Abstract
The effects of regular exercise versus a single bout of exercise on cognition, anxiety, and mood were systematically examined in healthy, sedentary young adults who were genotyped to determine brain-derived neurotrophic factor (BDNF) allelic status (i.e., Val-Val or Val66Met polymorphism). Participants were evaluated on novel object recognition (NOR) memory and a battery of mental health surveys before and after engaging in either a) a four-week exercise program, with exercise on the final test day, b) a four-week exercise program, without exercise on the final test day, c) a single bout of exercise on the final test day, or d) remaining sedentary between test days. Exercise enhanced object recognition memory and produced a beneficial decrease in perceived stress, but only in participants who exercised for four weeks including the final day of testing. In contrast, a single bout of exercise did not affect recognition memory and resulted in increased perceived stress levels. An additional novel finding was that the improvements on the NOR task were observed exclusively in participants who were homozygous for the BDNF Val allele, indicating that altered activity-dependent release of BDNF in Met allele carriers may attenuate the cognitive benefits of exercise. Importantly, exercise-induced changes in cognition were not correlated with changes in mood/anxiety, suggesting that separate neural systems mediate these effects. These data in humans mirror recent data from our group in rodents. Taken together, these current findings provide new insights into the behavioral and neural mechanisms that mediate the effects of physical exercise on memory and mental health in humans.
Keywords: BDNF, genotype, object recognition memory, mood, anxiety, stress
Physical exercise of various intensities and durations can enhance cognition across the lifespan of humans (Cotman and Berchtold, 2002). For example, the amount of exercise during young adulthood can predict cognitive performance later in life (Dik et al., 2003). Similarly, long-term exercise can improve cognition (Davis et al., 2011), including executive function (Erikson and Kramer, 2009; Angevaren et al., 2008), and memory (Periera et al., 1997; Flöel et al., 2010), and decrease the risk for dementia (Colcombe and Kramer, 2003; Larson, 2008). However, there is significant variability in these findings due to differences in the exercise regimen and cognitive assessment (Kramer et al., 2005). Moreover, the literature is primarily comprised of retrospective rather than prospective studies (Lawlor and Hopker, 2001; Smith et al., 2010).
Among the few studies designed to test for a causal relationship between exercise and cognition, most used a single bout of exercise (Coles and Tomporowski, 2008; Hillman et al., 2009) and focused on executive function more than memory per se. Moreover, most studies report effects within 30 minutes of exercising, when effects on physiological arousal are still increased (Ferris et al., 2007; Winter et al., 2007). Thus, it is difficult to determine whether changes in cognition are due to mechanism(s) that are unique to exercise per se, or simply reflect differences due to generalized heightened arousal. The distinction between the effects of exercise and arousal is particularly important because of the unique constellation of neural mechanisms that are activated by each. For instance, the metabolic demands associated with exercise are associated with changes in MAP/ERK and CAMKII signaling as well as increases in ghrelin and UCP-2, all of which are regulated by brain-derived neurotrophic factor (BDNF; Ding et al., 2002; Molteni et al., 2006). By comparison, these neural changes would not necessarily be activated - or not in the same pattern - as a result of general autonomic arousal, which is typically associated with central and peripheral catcholaminergic regulation (Audiffren, 2009). Moreover, there has been no systematic and direct comparison between the effects of acute and regular exercise in the same study, which would further provide an opportunity to draw distinctions between any underlying mechanisms that are unique to exercise (which may develop over time in response to repeated bouts of exercise) versus those that result from arousal.
It also remains unclear whether the effects of exercise on cognition can be dissociated from changes in mood and anxiety. Several studies have demonstrated that exercise can positively affect mental health (Annesi, 2004; Larun et al., 2006; Tkacz et al., 2008), and a recent meta-analysis reported that exercise interventions lasting 3–12 weeks effectively reduce anxiety measures in sedentary participants with a chronic illness (Herring et al., 2010). However, not all exercise interventions have been shown to produce positive affective changes (Lennox et al., 1990). Further, although single bouts of exercise affect mood and anxiety (Berger and Owen, 1998; Hansen et al., 2001), assessment has typically taken place immediately after exercise. Finally, though previous studies have examined the efficacy of exercise to treat emotional disorders, little is known about the effects of exercise on psychiatrically healthy participants.
Substantial research has also focused on the neural substrates that underlie the behavioral effects of exercise. Rodent studies have demonstrated that an increase in brain-derived neurotrophic factor (BDNF) mediates the cognitive effects of exercise (Van Hoomissen et al., 2004; Vaynman et al., 2004; Hopkins and Bucci, 2010). Moreover, a polymorphism in the human BDNF gene (Val66Met; Egan et al., 2003; Frielingsdorf et al., 2010) alters activity-dependent release of BDNF and affects learning, memory, and emotion (Egan et al., 2003; Hajcak et al., 2009; Lau et al., 2010; Soliman et al., 2010). It is currently unknown whether the allelic status of BDNF influences the degree to which an individual may benefit from exercise. We addressed several of these issues by testing the effects of a single bout of exercise versus a four-week exercise regimen on cognition and mood/anxiety in healthy, sedentary young adults. Participants were evaluated on a recognition memory task modified from one used to demonstrate exercise-induced improvements in rats (Hopkins and Bucci, 2010, Hopkins et al., 2011), thus enhancing the translational value of the study. Evaluations were conducted before and after the exercise intervention (or no exercise), and DNA samples were collected to determine if BDNF genotype influences the effects of exercise.
EXPERIMENTAL PROCEDURES
Participants
Seventy-five healthy young adults (ages 18–36) were recruited for this study based on sedentary lifestyle, which was defined as not having engaged in 20 minutes or more of purposeful physical activity more than two times a month over the previous six months; a definition at least strict as reported in other studies (Pèrusse et al., 1997; Schachter et al., 2003). All participants were undergraduates from Dartmouth College or individuals recruited from the local Hanover, NH community. Prior to enrollment, all participants were screened for history of neurological or psychiatric disorders, psychotropic medications, pregnancy, nicotine use, or contraindications to physical activity to ensure that they were physically healthy and able to complete the exercise regimen. Written informed consent was obtained prior to the experiment in accordance with the Committee for Protection of Human Subjects of Dartmouth College. Participants received monetary compensation or course credit for participating.
