Abstract
The emerging role of astrocytes in neural communication represents a conceptual challenge. In striking contrast to the rapid and highly space- and time-constrained machinery of neuronal spike propagation and synaptic release, astroglia appear slow and imprecise. Although a large body of independent experiments documents active signal exchange between astrocytes and neurons, some genetic models have raised doubts about the major Ca2+-dependent molecular mechanism routinely associated with release of “gliotransmitters.” A limited understanding of astrocytic Ca2+ signaling and the imperfect compatibility between physiology and experimental manipulations seem to have contributed to this conceptual bottleneck. Experimental approaches providing mechanistic insights into the diverse mechanisms of intra-astrocyte Ca2+ signaling on the nanoscale are needed to understand Ca2+-dependent astrocytic function in vivo. This review highlights limitations and potential advantages of such approaches from the current methodological perspective.
Keywords: astrocyte, glia-neuron communication, synaptic plasticity
The emerging importance of astroglia for neural communication in the brain has taken neurophysiology by storm. The discovery of Ca2+ waves in cultured astrocytes (Cornell-Bell and others 1990; Nedergaard 1994; Parpura and others 1994) and the demonstration of mechanisms enabling Ca2+ entry, propagation, and removal inside glial cells (Verkhratsky and others 1990; Porter and McCarthy 1996; Pasti and others 1997; Bezzi and others 1998) had laid early foundations for the era in which glial cells would have become a recognized functional partner of neurons (for early reviews, see Attwell 1994; Kostyuk and Verkhratsky 1994; Verkhratsky and Kettenmann 1996; Porter and McCarthy 1997). The classical view of astroglia as an entity dealing mainly with tissue metabolism and potassium homeostasis had also been challenged by the studies showing the importance of neurotransmitter uptake enacted by glial transporters, for shaping synaptic signals in space and time (reviewed in Barbour and Hausser 1997; Billups and others 1998; Rusakov and Kullmann 1998; Bergles and others 1999; Danbolt 2001; Kullmann and others 2005). Over the past decade, the knowledge about the versatile role of astroglia in neural function has expanded dramatically (reviewed in Haydon 2001; Sykova 2001; Volterra and Meldolesi 2005; Fields and Burnstock 2006; Haydon and Carmignoto 2006), and several recent reviews provide an in-depth account of the reported cellular mechanisms controlling molecular signal exchange between astroglial and neural networks (Hamilton and Attwell 2010; Perea and Araque 2010; Reichenbach and others 2010).
We therefore did not intend in this review to discuss recently published research on glia-neuron communication, nor did we aim to provide a detailed analysis of a particular hypothesis in the field. Instead, we attempted to focus on what might be described as a conceptual gap between a rapidly growing body of experimental evidence pointing to active communication from passive (protoplasmic) astrocytes to neurons, on one hand, and a poor understanding of the Ca2+-dependent microscopic mechanisms acting inside astrocytes, which could explain such communication, on the other hand. Such a gap appears to contribute to a recent controversy regarding the role of astrocytic Ca2+ signaling in synaptic transmission and plasticity. Indeed, numerous studies have associated Ca2+ elevations induced by exogenous activation of the inositol tris-phosphate (IP3)–dependent cascades with modulatory influences on the function of nearby excitatory synapses (Pasti and others 1997; Fiacco and McCarthy 2004; Navarrete and Araque 2008). In contrast, the astrocyte-specific genetic suppression or enhancement of the IP3R2 receptor signaling cascades has failed to exert any detectable effects on excitatory synaptic transmission or its plasticity (Agulhon and others 2008; Agulhon and others 2010). At the same time, however, suppression of endogenous Ca2+ activity in individual astrocytes has been reported to affect neighboring synapses in organized brain tissue (Jourdain and others 2007; Perea and Araque 2007; Andersson and Hanse 2010; Gomez-Gonzalo and others 2010; Henneberger and others 2010), and a similar causality has recently been reported between Schwann cells and the neuromuscular junction (Todd and others 2010). Because much of the current debate refers to the interpretation of astroglial Ca2+ signals triggered and recorded using particular experimental approaches, we aimed here to discuss, first, some physiological constraints for efficient signal transfer between neurons and astrocytes and, second, to what degree astrocytic Ca2+ signaling can be evaluated and interpreted using the currently available methodologies.
