative, blue-tone voxels, 2,160 mm3). Brain regions showing significantly more connectivity for the left versus right hemisphere seed included the putamen, insula, inferior, middle and superior frontal gyrus, anterior cingulate, and the precentral gyrus regions in the left hemisphere. Brain regions showing significantly more connectivity for the right versus left hemisphere seed were limited to the cerebellum and insula in the right hemisphere. However, interpretation of cerebellar connectivity is complicated by the fact that cortico-cerebellar fibers cross over to the contralateral side.
Fig. 1.
Anterior (a) and posterior (b) insula seed, left versus right seed contrast for volitional swallowing. Significant Z score clusters of connectivity are displayed (P < 0.05, corrected). Left > right shown as positive values, right > left shown as negative values
Posterior insula
The LI for the posterior insula during volitional swallowing was 0.33. When considering the left and right hemisphere seeds, regions that were significantly connected to the posterior insula were similar to those that were described for the anterior insula. Connectivity for the left posterior insula as seed was symmetrically represented in the left and right hemispheres, whereas connectivity for the right posterior insula as seed showed greater volume in the ipsilateral hemisphere.
A contrast was performed to determine significant differences in brain connectivity for the posterior insula, left versus right hemisphere seeds (Fig. 1b). Surviving voxels for the left > right contrast showed substantially more brain regions and greater volume of connected voxels (7,317 mm3) than the right > left contrast (1,485 mm3). Brain regions showing significantly more connectivity for the left versus right hemisphere seed included the amygdala, lingual gyrus, putamen, insula, IFG, and the precentral gyrus regions in the left hemisphere. Brain regions showing significantly more connectivity for the right versus left hemisphere seed were limited to the insula in the right hemisphere.
Inferior frontal gyrus (BA44)
The LI for the IFG (BA44) during volitional swallowing was 0.12. Volume of connectivity for the IFG included large regions throughout the brain and was represented bilaterally and relatively symmetrically in both hemispheres. Regions with significant connectivity included the cerebellum, thalamus and basal ganglia regions, claustrum, lingual gyrus and cuneus/precuneus, multiple occipital lobe regions, insula, inferior, middle and superior frontal gyrus, inferior, middle and superior temporal gyrus, primary sensorimotor cortex, premotor cortex, secondary somatosensory cortex, cingulate gyrus (all portions), inferior parietal lobe, and the SMA.
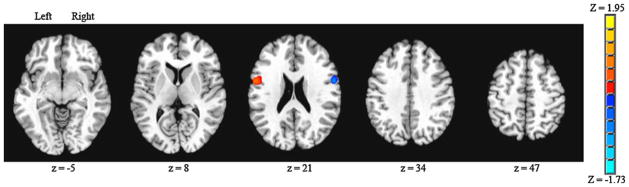
A contrast was performed to determine significant differences in brain connectivity for the IFG (BA44), left versus right hemisphere seeds (Fig. 2). Surviving volume of voxels for the left > right contrast (1,377 mm3) was numerically greater than the volume of connected voxels for the right > left contrast (918 mm3). Brain regions that showed significantly greater connectivity for either contrast were in symmetrical locations of each hemisphere that only included the BA44 region.
Fig. 2.
Inferior frontal gyrus (Brodmann’s area 44) seed, left versus right seed contrast for volitional swallowing. Significant Z score clusters of connectivity are displayed (P < 0.05, corrected). Left > right shown as positive values, right > left shown as negative values
Primary sensory cortex (area 3b)
The LI for S1 (area 3b) during volitional swallowing was 0.02. Volume of connectivity for S1 included regions throughout the brain and was represented bilaterally and generally symmetrically in both hemispheres. When considering the left and right hemisphere seeds, bilateral regions that were significantly connected to S1 included the cerebellum, thalamus and basal ganglia regions, lingual gyrus and cuneus/precuneus and other occipital lobe regions, insula, inferior, and middle frontal gyrus, superior temporal gyrus, supramarginal gyrus, right and left inferior parietal lobe, primary sensorimotor cortex, secondary somatosensory cortex, middle cingulate gyrus, and the SMA.