Procedures
Experimental Design
Participants visited the laboratory twice, with four weeks between Visit 1 and Visit 2. During each visit, participants completed the informed consent form, confirmed the accuracy of their health screening form, and completed the novel object recognition (NOR) task described below. Between the acquisition and recall phases of the task, participants also completed a battery of surveys and questionnaires (described below). At the end of Visit 1, participants were randomly assigned to either a no-exercise control group or one of the three exercise conditions (described below), were given pedometers to monitor their daily activity, and received instructions regarding their group assignment to be followed for the remainder of the study. During Visit 2, saliva samples were collected from each participant using an Oragene ™ DNA collection kit (DNA Genotek, Kanata, Ontario, CA) and self-reported aerobic capacity (VO2 max) was obtained.
At each visit, participants reported their physical activity levels using the Physical Ability Questionnaire (George, Stone & Burkett, 1997). The surveys included questions on participants’ perceived functional ability to walk, jog, or run given distances (PFA) and habitual physical activity (PA-R). BMI was calculated from self-reported body weight (pounds) and self-reported body height (inches). Self-reported BMI and PAQ were used to estimate an approximate VO2max for each participant using the method developed by George and colleagues (1997). This measure provided an index of self-reported physical fitness level for each participant, which was compared between Visit 1 and Visit 2. We also compared baseline measures of VO2max between exercise groups in order to ensure that participants were of the same overall fitness ability at the beginning of the study.
Novel Object Recognition Task (Visits 1 and 2)
A computer-based NOR task was developed to test visual recognition memory ability. All images used in the task were compiled from Clip Art in Microsoft Excel (Microsoft Office 2011) and Google image searches. Images were selected based on ratings of recognizability and nameability (all objects were highly recognizable and readily nameable), neutrality (all objects rated as neutral, not positive or negative) and arousal (all objects rated as non-arousing) as assessed in a pilot study with 23 graduate students (16 female), for a final set of 200 images. During pilot testing, participants were asked to flag any items that were not readily nameable or were not perceived as neutral in emotional valence. Any flagged objects were removed from the final set of images. In order to prevent ceiling effects, the task parameters (i.e. duration of stimulus presentation, inter-trial interval, etc.) were designed to be relatively difficult, with an average percent accuracy between 70% and 80%.
In the first phase of the task, participants passively viewed a series of images (encoding phase; 50 images). Images were presented for 250 milliseconds interspersed with a 1000 msec fixation cross (see Figure 1A). The images and fixation cross were presented in the center of the screen on a white background. The images were created in PowerPoint and they were size adjusted to take up approximately 1/4–1/2 of the computer screen. All of the images ranged between 12–22cm along their longest axis and ranged from 5–20cm on their shortest axis. In the second phase of the task, participants completed the mental health surveys and questionnaires (described below). This phase also served as a distracter and took approximately 15 minutes to complete. In the final phase, participants completed the recognition memory task (retrieval phase; 100 images). Fifty images were “old” objects that participants had seen during the encoding phase, and 50 were “new” objects that participants had not previously seen (see Figure 1B). Participants were instructed to indicate by key presses whether each object was a member of the original set, or whether it was a new object. The order of “old” and “new” images was randomized for each retrieval block (four different versions). The duration of the images was controlled by participant’s response time (there was no fixation during the retrieval block). Participants used both hands to respond on the keyboard to indicate whether each object was “old” (press 1 or 9) or “new” (press 1 or 9), counterbalanced across participants. Accuracy served as the primary variable of interest and was defined as the percentage of objects correctly identified as old or new. There were four practice trials (four images) before the retrieval block began (100 images; 50 “old” and 50 “new”), and participants were randomly assigned to one of four different versions of the task at Visit 1 and Visit 2 so that they were not tested on the same set of images twice.
Figure 1.
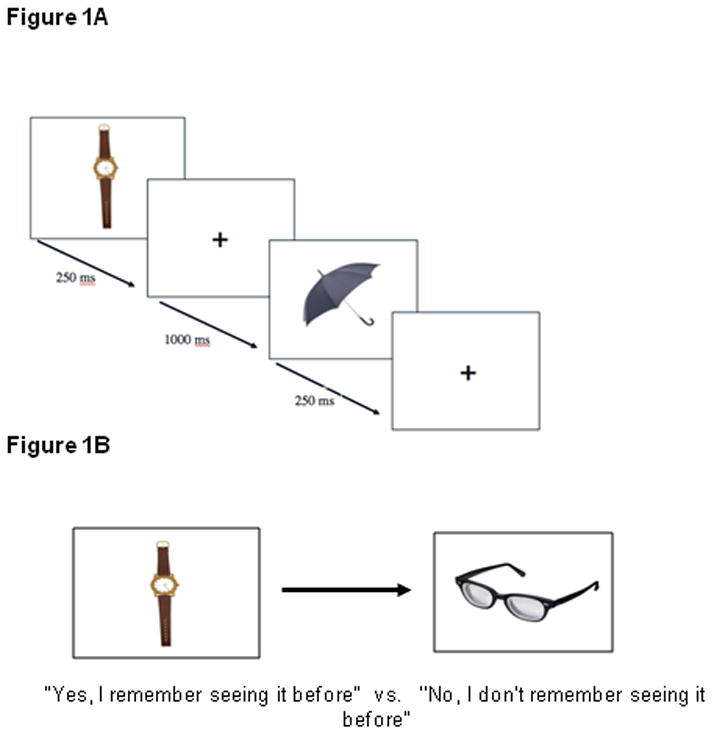
A) Encoding block of the NOR task. Participants were told to pay attention to the objects because they would be asked about them later (passive viewing). B) Retrieval block of the NOR task. Participants responded with button press “1” for “yes, I remember seeing that before” and “9” for “no, I do not remember seeing that before”.