Glutamatergic Neuron-Glia Signaling: Strategic Communication Loci versus Global Glutamate Uptake
Modulation of internal Ca2+ in space and time has commonly been perceived as a universal communication medium for glial cells. Experimental studies in vitro and in situ have detected numerous sources of Ca2+ and a variety of both converging and diverging Ca2+ signaling pathways operated by electrically nonexcitable (passive) astrocytes. Among those sources are Ca2+-permeable channels, receptors that initiate or modulate Ca2+ store—dependent signaling, exchanger-type Ca2+ transport, metabolically triggered Ca2+ signaling, and influx of Ca2+ through gap junctions (see references above). Some of these mechanisms appear well suited for translation of neuronal network activity into Ca2+ signals inside astroglia. Indeed, astrocytes in various brain regions host a number of receptors sensitive to the common excitatory neurotransmitter glutamate, such as Ca2+-permeable AMPA receptors, N-methyl-d-aspartate (NMDA) receptors, and group I metabotropic glutamate receptors (mGluRs) (Cornell-Bell and others 1990; Burnashev and others 1992; Evans and others 1992; Porter and McCarthy 1996; Schipke and others 2001), as discussed in recent reviews (Verkhratsky and Kirchhoff 2007; Agulhon and others 2008).
At the same time, however, passive astrocytes are believed to take up >90% of glutamate released into the extracellular space (Bergles and Jahr 1998; Danbolt 2001), a process essential for the normal functioning of excitatory synaptic circuits (Bergles and others 1999; Zheng and others 2008). Indeed, astrocytic plasma membranes in the hippocampus or cerebellum are enriched in high-affinity glutamate transporters, notably GLAST-GLT types, at a surface density of up to 104 molecules per square micron, providing an average extracellular concentration of ~0.2 mM (Lehre and Danbolt 1998). Because these glial transporters buffer glutamate on a rapid time scale (Bergles and Jahr 1997; Diamond and Jahr 1997; Rusakov 2001), successful signal transfer between glutamate release sites and designated receptors will only occur when the number of diffusing glutamate molecules exceeds the number of local transporter molecules. This may happen, for instance, during intense synaptic activity (Lozovaya and others 1999; Lozovaya and others 2004; Scimemi and others 2004) or when the target receptors are in the immediate proximity of the glutamate release site. Therefore, spatial juxtaposition of release sites and target receptors should be an important factor in enabling efficient glutamatergic signal exchange between neuronal and astroglial compartments. Indeed, high-affinity NMDA receptor (NMDAR) subunits have been detected in neuronal terminals strategically apposing astroglial compartments featuring putative glutamate-containing vesicles (Jourdain and others 2007). Similarly, quantal AMPA receptor-mediated synaptic events in Bergmann glia have been associated with glutamate release from ectopic (nonsynaptic) sites directly facing the target AMPA receptors in glial membranes (Matsui and others 2005). However, little is known about the density and distribution patterns of high-affinity glial transporters on the nanoscale. Careful immunoelectron microscopy studies have revealed only some general trends in the layout of GLAST-GLT expressed by astrocytes: Higher expression has been documented near excitatory synapses, as opposed to other neuronal compartments or vascular endothelium (reviewed in Danbolt 2001). Clearly, further investigation is needed to identify circumstances in which the subcellular localization of glutamate receptors is compatible with their roles in astroglia-neuron exchange.
Importantly, astrocytic Ca2+ signaling can be triggered not only by glutamate receptors but also through the glutamate uptake machinery. Glutamate transport into glia is dependent on an electrochemical gradient of sodium (as well as on K+ concentration), and co-transported sodium ions could therefore accumulate in the astrocyte cytosol during neuronal activity (Brew and Attwell 1987; Langer and Rose 2009). This is likely to create favorable conditions for the reversal of the astrocytic sodium-calcium exchanger (NCX), leading to an increase in the internal Ca2+ level (Kirischuk and others 1997) (Fig. 1). Strikingly, small astrocytic processes that contact synapses in the hippocampus are particularly enriched in NCX molecules (Minelli and others 2007). Because such thin processes have a relatively high surface-to-volume ratio and because diffusion exchange within such processes should be relatively slow, they might represent a favorable environment for sustained and localized sodium accumulation (see below), boosting local Ca2+ entry via the exchanger.
Figure 1.
A decrease in external Na+ or an increase in internal Na+ elevates intracellular Ca2+ in Bergmann glia, potentially implicating the sodium-calcium Ca2+ exchanger (NCX) mechanism. (A) Fluorescence intensity image (left, fura-2 AM, λx = 380 nm) of the cerebellar slice. White circle, region of interest (Bergmann glia cell body); PN, Purkinje cell. (B, C) Intracellular Ca2+ concentration reversibly increases in response to application of an Na+-free bath solution (B) or to intracellular whole-cell loading of bulk-loaded cells with a high Na+ solution (C). Modified from Kirischuk and others (1997).