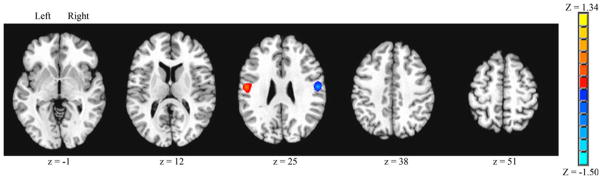
A contrast was performed to determine significant differences in brain connectivity for S1 (area 3b), left versus right hemisphere seeds (Fig. 3). Surviving voxels for the left > right contrast were nearly identical in volume (1,215 mm3) to the right > left contrast (1,269 mm3). Brain regions that showed significantly greater connectivity for each contrast were symmetrically represented and were limited to a focal area that included areas 3a and 3b of S1, areas OP1 and OP4 of the secondary somatosensory cortex, and area 4p of M1.
Fig. 3.
Primary sensory cortex (area 3b) seed, left versus right seed contrast for volitional swallowing. Significant Z score clusters of connectivity are displayed (P < 0.05, corrected). Left > right shown as positive values, right > left shown as negative values
Primary motor cortex (area 4p)
The LI for M1 (area 4p) during volitional swallowing was −0.19. Volume of connectivity for M1 included regions throughout the brain and was represented bilaterally and relatively symmetrically in both hemispheres. When considering the left and right hemisphere seeds, bilateral regions that were significantly connected to M1were nearly identical to those regions described for area 3b as seed, although connectivity to the cingulate cortex was more extensive from area 4p as the seed than it was for area 3b.
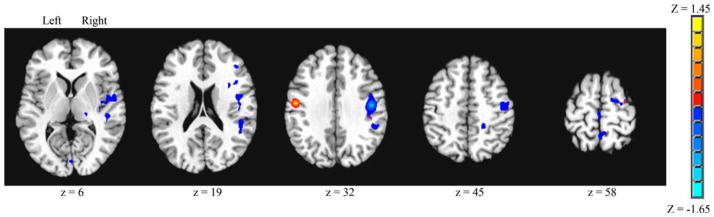
A contrast was performed to determine significant differences in brain connectivity for M1 (area 4p), left versus right hemisphere seeds (Fig. 4). Surviving voxels for the left > right contrast were minimal (702 mm3) as compared to the volume of connected voxels for the right > left contrast (19,359 mm3). Brain regions that showed significantly greater connectivity for the left hemisphere seed were limited to a focal area that included M1 (area 4p) and premotor cortex (BA6). Brain regions that showed significantly greater connectivity for the right hemisphere seed spanned multiple brain areas, including the cerebellum, thalamus, insula, superior temporal gyrus (STG), secondary somatosensory cortex (OP1, OP4), inferior, middle, and superior frontal gyrus, BA44, S1 (areas 3a, 3b, 2), M1 (areas 4a, 4p), precuneus, supramarginal gyrus, premotor cortex (BA6), and SMA.
Fig. 4.
Primary motor cortex (area 4p) seed, left versus right seed contrast for volitional swallowing. Significant Z score clusters of connectivity are displayed (P < 0.05, corrected). Left > right shown as positive values, right > left shown as negative values
Individual laterality of swallowing connectivity
Although computations for this study focused on group data, the individual patterns of lateralized connectivity were also of interest. Using the same LI computation method described above for the group data, we computed the LIs of each individual participant for the five seed ROIs. A stringent voxel-wise threshold of P = 0.000001 was applied, with an additional cluster threshold to correct for multiple comparisons that produced a family-wise, corrected P < 0.001 (based on Monte Carlo simulation). Only the voxels that survived this stringent thresholding were included in the LI computations.
As seen in Table 2, all seed regions showed individual LIs that included negative and positive values, indicating a mixture of right and left hemisphere predominance in swallowing connectivity. Area 4p within the primary motor cortex showed the greatest trend toward right hemisphere predominance of connectivity across individuals, with 13 of the 14 participants demonstrating negative LI values and no participant showing LI values greater than 0.30. In area 3b of the somatosensory cortex, 10 of the 14 participants showed negative LI values. In BA44, 9 of the 14 participants showed positive LI values. Finally, for the anterior and posterior insula, 8 and 9 of the 14 participants, respectively, showed positive LI values. No participant showed consistent hemispheric laterality across all ROIs; a mixture of positive and negative LI values was evidenced within each individual for the five seed regions studied.
Table 2.