Mood and Anxiety Measures during Visits 1 and 2
During both visits, participants completed surveys for state anxiety (STAI-Y1; Speilberger et al., 1983; depression (Beck Depression Inventory, BDI; Beck et al., 1961), perceived stress (Perceived Stress Scale, PSS; Cohen et al., 1983), and positive and negative mood (Positive and Negative Affect Schedule, PANAS; Watson et al., 1988). Any participant scoring above 15 on the BDI at either Visit 1 or Visit 2 was excluded from the study. During Visit 1, participants also completed a trait anxiety survey (STAIY; Speilberger et al., 1983).
Exercise Procedure
During the 4 weeks between Visits 1 and 2, two groups of participants were required to go to the gym at least 4X/week and to walk or jog continuously on a treadmill for a minimum of 30 minutes, at a minimum speed of 3.5mph (equivalent to a brisk walk). The treadmill guidelines were determined for each participant based on their individual stride length. This exercise regimen was chosen based on the recommended guidelines of the United States of America Department of Health and Human Services and the Centers for Disease Control and Prevention (Healthy People 2000, 2010), as well as previous data suggesting that a mild to moderate exercise regimen may be optimal for achieving mental health benefits (Kamijo et al., 2004; Ploughman et al., 2005; García-Capdevila et al., 2009; Herring et al., 2010). This exercise intervention also corresponds with our previous rodent studies, which consisted of voluntary wheel-running every other day for four weeks (Hopkins and Bucci, 2010, Hopkins et al., 2011). The participants in one of the two groups (group 4W+) were required to exercise on the final day of the four week experiment, a minimum of two hours prior to the second visit to the laboratory (range was 2–4 hours). Participants in the other group (group 4W−) were required not to exercise on the day of the second visit.
As an acute-exercise control, a third group of participants (group 0W+) were instructed to maintain the same level of physical activity they had engaged in for the previous six months, but to exercise once on the final day of the study, at least two hours prior to second testing (walk or jog continuously on a treadmill for 30 minutes at a minimum speed of 3.5 mph). Finally, a fourth set of participants (group 0W−) served as a no-exercise control group and were required to maintain the same level of physical activity they had been engaged in for the previous 6 months. Specifically, participants in groups 0W+ and 0W− were instructed that aerobic exercise exceeding 20 mins on more than 2 occasions during the four weeks between test days would exclude them from the study.
Daily Data Collection between Visits 1 and 2
Each participant was provided with a pedometer (HJ-113, Omron®) that they were instructed to wear at all times for the duration of the four-week study. The pedometers automatically recorded and stored the total number steps, distance, and aerobic steps every 24 hours. The pedometer aerobic mode was activated after 10 minutes of walking more than 60 steps per minute and deactivated after a 1-minute break from steps. Thus, total number of aerobic steps represents a measurement of aerobic activity for each individual. Participants reported these data on a daily basis through email correspondence and also completed a STAI-Y1 survey and the PANAS-P/N survey.
Genetic Screening
DNA was extracted and pelleted according to the manufacturer’s protocol (DNA Genotek, Kanata, Ontario, CA). Briefly, samples were incubated at 50°C for one hour. Next 500ul of each sample was transferred to a 1.5 mL microcentrifuge tube, 20 μL of Oragene•Purifier solution (OG-L2P) was added, and samples were mixed by vortexing for a few seconds. Samples were then incubated on ice for 10 minutes, followed by centrifugation at room temperature for 5 minutes at 13,000 RPM. Next, the supernatants were transferred into fresh microcentrifuge tubes, and the DNA was precipitated using 500ul of 95% ethanol. Samples were centrifuged at room temperature for 2 minutes at 13,000 rpm. Supernatants were discarded and the DNA pellets were washed once with 250ul of 70% ethanol, followed by resuspension in 100ul of TE solution. BDNF allelic status was determined using the TaqMan® SNP Genotyping Assays protocol (Applied Biosystems, Carlsbad, CA) by the Translational Research Program in the Department of Pathology at Dartmouth Hitchcock Medical Center.
Data Analysis
Data from Visits 1 and 2
Data obtained for the recognition memory task and mental health measures during Visits 1 and 2 were subjected to a repeated measures analysis of variance (ANOVA) using Group (0W−, 0W+, 4W−, 4W+) as the independent variable and Visit as the within-subjects variable. Significant interactions were decomposed using one-way ANOVAs and pair-wise comparisons (t-tests). A primary question of interest was whether the exercise intervention between visits would affect cognitive and psychological measures; thus, difference scores were calculated for each dependent measure by subtracting performance during Visit 2 from Visit 1 when comparing groups following a significant interaction. The use of difference scores also allowed us to minimize the variability due to individual differences in task performance. In addition, planned comparisons (independent measures t-tests) were used to determine if there were any differences between participants with the Val/Val genotype versus Met carriers in each exercise condition. Lastly, simple regressions were carried out to test for relationships between any measures that were affected by exercise.
Daily Measures
To determine whether exercise affected daily mood or anxiety during the four week period between Visits 1 and 2, daily email data from participants in the 4W− and 4W+ groups were pooled (since participants in both groups were given identical exercise instructions throughout the four week study). The daily email data were separated into two groups depending on whether the individual had exercised that day. A paired t-test then carried out for each measure comparing the data on no-exercise days (No-EX) and exercise days (EX).
For the daily exercise data, the average number of aerobic steps taken by participants in each group across the 4 week period between visits was subjected to a one-way ANOVA to test for group differences in the amount of exercise. In addition, data were pooled for participants in the 4W− and 4W+ groups and a paired t-test was used to compare the number of aerobic steps taken on exercise versus non-exercise days. Lastly, a simple regression was used to examine the relationship between aerobic steps taken and aerobic capacity (VO2 max). An alpha level of 0.05 was used for all analyses in the study.