As for the downstream Ca2+-dependent cascades involving internal Ca2+ stores, the key Ca2+-regulating sensor in this system is the IP3 receptor (IP3R) acting as a Ca2+ channel, which is sensitive both to IP and to free Ca2+ (reviewed in Foskett and others 2007). In this environment, in which Ca2+ sources and sinks can be sensitive to Ca2+ in a highly nonlinear fashion, formation of internal Ca2+ signals in space and time is likely to depend strongly on the spatial layout of Ca2+ stores and IP3Rs. Furthermore, store-dependent Ca2+ signaling does not always require upstream activation of membrane-bound receptors. A recent study has documented dopamine-induced, receptor-independent Ca2+ astrocytic signals (in the cortex, hippocampus, and midbrain) mediated by the monoamine oxidase–dependent production of reactive oxygen species, by lipid peroxidation and activation of lipase C, all leading to Ca2+ store release via an IP3R-dependent mechanism (Vaarmann and others 2010). With or without receptor-mediated Ca2+ signaling, it would seem therefore crucial to gather experimental evidence on the subcellular distribution of Ca2+ storage in astrocytes, not only for a better understanding of the underlying molecular mechanism per se but also to provide insights into the origin and regulation of the uneven Ca2+ landscape inside astrocytes.
Current Methods of Ca2+ Imaging Mask the Diversity of the Nanoscopic Mechanisms Involved
The vast majority of experimental data regarding Ca2+ signaling in astroglia have come from studies involving fluorescence imaging with Ca2+-sensitive indicators. Although this approach has undoubtedly revolutionized our understanding of the astrocytic Ca2+-dependent activity, it is important to appreciate its inherent technical limitations, especially when interpreting such data in terms of the microscopic mechanisms involved.
First, spatial resolution of optical imaging is limited both by light diffraction and by optical distortions in the excitation and detection systems. Because of these two factors, any physical fluorescence point source in the specimen will be represented by a point-spread function (PSF) characteristic for a particular imaging system (see below for a brief survey on super-resolution imaging). In fluorescence imaging experiments that involve organized brain tissue and therefore require two-photon excitation with infrared laser pulses, this PSF-associated “blurring” factor might well exceed one micron, at least in the z direction (see Zipfel and Webb 2001 for background reference and Fig. 4 in Scott and Rusakov 2006 for an experimental example). Second, optical registration with highly sensitive detectors (such as photo-multipliers routinely used in confocal microscopes) involves the shot noise arising from the fact that the number of emission photons registered over a brief period within a small sampled volume is limited. The latter may result in stochastic, rather than smooth and continuous, representation of the signal intensity, thus generating an inherently “noisy” image over a single duty cycle (for background reference, see Scheppard and others 2006). Because astrocytic processes in the brain neuropil could be as thin as 30 to 50 nm while being separated from one another by a distance of less than 0.5 μm (Ventura and Harris 1999; Lehre and Rusakov 2002; Witcher and others 2007; Lushnikova and others 2009), optical fluorescence imaging is unlikely to resolve Ca2+ signals between individual processes, let alone to discriminate among rapidly evolving Ca2+ signaling domains within individual thin protrusions.
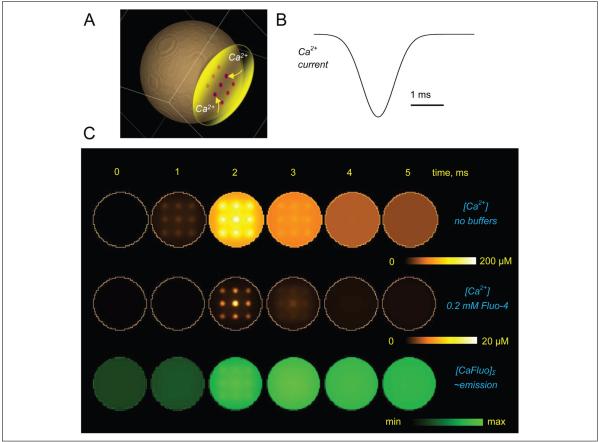
In addition to these well-recognized limiting factors, it is also important to understand the relationship between endogenous intracellular Ca2+ dynamics and the fluorescence emission signal reported by fluorescent Ca2+ indicators. By definition, such indicators are powerful Ca2+ buffers, and they are routinely used in a relatively high intracellular concentration (50–500 μM). Therefore, two important aspects should be considered when interpreting their emission recordings. First, by binding to Ca2+, such indicators interfere with the endogenous Ca2+ signaling. The potential ensuing effect on Ca2+ nanodomains (“hotspots”) in the vicinity of Ca2+ sources may be difficult to predict: In some cases, the number of entering Ca2+ ions could actually dwarf the number of locally available indicator molecules. However, Ca2+ indicators are likely to consistently influence Ca2+ waves evolving on a longer and larger scale, by effectively providing a space and time filter to such waves. Second, the fluorescence signal has to be separated from the underlying evolution of free Ca2+. For a number of technical reasons, nonratiometric Ca2+ dyes (such as Oregon Green BAPTA-1 or Fluo-4), which increase their emission intensity sharply upon Ca2+ binding, have been a preferred choice in monitoring rapid intracellular Ca2+ transients in organized brain tissue (Maravall and others 2000). In many cases, therefore, detailed computer simulations have to be employed to understand the relationship between endogenous Ca2+ signals, the effect of an exogenous indicator, and the reported fluorescence intensity. Diagrams in Figure 2 show an example of such a relationship computed using a high-resolution, multicompartmental diffusion model of rapid Ca2+ entry into the cell cytosol through a local grouping of Ca2+ channels (Scott, Ruiz, and others 2008; Henneberger and others 2010), with and without the common Ca2+ indicator Fluo-4. These data illustrate the extent of filtering in space and time domains that should be contemplated when interpreting Ca2+ imaging experiments in astroglia. Adding endogenous astrocytic Ca2+ buffers (of which little is known at present) to the picture could further complicate the relationship between recorded signals and the underlying Ca2+ dynamics.