Laterality indices (LIs) of swallowing connectivity in individual participants (based on corrected P < 0.001)
| Individual laterality index (LI) | Number of participants (of 14 total) within LI range for each seed region
|
||||
|---|---|---|---|---|---|
| Area 3b | Area 4p | BA44 | Anterior insula | Posterior insula | |
| LI < −0.3, right hem. laterality | 2 | 6 | 1 | 3 | 4 |
| LI −0.3 to < 0.0, right hem. predominance | 8 | 7 | 4 | 3 | 1 |
| LI 0.0 to 0.3, left hem. predominance | 3 | 1 | 5 | 4 | 4 |
| LI > 0.3, left hem. laterality | 1 | 0 | 4 | 4 | 5 |
For each seed region, the number of participants showing LI values in the ranges of < −0.3 (right laterality), −0.3 to < 0.0 (right predominance), 0.0 to 0.3 (left predominance), and > 0.3 (left laterality) are given
Hem hemisphere, BA Brodmann’s area
Discussion
This study is the first to address laterality of functional interactions between brain regions during swallowing. Degree of lateralized, functional connectivity for designated seed regions to areas throughout the brain was studied by computing the correlations of the residuals after all task and other modeled effects were removed. Functional connectivity at the group level was lateralized to the left hemisphere for the posterior insula and to a lesser extent the anterior insula, with the left IFG also showing somewhat greater interactions than the right. Primary somatosensory cortex showed no lateralization and primary motor cortex showed some right-hemisphere predominance of functional interactions. Volume of connectivity from the left insula to areas throughout the brain was numerically highest among the bilateral ROIs that were studied.
The insula is a multifunctional region, with roles that may relate to both sensory and motor components of swallowing. Univariate fMRI swallowing studies have consistently demonstrated the involvement of the insula, but findings are mixed as to whether there is a right or left-hemisphere lateralization of this region (Martin et al. 2001a; Watanabe et al. 2004). Watanabe et al. (2004) found that activation of the insula was strongly lateralized to the left in the preparation and initiation stages of swallowing as studied through MEG, whereas several studies using fMRI have shown lateralization to the right insula (Hamdy et al. 1999; Martin et al. 2001a). Interestingly, our own previous univariate analysis of the current data also showed lateralization to the right insula during volitional swallowing. In that study, a univariate analysis showed nearly equivalent activation of the right and left hemispheres for the majority of regions of interest except the insula, which showed a volume of activation that was nearly three times greater in the right hemisphere (5,616 mm3, P < 0.01 corrected) than in the left (2,079 mm3, P < 0.01 corrected). Our current finding that functional interactions for these data are lateralized to the left insula emphasizes the differences between univariate analyses that present the contributions of multiple regions in isolation, and the current analysis of functional connections between interacting brain regions involved in volitional swallowing. In our current study, interactions were not only greater for the left insula but also were distributed substantially both within and across hemispheres, indicating that the left insula is involved in greater neural cross-talk to ipsilateral and contralateral brain regions than the right insula. The posterior insula showed definitive lateralization, with the anterior insula showing less pronounced lateralization to the left. In previous univariate studies, swallowing has evoked activation of both anterior and posterior portions of the insula (Martin et al. 2001b, 2004; Malandraki et al. 2009).
Primary taste cortex is located within the insula, and taste stimulation strongly activates this region (Francis et al. 1999). The insula is also involved in the processing of touch (Rolls 2004), sense of volitional movement and proprioception (Hallett 2007), and processing of temperature stimuli (Brooks et al. 2005). Sensory inputs are critical in swallowing (Jean and Car 1979; Jean 2001), and their ability to evoke a motor swallowing response is dependent upon the processing and integration of that sensory information. Thus, the specialized functions of the insula may be important to the processing of taste, texture, and size aspects of the bolus being swallowed while sensing awareness of the tongue and other structures that are moving the bolus through the oral cavity. The neural connections of the insula to primary somatosensory cortex (Mesulam and Mufson 1984; Rolls 2004) and premotor cortex (Mesulam and Mufson 1984) are well suited for processing oral-tactile sensory information and communicating with both sensory and motor-related regions. Our results therefore support the findings by Watanabe et al. (2004), suggesting that the left insula contributes heavily to the initiation of the swallow by processing sensory elements and interacting with widespread brain regions in both hemispheres that include primary sensorimotor cortex. The importance of left hemisphere anatomical connectivity in swallowing is supported by the findings of Cola et al. (2010), in which disruption of anatomical tracts in the left periventricular white matter was associated with dysphagia in 60 % of acute stroke patients, whereas no patients who had right hemisphere damage to the same region showed subsequent dysphagia.