RESULTS
Of the 75 participants initially enrolled in the study, 21 were excluded from the analyses due to discontinuation (n = 10), compliance issues (n = 3), depression level (BDI score >15; n = 4), incorrect task assignment (n=3), and outlier on task performance (n=1; object recognition accuracy data were more than 2 standard deviations from the mean on both Visits 1 and 2, as were BDI and STAI-Y1 data). Of the remaining 54 participants (average age = 20.6 ± 0.4 years), 13 were in the 0W− group (12 females/1 male, mean age = 20.9 ± 0.5), 14 were in the 4W− group (9 females/5 males, mean age=20.4 ± 0.9), 12 were in the 4W+ group (8 females/4 males, mean age=21.3 ± 0.9), and 15 were in the 0W+ group (11 females/4 males, mean age=19.8 ± 0.7). There were no gender differences on any behavioral measure (Ps > 0.1) and so the data were collapsed across gender in each treatment condition.
Thirty-one participants were Val homozygous genotype (Val/Val), and 23 were Met carriers (19 Val/Met; 4 Met/Met). Participants with the Met allele were combined into one group because of the low incidence of Met/Met genotype. The incidence of Val/Val and Met carriers in each exercise group is noted in Table 1. The population frequency for carrying at least one Met allele is 50% in Asians, 30% in Caucasians, and 4% in African-Americans (Shimizu et al., 2004).
Table 1.
Sample sizes of Val/Val and Met-carrying participants
| 0W− | 4W− | 4W+ | 0W+ | |
|---|---|---|---|---|
| Val/Val | 7 | 10 | 6 | 8 |
| Met carrier | 6 | 4 | 6 | 7 |
Daily Exercise
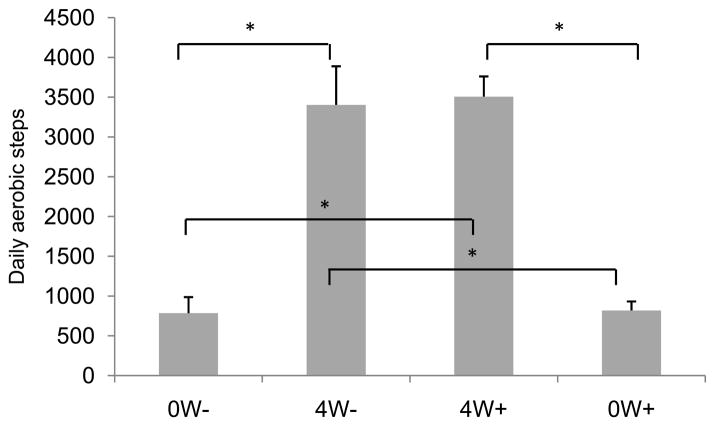
The pedometer data for 4 participants was not included in the analysis because of technical difficulties with their pedometers (1 participant in each of the 0W− and 4W− groups, and 2 participants in the 0W+ group). A one-way ANOVA conducted on the remaining data revealed a significant group difference in the average number of aerobic steps taken each day during the 4 week period between Visits 1 and 2 [F(3,46) = 25.5, P < 0.0001]. As expected, participants in the two groups that exercised during the 4 week period (4W−, 4W+) each took more aerobic steps than those in the 0W− or 0W+ groups (Ps < 0.0001) as shown in Figure 2. There were no significant differences between groups 0W− and 0W+ (P > 0.9) or between groups 4W− and 4W+ (P > 0.8). Furthermore, a paired t-test that compared the average number of aerobic steps taken on exercise (EX) versus no-exercise (No-EX) days for participants in groups 4W− and 4W+ (pooled together) confirmed that participants took more aerobic steps on days that they exercised compared to non-exercise days [t(24) = 8.3, P < 0.0001]. The mean number of aerobic steps taken on No-EX days and EX days was 5218.4 ± 412.1 and 1333.8 ± 248.2, respectively. In addition, there was a statistically significant relationship between the average daily number of aerobic steps and VO2 max (r = 0.3, P < 0.02).
Figure 2.
Aerobic steps taken by participants in each group. Individuals in the 4-week exercise groups (4W− and 4W+) took significantly more aerobic steps than those in the 0W− or 0W+ groups. Data are the mean ± SEM steps taken over the 30 day experiment, including days that individuals in the exercise group chose not to exercise. (*indicates P < 0.05 in all figures).
Novel Object Recognition Task
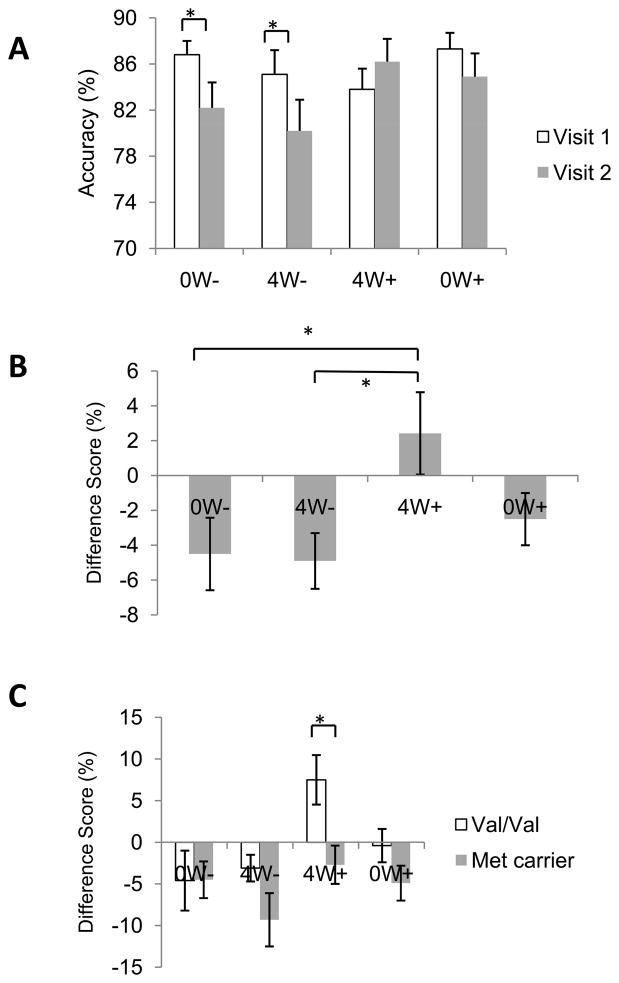