Figure 2.
An example (diffusion computation) illustrating the effect of Ca2+ indicators on Ca2+ signals inside a small cellular compartment and the relationship between fluorescence recordings and the underlying kinetics of free Ca2+. (A) Diagram depicting the modeled Ca2+ store (yellow) with Ca2+ channels (nine 50-nm channel clusters depicted by dots) facing the cell cytoplasm (~1-μm wide compartment). (B) Simulated Ca2+ entry time course; the total entry corresponds to ~104 Ca2+ ions (which is approximately twice the spike-evoked presynaptic Ca2+ influx in small central synapses [Koester and Sakmann 2000], consistent with the estimate for presynaptic Ca2+ store release reported earlier [Scott, Lalic, and others 2008]). (C) Concentration profiles at five time points during Ca2+ entry. Top row: free Ca2+ landscapes, with no Ca2+ buffers present, shown within the 10-nm layer adjacent to the Ca2+ store membrane. Middle row: same as top row but in the presence of 0.2 mM Fluo-4 (typical imaging protocol). Bottom row: concentration profile of Ca2+-bound Fluo-4 averaged over a 100-nm depth adjacent to the Ca2+ store interface, [CaF]Σ, to reflect recorded fluorescence. False color scale bars as shown. Models adapted and modified from Scott, Lalic, and others (2008) and Henneberger and others (2010) were constructed and run online using Virtual Cell (VCell) version 4.7 at the National Resource for Analysis and Modeling, National Institutes of Health, Connecticut.
Distinct Molecular Cascades May Result in Similar Macroscopic Ca2+ Waves
It is therefore expected that fluorescence Ca2+ imaging will report Ca2+ signals averaged, in time and space, on a scale that may exceed the underlying Ca2+ fluctuations by an order of magnitude. In other words, distinct Ca2+ sources and sinks separated on a submicron-millisecond scale cannot be successfully resolved at present. One important consequence of this technical limitation is that similar recordings of Ca2+ activity in astrocytes could in fact represent distinct and spatially separated molecular cascades. Indeed, both uncaging of IP3 inside astrocytes and activation of MrgA1 could evoke comparable, in amplitudes and durations, increases of Ca2+-dependent fluorescence in astrocytic somata or large proximal processes (Fiacco and McCarthy 2004; Fiacco and others 2007; Agulhon and others 2010). However, IP3 uncaging does affect spontaneous synaptic transmission in the hippocampus (Fiacco and McCarthy 2004), whereas activation of MrgA1 does not (Fiacco and others 2007; Agulhon and others 2010). Similarly, excitatory synaptic responses routinely recorded from principal hippocampal neurons remain relatively stable in baseline conditions over dozens of minutes even though on the same time scale the nearby astrocytes show well-documented prominent spontaneous Ca2+ elevations; in contrast, similar Ca2+ elevations associated with IP3 uncaging increase release probability in excitatory terminals (Perea and Araque 2007). There is no inherent conceptual discrepancy between these sets of observations because “global” Ca2+ transients documented in such experiments are likely to represent distinct Ca2+ signaling mechanisms acting on a microscopic or nanoscopic scale. Further studies focusing on the subcellular distribution of such mechanisms (Ca2+ stores, mitochondria, G-protein-coupled receptor [GPCR] complexes, Ca2+-permeable channels, and receptors in plasma or organellar membranes) are therefore required to understand how the ensuing local Ca2+ signals interact and evolve on a global scale.