Although not as pronounced as the insula, the results of the current study showed that functional interactions from the IFG to other brain regions were somewhat greater in the left hemisphere as compared to the right. Neural connectivity between closely approximated regions such as the insula and IFG, and from those regions to other brain regions, is likely to be more similar than connectivity from disparate regions. Therefore, our findings of somewhat greater functional interactions for the left BA44 within the IFG, which is closely situated to the insula, may be associated with the increased likelihood of connecting neural pathways. Similar regions of the frontal operculum have shown left lateralization during univariate studies of swallowing (Dziewas et al. 2003). Functional connectivity of the IFG has been demonstrated in other laryngeal control activities such as voicing, and the lateralization to the left hemisphere for the overall laryngeal motor cortex is demonstrated in simple voicing activities involving speech (Simonyan et al. 2009).
Comparisons of hemispheric differences in the functional interactions between the primary sensorimotor cortex and other brain regions showed differing patterns than those for the insula and IFG. For the region within S1 as seed, functional connectivity was equivalent between the left and right hemispheres, indicating that neural connectivity for primary somatosensory cortex was similar in both hemispheres. Connectivity for the M1 cortical region was somewhat greater in the right hemisphere in the current study (LI = −0.19). Several previous imaging studies have addressed lateralization of volume or degree of univariate activation of the combined primary sensorimotor cortex. This research has in some cases shown a strong left-lateralization of the primary sensorimotor cortex (Dziewas et al. 2003; Martin et al. 2004, 2007), and in other cases shown bilateral activation with variability across tasks or individuals (Hamdy et al. 1999; Mosier et al. 1999b). Some studies that included lesion-based analysis of patients post-stroke have shown that right hemisphere deficits are associated with longer durations of pharyngeal stage events and greater frequency of aspiration when swallowing liquids (Robbins and Levin 1988; Robbins et al. 1993). However, other researchers have found that pharyngeal phase swallowing deficits can occur at similar frequency after either right or left hemisphere stroke (Alberts et al. 1992; Daniels et al. 1996; Daniels and Foundas 1999).
Teismann et al. (2009) used MEG to study the timing of lateralization patterns for univariate brain activity, capitalizing on the substantially better temporal resolution of MEG relative to fMRI. These researchers suggested that there is a time-dependent shift in lateralized swallowing activity of the primary sensorimotor cortex, with the left hemisphere predominating in the early stages of swallowing followed by bilateral activity and finally right-lateralized activity in the later swallowing stages. It is possible that the functional connections from the primary sensorimotor cortex are greatest in the mid to later stages of the swallow process, producing the bilateral and right-hemisphere focused connectivity patterns evidenced in our results. However, in the present study we addressed the functional connectivity of specific sub-regions (areas 3b and 4p) of the primary sensorimotor cortex to other brain regions; an alternative possibility is that functional connectivity for other areas of S1/M1 would yield different patterns of lateralization.
In studies of acute, post-stroke patients, damage to the insula in either hemisphere is frequently associated with dysphagia (Daniels et al. 1996; Daniels and Foundas 1997, 1999) although not always associated with risk of aspiration (Daniels and Foundas 1999). These studies have mostly addressed the swallowing deficits of patients that occur within 1 month of stroke onset, and indicate that left/right differences in the association of insula damage with dysphagia are not evidenced. However, it is possible that hemispheric differences after damage to the insula may occur in the swallowing recovery stage, several months after the onset of the stroke. Recovery from dysphagia after stroke is associated with increased evoked motor responses in the side contralateral to the lesion (Hamdy et al. 1998), implying a compensatory role of the contralateral hemisphere (Hamdy and Rothwell 1998). Based on our findings, it is possible that the compensatory capacity of the left insula during swallowing recovery may be greater than that of the right insula due to its greater connectivity to areas throughout the brain that span both the right and left hemisphere. However, our results pertain to people with normal swallowing skills only. Lesion localization studies that determine the prevalence of dysphagia in patients 6 months or more post-onset of stroke, and studies that determine hemispheric dominance for swallowing before and after stroke in people with dysphagia, are needed to determine the compensatory role of functional connectivity in dysphagia.
Examination of the individual data showed that lateralization patterns were generally consistent with the group results, but that multiple combinations of hemispheric predominance contributed to the group laterality findings. All seed regions that we studied showed individuals with LIs that included negative (right hemisphere connectivity greater than left) and positive (left hemisphere connectivity greater than right) values. The primary motor cortex showed the greatest consistency of right hemisphere predominance at the individual level, and a similar right hemisphere focus was seen for this region at the group level. For the other seed regions, the negative or positive sign value for the group LI matched the majority count of individuals within each ROI for all regions except area 3b, in which the group LI was near zero but the majority of individual LIs showed a right hemisphere predominance.