The percentage of objects each group accurately identified as old or new during Visits 1 and 2 is illustrated in Figure 3A. A repeated measures ANOVA did not detect a main effect of Group (P>0.5), but did reveal a significant main effect of Visit [F(1,50) = 6.3, P < 0.02] and a significant Group X Visit interaction [F(3,50) = 2.97, P < 0.04]. A follow up analysis found no group differences during Visit 1 (P < 0.4), indicating that accuracy was comparable in all groups prior to the exercise manipulation. Similarly, a one-way ANOVA of the Visit 2 data did not reveal any group differences (P > 0.2). Instead, the significant Group X Visit interaction was driven by differences between groups in the change in performance on Visit 2 compared to Visit 1. For the 0W− and 4W− groups, accuracy decreased significantly during Visit 2 compared to Visit 1 (Ps < 0.5); this was not the case for the 4W+ or 0W+ groups (Ps > 0.1). Moreover, as illustrated in Figure 3B and confirmed by a one-way ANOVA [F(3,50) = 2.97, P<0.04], the difference scores (accuracy during Visit 2 minus accuracy during Visit 1) were significantly different between groups. Post-hoc analyses indicated that the difference scores of participants in the 4W+ group were significantly higher than those in the 0W− group (P<0.02) or the 4W− group (P<0.01). The difference between the 4W+ and 0W+ groups did not reach statistical significance (P=0.07); no other pair-wise comparisons were statistically significant (Ps > 0.3). Notably, the 4W+ group was the only group with a positive accuracy difference score, indicating improvement from Visit 1 to Visit 2. As shown in Figure 3C, planned comparisons indicated that this was driven primarily by significantly higher difference scores in Val homozygotes compared to Met carriers in group 4W+ [t(10) = 2.7, P < 0.03]. Moreover, Val/Val participants exhibited a significant increase in accuracy during Visit 2 compared to Visit 1 (P < 0.05) but Met carriers did not (P > 0.3).
Figure 3.
Effects of exercise on object recognition memory. A) Accuracy in identifying objects as old or new during Visits 1 and 2. Groups 0W− and 4W− exhibited a decrease in accuracy from Visit 1 and Visit 2. B) Object recognition difference scores (Visit 2 – Visit 1). Group 4W+ exhibited a greater change in accuracy compared to non-exercising groups. C) Only Val homozygotes in group 4W+ exhibited an increase in accuracy during Visit 2 compared to Visit 1. Data are mean ± SEM.
Mood and Anxiety Measures During Visits 1 and 2
Perceived Stress (PSS)
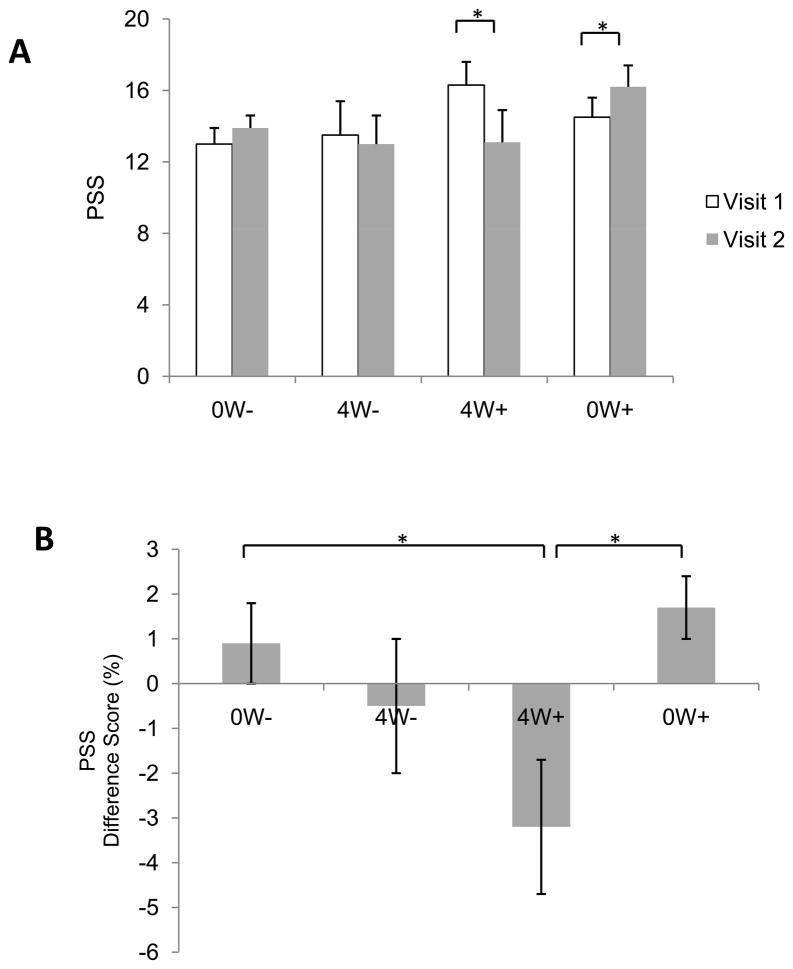
Data from the perceived stress surveys are illustrated in Figure 4A. There were no main effects of Group or Visit (Ps > 0.5), but there was a significant Group X Visit interaction. Follow up ANOVAs did not reveal any group differences during Visit 1 (P > 0.4) or Visit 2 (P > 0.3). However, there was a significant increase in perceived stress during Visit 2 compared to Visit 1 for the 0W+ group (P < 0.04) and a significant decrease in perceived stress in the 4W+ group (P < 0.05). This was also reflected in the difference scores, which differed significantly across groups [F(3,50) = 3.2, P < 0.03] with the 4W+ group exhibiting a significantly higher difference score compared to the 0W− group (P < 0.02) and 0W+ group (P < 0.005), as shown in Figure 4B. No other pair-wise comparisons achieved statistical significance (Ps > 0.1) and there were no differences between Val/Val and Met-carrying participants in any group (Ps > 0.3). There was also no significant relationship between accuracy on the NOR task and perceived stress (P > 0.1).