Thin Glial Processes Provide Favorable Conditions for Strong Compartmentalization of Molecular Reactions
Although early studies of protoplasmic astrocytes depict a relatively simple morphology, including the cell body and multiple dendrites, more recent examinations involving electron microscopy or high-resolution imaging have revealed that the vast majority of astrocytic processes are represented by thin, leaf-like structures capable of penetrating even the minute extracellular gaps in the brain neuropil (for recent reviews, see Matyash and Kettenmann 2010; Reichenbach and others 2010). Whole-cell astrocyte labeling with biocytine and subsequent conversion for electron microscopy reveal numerous individual astrocytic processes that are as thin as 20 to 30 nm (Medvedev, Henneberger, Rusakov and Stewart, unpublished data). Because such processes densely populate synaptic neuropil while being far beyond maximum optical resolution, they add a substantial “fuzzy background” component to the fluorescence images of individual astroglia (see examples in Henneberger and others 2010; Reichenbach and others 2010).
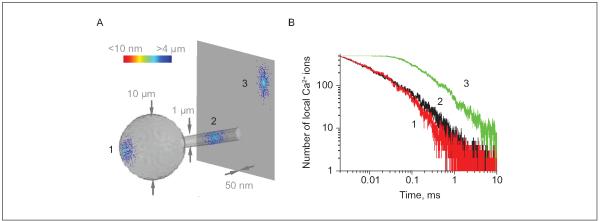
Most intracellular organelles, including small vesicles, are compatible with or larger than the width of these small processes, raising the question about an adaptive functional significance of such morphology. One important aspect attributed to the breadth of cell processes is the ability to transmit intracellular chemical signals via diffusion. Indeed, prominent chemical compartmentalization has been shown to play an important part in the functioning of thin dendritic spines (hosted by neurons) in which the spine head is relatively isolated, in terms of diffusion exchange, from the dendritic stem by an ultrathin spine neck (for review, see Yuste and others 2000). It would seem plausible, therefore, that thin processes of astrocytes could provide favorable conditions for retaining relatively high levels of signaling molecules, such as Ca2+ ions, for relatively longer time intervals postentry. To evaluate the plausibility of such a scenario, we adapted the Monte Carlo diffusion environment described earlier (Zheng and others 2008) and modeled rapid local entry and subsequent diffusion-driven dispersion of 500 small molecules within three characteristic, morphologically distinct astrocytic compartments: the 10-μm wide soma, a 1-μm thick primary dendritic stem, and an ultrathin (50-nm wide) flat process (Fig. 3). Simulations point to a striking, 5- to 10-fold increase in the amplitude and decay times for the hotspots of diffusing molecules in thin, flat processes compared to the other, more conventional cell compartments, such as dendritic stems or the cell body (Fig. 3). These considerations suggest that a strong chemical signal could be generated inside thin perisynaptic processes with a relatively small number of participating molecules. As discussed above, current fluorescence imaging methods are unlikely to distinguish such signals from larger, slower waves.
Figure 3.
Flat ultrathin processes of astrocytes provide favorable conditions for chemical compartmentalization (extended lifetime of concentration hotspots): a Monte Carlo example. (A) A diagram depicting three scatters of 500 small molecules released, from a single-point source, into three representative cellular compartments of an astrocyte, 5 ms postrelease: the soma (represented by a 10-μm-wide sphere, denoted 1), a large primary process (1-μm-wide cylinder, 2), and an ultrathin flat process (50-nm-thick planar slab, 3). In the scatters, individual diffusing molecules are color-coded to report their distance to the release site (as shown by the color scale bar). A dense cluster of molecules near the release site can clearly be seen in the flat process; intracellular diffusion coefficient, D = 0.1 μm2/ms. (B) Time course of the number of molecules (the effective local concentration) remaining within a 200-nm-wide, 50-nm-high “hotspot nanodomain” centered at the release point, for the three loci depicted in A. The life span of the hotspot at the ultrathin flat process appears 5- to 10-fold of that at the other two sites. Modeling environment modified and adapted from Zheng and others 2008.
Sensitivity versus Resolution: FLIM and STED?
Because of such highly compartmentalized cell morphology, local changes in Ca2+ signaling in astrocytes in situ may well be too small and too fast to be analyzed reliably using regular two-photon excitation fluorescent microscopy. The nanoscale volume poses serious challenges in obtaining high spatial and temporal resolution, which is essential to the study of Ca2+ dynamics in astroglia, especially in the context of the “tripartite” synaptic interaction.