Of interest was the individual variability in hemispheric dominance for swallowing connectivity, which is consistent with previous univariate, neural swallowing studies that indicate substantial variability in overall hemispheric dominance for swallowing (Hamdy et al. 1996; Martin et al. 2001a). Furthermore, the lack of consistency in hemispheric predominance that was evidenced within each individual across the five seed ROIs has been previously demonstrated in a univariate fMRI study (Mosier et al. 1999b). Mosier et al. (1999b) found that within an individual, the dominant hemisphere varied for multiple swallowing-related cerebral regions. Future studies are needed to further examine the individual patterns of hemispheric dominance for the neural connectivity of swallowing regions (Hamdy et al. 1996; Mosier et al. 1999b).
This study addressed the lateralized connectivity of several cortical brain regions that are known to be important in swallowing; the scope of cortical regions was purposely limited to those associated with sensory, motor and combined sensorimotor functions to facilitate interpretation of results. However, other cortical regions are known to be important to swallowing, including the supplemental motor area and cingulate cortex (Hamdy et al. 1999; Mosier et al. 1999a; Mosier and Bereznaya 2001; Martin et al. 2007). Likewise, multiple subcortical and white matter regions of the brain are involved in normal swallowing (Mosier et al. 1999b; Mosier and Bereznaya 2001; Martin et al. 2004; Malandraki et al. 2009) and when damaged after stroke, may be associated with dysphagia (Alberts et al. 1992; Daniels and Foundas 1999; Gonzalez-Fernandez et al. 2008; Cola et al. 2010). Lesions that affect subcortical white matter regions such as the internal capsule and periventricular white matter have shown a higher association with dysphagia after CVA than several other cortical and subcortical regions (Daniels and Foundas 1999; Gonzalez-Fernandez et al. 2008), with dysphagia frequently following damage to the left periventricular lesions but not right periventricular regions (Cola et al. 2010). These white matter tracts may provide critical connections between brainstem, subcortical and cortical areas and when a functional disconnection of these tracts occurs, dysphagia is a likely consequence (Cola et al. 2010). Future studies that implement tractography would be needed to address laterality of the internal capsule and periventricular white matter connections with cortical regions.
In conclusion, we found that at the group level, the insula showed left lateralization during volitional saliva swallowing, with the posterior insula having the most pronounced LI. The IFG region showed somewhat greater functional interactions for the left hemisphere, without definitive lateralization. In contrast, S1 did not show any lateralization and M1 showed some predominance of connectivity in the right hemisphere. Our results suggest that unilateral brain damage to the primary somatosensory cortex may allow greater compensatory mechanisms in recovery than unilateral damage to the insula, which appears to have greater left-lateralized, functional connections both within and across hemispheres. Future studies incorporating techniques such as diffusion tensor imaging with fMRI would be useful for specifying the neural pathways and their directionality in human swallowing. This knowledge of directionality in human swallowing pathways would then allow effective connectivity analysis to determine the effect of one brain region on another. Furthermore, comparisons of volitional to automatic swallowing and water to saliva swallowing would be useful for determining whether lateralization patterns for specific brain regions vary across tasks.
Acknowledgments
We thank Adam Gerson for his assistance on initial methods for this study. This research was supported by the Divisions of Intramural Research of the National Institute of Neurological Disorders and Stroke and of the National Institute on Deafness and Other Communication Disorders.
Contributor Information
Soren Y. Lowell, Email: slowell@syr.edu, Laryngeal and Speech Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Building 10, 5D-38, 10 Center Drive, MSC 1416, Bethesda, MD 20892-1416, USA. Department of Communication Sciences and Disorders, Syracuse University, 805 S. Crouse Ave., Syracuse, NY 13210, USA
Richard C. Reynolds, Scientific and Statistical Computing Core, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA
Gang Chen, Scientific and Statistical Computing Core, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Barry Horwitz, Brain Imaging and Modeling Section, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, MD 20892, USA.