Figure 4.
A) Perceived stress (PSS) during Visits 1 and 2, and B) difference scores between visits. Group 2 exhibited a significant decrease in stress from Visit 1 to Visit 2. Data are mean ± SEM.
Positive Mood (PANASP)
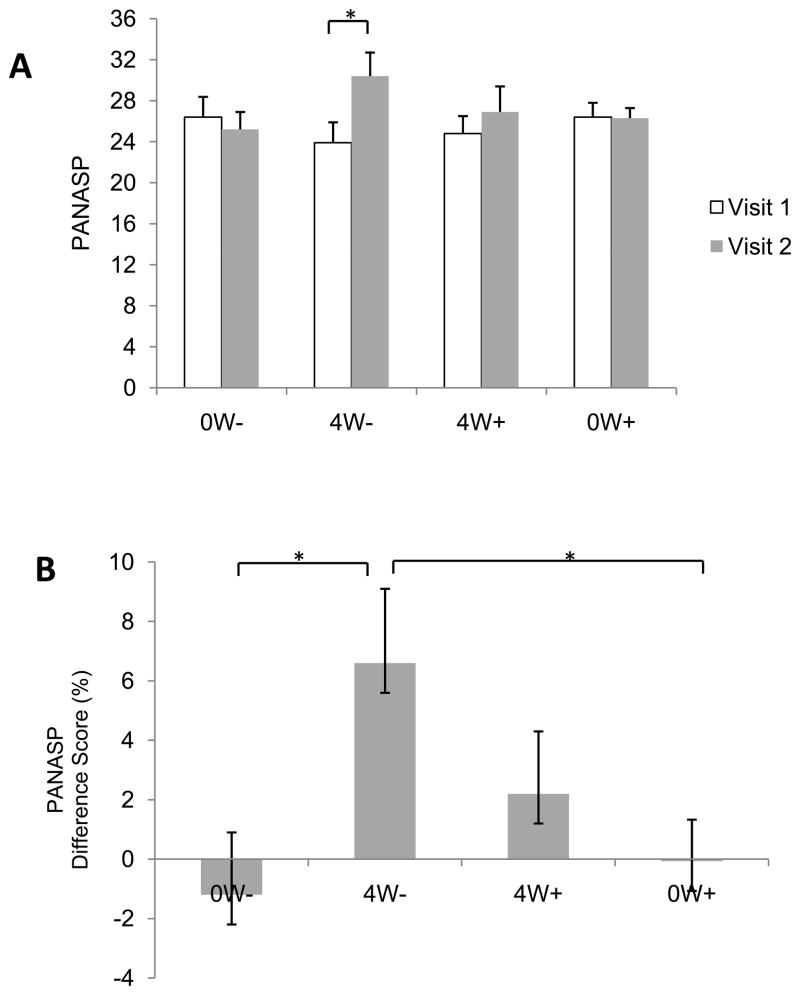
The positive mood ratings for each group are depicted in Figure 5A. Although there was no main effect of Group (P > 0.9) or Visit (P > 0.07), there was a significant Group X Visit interaction [F(3,50) = 3.0, p < 0.04]. One-way ANOVA indicated that there were no group differences during Visit 1 (P > 0.6) or during Visit 2 (P > 0.2). However, there was a significant increase in positive mood from Visit 1 to Visit 2 in participants in the 4W− group (P < 0.02). Analysis of the difference scores also revealed a significant difference between groups [F(3,50) = 3.0, p< 0.04], and post-hoc tests indicated that difference score for the 4W− group was significantly higher than scores for the 0W− (P < 0.01) and 0W+ groups (P < 0.02), as illustrated in Figure 5B. No other pair-wise comparisons achieved statistical significance (Ps > 0.3) and there were no differences between Val/Val and Met-carrying participants in any group (Ps > 0.1). There was also no significant relationship between accuracy on the NOR task and positive mood (P > 0.2).
Figure 5.
A) Positive mood (PANASP) during Visits 1 and 2, and B) the differences scores between visits. Participants in group 4W− exhibited an increase in positive mood from Visit 1 to Visit 2. Data are mean ± SEM.
Depression Index (BDI)
Depression scores for each group during Visits 1 and 2 are indicated in Table 2. The main effect of Visit approached statistical significance (P = 0.06), as did the Group X Visit interaction (P = 0.06). However, there was no significant relationship between BDI and object memory performance (Ps > 0.3). There was also no significant main effect of Group (P > 0.3).
Table 2.
Depression Index (BDI) during Visits 1 and 2
| 0W− | 4W− | 4W+ | 0W+ | |
|---|---|---|---|---|
| Visit 1 | 2.5 ± 0.7 | 6.5 ± 1.6 | 6.7 ± 1.1 | 4.9 ± 1.0 |
| Visit 2 | 3.2 ± 0.8 | 4.1 ± 1.4 | 4.8 ± 1.2 | 5.0 ± 1.3 |
State Anxiety (STAI-Y1)
Data from the state anxiety inventory during Visits 1 and 2 are shown in Table 3. There were no differences between groups at either visit, as confirmed by a repeated measures ANOVA that failed to detect any main effects or interactions (Ps > 0.5).
Table 3.
State Anxiety (STAI-Y1) during Visits 1 and 2
| 0W− | 4W− | 4W+ | 0W+ | |
|---|---|---|---|---|
| Visit 1 | 30.7 ± 2.2 | 32.1 ± 2.5 | 33.5 ± 2.3 | 32.3 ± 2.1 |
| Visit 2 | 32.5 ± 1.8 | 30.4 ± 2.3 | 34.3 ± 3.2 | 35.1 ± 2.2 |
Negative Mood (PANASN)
Negative mood ratings are included in Table 4. There were no significant group differences, main effects of Visit, or a Group X Visit interaction (Ps > 0.3).
Table 4.