There have been several promising developments ext ending the boundaries of diffraction-limited far-field imaging and video-rate fluorescent imaging techniques. These could be divided broadly into the two main approaches to nanoscale imaging under far field. First, these are statistical methods, such as fluorescent correlation spectroscopy (FCS), photoactivated localization microscopy (PALM), and stochastic optical reconstruction microscopy (STORM). Such methods are based on the ability to de-convolute individual, nonoverlapping PSFs to a pair of relatively precise coordinates, thus achieving effective resolution far beyond the diffraction limit (Wilt and others 2009). This, however, requires relatively long acquisition duty cycles, mainly because of the low concentration of activated fluorescence indicators and because of the requirement of time averaging when calculating correlation functions. To date, such methods have not yet been implemented at a speed sufficient to resolve small and rapid Ca2+ transients. For the purpose of this review, we will therefore focus on optical manipulation of the excitation-emission volume on the nanoscale, which has been most prominently implemented using stimulated emission depletion (STED), a potentially plausible option for both morphological and dynamical imaging in tackling Ca2+ dynamics in astrocyte nanodomains.
STED was historically the first method to break the diffraction limit in far-field imaging (Hell and Wichmann 1994). The method is based on introducing a second excitation laser beam, the STED beam, which has a donut-shaped PSF concentric to the spherical PSF of the first excitation laser beam. The STED beam has a wavelength that corresponds to the energy-level difference between relaxed excited singlet state and excited ground singlet state. Therefore, by exposing the sample to the STED beam immediately after the excitation beam pulse, one could de-excite the fluorescence within the donut-shaped, outer regions of the PSF. This de-excitation, termed stimulated emission, helps to create an excited fluorophore region in the center that is much smaller than the original PSF. This method allows resolution as high as ~30 nm in the x-y plane (Westphal and others 2003) and ~100 nm in the z-axis using a STED 4pi system (Hell and others 1997), the latter being also achieved in experiments in living cell cultures (Hein and others 2008).
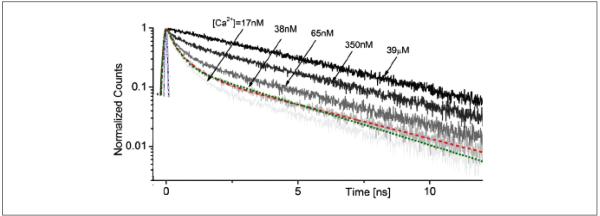
Although STED thus allows high-resolution imaging, it also requires a high-sensitivity detection system to reliably document weak fluorescence signals represented by nanoliter emission volumes. Furthermore, within such small volumes, the numbers of mobile (soluble or diffusable) fluorophore molecules contributing to the useful emission signal could fluctuate considerably with time, thus adding complexity to the interpretation of dynamic (real-time) STED measurements. The latter could be particularly important when using STED to monitor ion concentrations with ion-sensitive indicators, which is a standard approach in Ca2+ imaging. Ideally, one would prefer to remove completely the factor of the fluorophore concentration/emission intensity from such measurements. One way to achieve this is to monitor fluorescence lifetime. Fortuitously, the lifetime of some common Ca2+ indicators, such as Oregon Green BAPTA-1, is sensitive to the free Ca2+ concentration, especially in the 10- to 100-nM range (Fig. 4) (Wilms and others 2006; Gersbach and others 2009). Some commercially available fluorescence lifetime imaging microscopy (FLIM) systems (e.g., one from Becker & Hickl, Berlin, Germany) employ a single-photon counting system, which can use four or more detectors achieving a detection rate of 106 to 107 photons per second: Because photon counting is associated with very brief pulses, typically from a pulsed laser source, the background noise in FLIM experiments is often no greater than the dark current of the detector (Becker and others 2004).
Figure 4.
Fluorescence decay of OGB-1 (O6806; Molecular Probes, Eugene, OR) at various calcium concentrations (calibration kit C3008MP; Molecular Probes), as indicated. Two-photon excitation at (~100-fs pulses; laser: MaiTai, Spectra Physics, Mountain View, CA), attenuated beam power 9 mW; 20× objective (XLUMPlanFL; Olympus, Tokyo, Japan). Modified from Gersbach and others (2009).
It would therefore seem advantageous to combine the high spatial resolution of STED with the high sensitivity and the fluorophore-concentration independence of FLIM to measure Ca2+ concentration in thin astrocyte protrusions. Indeed, it has been shown that the STED-FLIM combination can achieve <200-nm resolution with a conventional laser-scanning two-photon excitation setup (Auksorius and others 2008). Furthermore, adapting video rate STED and FLIM in cell culture experiments has enabled monitoring synaptic vesicle movements within small presynaptic axonal boutons (Westphal and others 2008).