Christy L. Ludlow, Laryngeal and Speech Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Building 10, 5D-38, 10 Center Drive, MSC 1416, Bethesda, MD 20892-1416, USA
References
- Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia. 1992;7:170–173. doi: 10.1007/BF02493452. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and inter-subject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Car A. Cortical control of swallowing [in French] J Physiol Paris. 1973;66:531–551. [PubMed] [Google Scholar]
- Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–486. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging. 1999;9:91–98. doi: 10.1111/jon19999291. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis. 1996;6:30–34. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Dziewas R, Soros P, Ishii R, et al. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20:135–144. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006a;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006b;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Gaillard WD, Berl MM, Duke ES, et al. fMRI language dominance and FDG-PET hypometabolism. Neurology. 2011;76:1322–1329. doi: 10.1212/WNL.0b013e31821527b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez M, Kleinman JT, Ky PK, Palmer JB, Hillis AE. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: a pilot study. Stroke. 2008;39:3022–3028. doi: 10.1161/STROKEAHA.108.518969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Volitional control of movement: the physiology of free will. Clin Neurophysiol. 2007;118:1179–1192. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC. Gut feelings about recovery after stroke: the organization and reorganization of human swallowing motor cortex. Trends Neurosci. 1998;21:278–282. doi: 10.1016/s0166-2236(97)01212-5. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115:1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jean A, Car A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979;178:567–572. doi: 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. McGraw-Hill, Health Professions Division; New York: 2000. [Google Scholar]
- Kern MK, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001a;280:G531–G538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001b;280:G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- Kleinjan KJ, Logemann JA. Effects of repeated wet and dry swallows in healthy adult females. Dysphagia. 2002;17:50–56. doi: 10.1007/s00455-001-0101-9. [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–295. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G, Bellec P, Benali H. Exploring large-scale brain networks in functional MRI. J Physiol Paris. 2006a;100:171–181. doi: 10.1016/j.jphysparis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Krainik A, Duffau H, Pelegrini-Issac M, Lehericy S, Doyon J, Benali H. Partial correlation for functional brain interactivity investigation in functional MRI. Neuroimage. 2006b;32:228–237. doi: 10.1016/j.neuroimage.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001a;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, Letsos P, Taves DH, Inculet RI, Johnston H, Preiksaitis HG. Oropharyngeal dysphagia in esophageal cancer before and after transhiatal esophagectomy. Dysphagia. 2001b;16:23–31. doi: 10.1007/s004550000044. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Martin RE, Barr A, MacIntosh B, et al. Cerebral cortical processing of swallowing in older adults. Exp Brain Res. 2007;176:12–22. doi: 10.1007/s00221-006-0592-6. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows JC. Dysphagia in unilateral cerebral lesions. J Neurol Neurosurg Psychiatry. 1973;36:853–860. doi: 10.1136/jnnp.36.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey: architectonics, connectivity and function. In: Peters A, Jones EG, editors. Cerebral cortex. Plenum Press; New York: 1984. pp. 179–226. [Google Scholar]
- Miller AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1:91–100. [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999a;109:1417–1423. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J Neuroradiol. 1999b;20:1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Niskanen E, Kononen M, Villberg V, et al. The effect of fMRI task combinations on determining the hemispheric dominance of language functions. Neuroradiology. 2012;54(4):393–405. doi: 10.1007/s00234-011-0959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender MP, Ferguson SM. Dysarthria and dysphagia due to the opercular syndrome in multiple sclerosis. Mult Scler. 2007;13:817–819. doi: 10.1177/1352458506073481. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldery E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Riecker A, Gastl R, Kuhnlein P, Kassubek J, Prosiegel M. Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. 2009;24:114–118. doi: 10.1007/s00455-008-9164-1. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levin RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3:11–17. doi: 10.1007/BF02406275. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–1300. doi: 10.1016/0003-9993(93)90082-l. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci. 2009;29:14912–14923. doi: 10.1523/JNEUROSCI.4897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysik DA, Hyde JS. Strategies for block-design fMRI experiments during task-related motion of structures of the oral cavity. Neuroimage. 2006;29:1260–1271. doi: 10.1016/j.neuroimage.2005.08.063. [DOI] [PubMed] [Google Scholar]
- Soros P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS, Martin RE. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience. 2008;153:1300–1308. doi: 10.1016/j.neuroscience.2008.02.079. [DOI] [PubMed] [Google Scholar]
- Sumi T. Some properties of cortically-evoked swallowing and chewing in rabbits. Brain Res. 1969;15:107–120. doi: 10.1016/0006-8993(69)90313-8. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Teismann IK, Dziewas R, Steinstraeter O, Pantev C. Time-dependent hemispheric shift of the cortical control of volitional swallowing. Hum Brain Mapp. 2009;30:92–100. doi: 10.1002/hbm.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane GY. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19:100–108. doi: 10.1007/s00455-003-0509-5. [DOI] [PubMed] [Google Scholar]