Negative Mood (PANASN) during Visits 1 and 2
| 0W− | 4W− | 4W+ | 0W+ | |
|---|---|---|---|---|
| Visit 1 | 12.4 ± 0.7 | 11.8 ± 0.7 | 11.3 ± 0.9 | 11.5 ± 0.7 |
| Visit 2 | 11.2 ± 0.5 | 12.6 ± 1.2 | 11.8 ± 0.8 | 12.3 ± 0.8 |
Trait Anxiety (STAI-Y2)
A one-way ANOVA indicated that there were no significant differences between groups on the trait anxiety inventory administered during Visit 1 (P > 0.8). The mean for each group was 30.7 ± 2.2 (0W−), 32.1 ± 2.5 (4W−), 33.5 ± 2.3 (4W+), and 32.3 ± 2.1 (0W+).
Daily Mood/Anxiety Surveys
Data from the daily STAI-Y1, PANASP, and PANASN surveys on No-EX and EX days between Visits 1 and 2 is illustrated in Figure 6. State anxiety was significantly lower on EX days versus No-EX days [t(25)=3.2, P < 0.0001]. In addition, positive mood (PANASP) was higher on EX days compared to No-EX days [t(25) = −3.7, P < 0.001]. The group difference on the negative mood survey (PANASN) did not reach statistical significant (P > 0.09). There were no differences between Val/Val and Met-carrying participants on any of the daily mood/anxiety measures (Ps > 0.2).
Figure 6.
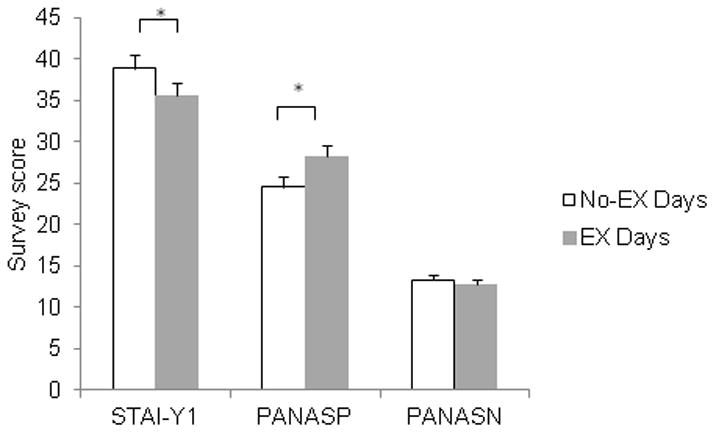
Daily measures of state anxiety (STAI-Y1), and positive (PANASP) and negative (PANASN) mood between visits. Stress levels were decreased and positive mood was increased in participants who exercised between Visits 1 and 2. Data are mean ± SEM.
DISCUSSION
We found that an acute exercise session combined with a regular exercise regimen augmented recognition memory and decreased perceived stress in sedentary, healthy young adults. In contrast, a single bout of exercise alone had no effect on recognition memory and increased self-reported stress. Further, the data indicate that a common genetic polymorphism may have an important role in the influence that exercise has on memory since the effects were only observed in participants who were homozygous for the Val allele of the BDNF gene. Here we discuss the implications and limitations associated with each of these results.
Effects of Exercise on Object Recognition Memory
Consistent with previous reports demonstrating that regular exercise can improve cognitive function in aging as well as diseased populations, (Cotman and Berchtold, 2002; Kramer and Erickson, 2007; Larson, 2008), we showed that regular exercise had a beneficial effect on memory in previously sedentary but otherwise healthy, young adults. However, this was only true for participants who exercised on the final day of testing after the 4-week exercise intervention (i.e., the 4W+ group). Considered in isolation, this finding raises the possibility that the effect could be attributed to the single bout of exercise on the testing day (Peyrin et al., 1987; Ferris et al., 2007; Winter et al., 2007; Coles and Tomporowski, 2008; Hillman et al., 2009). However, the 0W+ group, who also exercised on the final day of testing, did not exhibit any significant effects of exercise on recognition memory, similar to the 0W− group and the 4W− group.
These data may reflect a gradual development in the beneficial effects of regular exercise, whereby an acute bout of mild exercise can confer cognitive benefits to individuals who regularly engage in exercise. In other words, the degree to which an acute exercise session will influence cognitive performance may depend on the individual’s previous physical activity habits. Consistent with this notion, it is well-established that regular exercise facilitates long-term potentiation (LTP; van Praag et al., 1999), which could reflect a type of molecular priming that influences neural plasticity and long term memory formation after acute exercise. Further, it has been shown that regular exercise was related to increased BDNF levels when rats were re-exposed to a brief exercise regimen that was not sufficient to increase BDNF levels in naive rats (Berchtold et al., 2005). These data resonate with our findings and suggest that regular exercise has an enduring effect on the molecular machinery responsible for elevating BDNF. Future studies could enroll both sedentary controls and participants who are already regular exercisers (e.g., varsity athletes) to directly compare the effect of a single bout of exercise between these groups.
In the present study, we intentionally employed a 2-hour gap between acute exercise and the final testing session to minimize any effects on memory and/or mood resulting from physiological arousal. Previous studies have found increases in cognitive performance after more intense acute physical activity within 10–30 minutes of exercising (Tomporowski, 2003), and evidence suggests that exercise-induced increases in circulating catecholamines might mediate these short-term cognitive improvements (Ferris et al., 2007; Winter et al., 2007). Although we did not measure catecholamine levels in our current study, any acute physiological changes due to exercise would be expected to have returned to baseline by the time we tested participants (Lambert et al., 2000; Dietrich and Audiffren, 2011). Thus, our data emphasize the difference between immediate and delayed improvements in cognitive performance resulting from an acute exercise session.
Genetic Influences on the Effects of Exercise on Object Recognition Memory
The observed effect of exercise on recognition memory was associated with individual differences in BDNF genotype. Namely, participants who were homozygous for the Val allele within the 4W+ group that exhibited a significant increase in object recognition accuracy after exercise, while Met carriers did not. We note that although the Val66Met polymorphism does not affect BDNF protein function, it does result in altered intracellular trafficking of pro-BDNF and decreased availability of activity-dependent BDNF (Egan et al., 2003). In humans, BDNF Met carriers exhibit decreased experience-related neural plasticity (Kleim et al., 2006; Cheeran et al., 2008) and learning/memory impairments compared to individuals without the polymorphism (Egan et al., 2003; Hajcak et al., 2009). Thus, the altered activity-dependent release of BDNF in Met allele carriers may attenuate the cognitive benefits resulting from exercise.