However, significant obstacles remain in applying such techniques to imaging in organized brain tissue (be it in vivo or in acute slices). In such experiments, the tissue represents a highly scattering and birefrigent specimen (turbid medium), which effectively acts as part of the optical system. To date, several attempts have been made to apply the adaptive optics techniques originally developed for astronomy research, to compensate for the optical “disturbance” of the biological tissue (Marsh and others 2003; Ji and others 2010). Combining such methods with super-resolution approaches that allow intensity-independent concentration measurements could provide a basis for a methodological breakthrough in understanding nanoscale signaling in astrocytes in situ.
Concluding Remarks
Since the discovery of the quantal synaptic transmission, the neurotransmitter vesicles, and the strong dependence of their release on Ca2+, it has taken synaptic physiologists several decades to establish biophysical determinants and main molecular mechanisms underlying evoked neurotransmitter release. This progress has been facilitated by the experimental use of large (giant) axons and synapses, such as the calyx of Held or those in the chick ciliary ganglion, which allow direct experimental access to individual terminals with recording pipettes. Importantly, early comparative studies indicated that the principles and the building blocks of the Ca2+-dependent release machinery were similar across many synaptic types and among various animal species. Thus, the scene was set for the rapid progress of knowledge, which could be successfully extrapolated throughout the field of synaptic physiology.
Astroglia do not seem to be this fortunate. Although a groundbreaking series of experiments documented and carefully examined individual release events in cultured astrocytes a decade ago, there is still very little understanding of how, where, and in response to what local stimulus the signaling molecules are released from astrocytes in situ. Impressive progress has been made in gathering evidence for the causes and consequences of astroglial activity, as well as for the underlying molecular cascades and receptor-dependent mechanisms acting in organized brain tissue. It appears, however, that information about intracellular location and the temporal features of such mechanisms is still relatively scarce. The accumulated knowledge is yet to provide a unifying concept about the Ca2+ signaling machinery in astrocytes, and the potential difficulty is that there might be no such a concept in principle. The data gathered to date point to a diverse nature of astrocytic release mechanisms and to the possibility of their target- and/or purpose-oriented molecular specialization, as opposed to having a universal molecular apparatus distributed throughout the astrocyte arbor. The fact that individual processes of astrocytic dendrites could be as small in breadth as synaptic vesicles suggests a potential for strong chemical compartmentalization of local molecular events involving release and diffusion of signaling molecules such as Ca2+ ions. Similar to thin dendritic spines in nerve cells, the partitioning of the astrocytic arbor into tiny volumes enables rapid, many-fold changes in the local concentration of reaction components even when the signal source is represented by only a few molecules, be it channels, receptors, or individual molecular complexes. Although the knowledge about such nanoscopic mechanisms is likely to be obtained only in experiments in vitro, it should pave the way for a better understanding of astroglia-neuron communication in vivo.
Acknowledgments
Financial Disclosure/Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work is supported by the Wellcome Trust and the Medical Research Council (UK).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–4. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–46. doi: 10.1016/j.neuron.2008.09.004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Hanse E. Astrocytes impose postburst depression of release probability at hippocampal glutamate synapses. J Neurosci. 2010;30:5776–80. doi: 10.1523/JNEUROSCI.3957-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D. Glia and neurons in dialogue. Nature. 1994;369:707–8. doi: 10.1038/369707a0. [DOI] [PubMed] [Google Scholar]
- Auksorius E, Boruah BR, Dunsby C, Lanigan PMP, Kennedy G, Neil MAA. Stimulated emission depletion microscopy with a supercontinuum source and fluorescence lifetime imaging. Optics Lett. 2008;33:113–5. doi: 10.1364/ol.33.000113. others. [DOI] [PubMed] [Google Scholar]
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–84. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- Becker W, Bergmann A, Hink MA, Konig K, Benndorf K, Biskup C. Fluorescence lifetime imaging by time-correlated single-photon counting. Microsc Res Techn. 2004;63:58–66. doi: 10.1002/jemt.10421. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–8. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J Neurosci. 1998;18:7709–16. doi: 10.1523/JNEUROSCI.18-19-07709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–5. doi: 10.1038/34651. others. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. others. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–9. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–70. doi: 10.1126/science.1317970. others. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–3. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–87. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD, Reale V, Merzon RM, Villegas J. N-methyl-D-aspartate (NMDA) and non-NMDA (metabotropic) type glutamate receptors modulate the membrane potential of the Schwann cell of the squid giant nerve fibre. J Exp Biol. 1992;173:229–49. doi: 10.1242/jeb.173.1.229. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–26. doi: 10.1016/j.neuron.2007.04.032. others. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–32. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuronglia interactions. Nat Rev Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol tri-sphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersbach M, Boiko DL, Niclass C, Petersen CC, Charbon E. Fast-fluorescence dynamics in nonratiometric calcium indicators. Opt Lett. 2009;34:362–4. doi: 10.1364/ol.34.000362. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8:e1000352. doi: 10.1371/journal.pbio.1000352. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–38. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hein B, Willig KI, Hell SW. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc Natl Acad Sci U S A. 2008;105:14271–6. doi: 10.1073/pnas.0807705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell SW, Schrader M, van der Voort HT. Far-field fluorescence microscopy with three-dimensional resolution in the 100-nm range. J Microsc. 1997;187:1–7. doi: 10.1046/j.1365-2818.1997.2410797.x. [DOI] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–2. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–6. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N, Milkie DE, Betzig E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat Methods. 2010;7:141–84. doi: 10.1038/nmeth.1411. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–9. doi: 10.1038/nn1849. others. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. Faseb J. 1997;11:566–72. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529:625–46. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P, Verkhratsky A. Calcium stores in neurons and glia. Neurosci. 1994;63:381–404. doi: 10.1016/0306-4522(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DA, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABA(A) receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol. 2009;587:5859–77. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–7. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Rusakov DA. Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J. 2002;83:125–34. doi: 10.1016/S0006-3495(02)75154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–63. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Kopanitsa MV, Boychuk YA, Krishtal OA. Enhancement of glutamate release uncovers spillover-mediated transmission by N-methyl-D-aspartate receptors in the rat hippocampus. Neurosci. 1999;91:1321–30. doi: 10.1016/s0306-4522(98)00638-1. [DOI] [PubMed] [Google Scholar]
- Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–62. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–67. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PN, Burns D, Girkin JM. Practical implementation of adaptive optics in multiphoton microscopy. Opt Express. 2003;11:1123–30. doi: 10.1364/oe.11.001123. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–47. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–34. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–93. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–71. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–30. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–6. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2010;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–81. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51:439–55. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca2+ depletion. Biophys J. 2001;81:1947–59. doi: 10.1016/S0006-3495(01)75846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–70. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppard CJR, Gan X, Toy M. Signal-to-noise ratio in confocal microscopes. In: Pawley JB, editor. Handbook of biological confocal microscopy. Springer; New York: 2006. pp. 442–52. [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. Faseb J. 2001;15:1270–2. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24:4767–77. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Lalic T, Kullmann DM, Capogna M, Rusakov DA. Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses. J Neurosci. 2008;28:13139–49. doi: 10.1523/JNEUROSCI.2932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Ruiz A, Henneberger C, Kullmann DM, Rusakov DA. Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+ J Neurosci. 2008;28:7765–73. doi: 10.1523/JNEUROSCI.1296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Rusakov DA. Main determinants of presynaptic Ca2+ dynamics at individual mossy fiber-CA3 pyramidal cell synapses. J Neurosci. 2006;26:7071–81. doi: 10.1523/JNEUROSCI.0946-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E. Glial diffusion barriers during aging and pathological states. Prog Brain Res. 2001;132:339–63. doi: 10.1016/S0079-6123(01)32087-3. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci. 2010;30:11870–82. doi: 10.1523/JNEUROSCI.3165-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarmann A, Gandhi S, Abramov AY. Dopamine induces Ca2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. J Biol Chem. 2010;285:25018–23. doi: 10.1074/jbc.M110.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–52. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007;210:651–60. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky AN, Trotter J, Kettenmann H. Cultured glial precursor cells from mouse cortex express two types of calcium currents. Neurosci Lett. 1990;112:194–8. doi: 10.1016/0304-3940(90)90202-k. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Westphal V, Kastrup L, Hell SW. Lateral resolution of 28 nm (lambda/25) in far-field fluorescence microscopy. Appl Phys B Lasers Opt. 2003;77:377–80. [Google Scholar]
- Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–9. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- Wilms CD, Schmidt H, Eilers J. Quantitative two-photon Ca2+ imaging via fluorescence lifetime analysis. Cell Calcium. 2006;40:73–9. doi: 10.1016/j.ceca.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wilt BA, Burns LD, Wei Ho ET, Ghosh KK, Mukamel EA, Schnitzer MJ. Advances in light microscopy for neuroscience. Annu Rev Neurosci. 2009;32:435–506. doi: 10.1146/annurev.neuro.051508.135540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM. Plasticity of peri-synaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci. 2000;3:653. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]
- Zheng K, Scimemi A, Rusakov DA. Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophys J. 2008;95:4584–96. doi: 10.1529/biophysj.108.129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Webb WW. In vivo diffusion measurements using multiphoton excitation fluorescence photobleaching recovery and fluorescence correlation microscopy. In: Periasamy A, editor. Methods in cellular imaging. Oxford University Press; Oxford, UK: 2001. pp. 216–35. [Google Scholar]