Effects of Exercise on Mood and Anxiety
In addition to enhancing recognition memory, exercise reduced perceived stress only in the 4W+ group. In fact, the group that exercised only on the day of Visit 2 (0W+ group) experienced an increase in perceived stress compared to Visit 1. In contrast, the only group that exhibited a change in positive mood was group 4W−, which exercised for four weeks but did not exercise on the day of Visit 2. Further, our daily survey data provides evidence that the four-week exercise intervention improved psychological well-being since participants in groups 4W− and 4W+ experienced a decrease in state anxiety and an increase in positive mood on exercise days compared to non-exercise days.
In general, these data are consistent with the notion that regular exercise results in overall improvements in psychological well-being, while revealing that different exercise regimens have varying effects on specific psychological outcomes. There are several reasons why the acute exercise intervention employed here increased perceived stress. First, prior research shows that while acute exercise enhances both cognitive performance and psychological well-being in regular exercisers, sedentary individuals report decreased (Boutcher et al., 1997) or no change in mood (Hoffman and Hoffman, 2008). Furthermore, positive gains in mood have been observed in individuals who increase their physical activity levels after adjusting to regular exercise (Gauvin et al., 1996). Based on these findings, single bouts of exercise may be beneficial only for individuals who have been physically active over a longer period of time.
Importantly, we found no significant relationship between recognition memory and mood or anxiety, supporting the notion that the effects of exercise on cognition are not simply due to exercise-induced changes in affect. This is further supported by the finding that positive mood was only enhanced in the 4W− group. Moreover, the observation that exercise decreased stress and increased positive mood when measured at least 2 hours after the last bout of exercise extends prior research that carried out assessments either during or immediately after exercise (Berger and Owen, 1998; Hansen et al., 2001). Finally, the present effects on psychological well-being were observed in healthy young adults, data that extend previous studies showing similar effects in clinical populations (Blumenthal et al., 1999, 2007; Pinchasov et al., 2000).
Caveats and Future Directions
In the present study, NOR performance decreased over a four-week period for participants in the 0W− and 4W− groups. Because stimuli were counterbalanced across participants and testing sessions, this observation was not a function of changes in task difficulty. However, our aim was to test between group differences as a function of different exercise regimens. Indeed, our predictions were driven by object recognition studies in rodents showing that non-exercising rats were not able to successfully discriminate between novel and familiar objects, but exercise resulted in successful discrimination (Hopkins and Bucci, 2010, Hopkins et al., 2011; Griffin et al., 2009).
The finding that the effect of exercise on object recognition memory in group 4W+ was influenced by BDNF genotype is a potentially important new finding. Although the sample sizes of the Val-Val and Met-carrying participants in group 4W+ were relatively small (6/group), we note that effect size was 1.5 and the achieved power was 0.7 (G-power Statistical Software). Nonetheless, future studies are needed to further explore the intriguing notion that BDNF genotype may mitigate the effects of exercise. Likewise, it would be interesting to determine if there are sex differences in how BDNF genotype interacts with exercise.
Unlike these effects of exercise on recognition memory, we did not observe any differences between Val homozygotes and Met-carrying participants on mood or anxiety. Indeed, there is mixed evidence as to whether the Val66Met polymorphism leads to an increased risk of mental illness. While some studies have shown higher prevalence of depression (Blumenthal et al., 1999) and anxiety (Jiang et al., 2005; Montag et al., 2010) in Met carriers, others do not (Hashimoto et al., 2004; Lang et al., 2005; Frustaci et al., 2008; Duncan et al., 2009). Given the inconsistency of these findings, it is likely that other environmental factors (e.g., early life stress) may interact with BDNF genotype (Casey et al., 2009) to mediate the risk of mood disorders (Gatt et al., 2009; Frielingsdorf et al., 2010).
Conclusion
The present report presents data illustrating the beneficial effects of exercise on measures of cognition and psychological well-being in healthy individuals, and offers data identifying a genetic mediator of these effects. These data are more compelling because they broadly replicate previous findings in rats, showing a similar BDNF mediation of improvement in recognition memory as well as reduced anxiety-like behavior following exercise (Hopkins and Bucci, 2010; Hopkins et al., 2011). Given the functional relationship between exercise and BDNF activity now demonstrated in humans and non-human animals, a common polymorphism in the BDNF gene may exert influence over the degree to which an individual may benefit from exercise.
Acknowledgments
The authors thank Dr. Gregory Tsongalis and colleagues for processing the DNA samples, and Rachel Eggleston, Jennifer Buchholz, Matthew Miner, Dallis Fox, and Cynthia Akagbosu for assistance in collecting the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;16(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- Annesi JJ. Mood states of formerly sedentary younger and older women at weeks 1 and 10 of a moderate exercise program. Psychol Rep. 2004;94(3 Pt 2):1337–42. doi: 10.2466/pr0.94.3c.1337-1342. [DOI] [PubMed] [Google Scholar]
- Audiffren M. Acute exercise and psychological functions: a cognitive-energetic approach. In: McMorris T, Tomporowski PD, Audiffren M, editors. Exercise and cognitive function. Chichester, UK: John Wiley & Sons; 2009. pp. 3–39. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Berger BG, Owen DR. Relation of low and moderate intensity exercise with acute mood change in college joggers. Percept Mot Skills. 1998;87(2):611–21. doi: 10.2466/pms.1998.87.2.611. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead E, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–56. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Boutcher SH, McAuley E, Courney KS. Positive and negative affective response of trained and untrained subjects during and after aerobic exercise. Australian Journal of Psychology. 1997;49(1):28–32. [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Altemus M, Pattwell S, Jones R, Levita L, McEwen B, Magariños AM, Gunnar M, Thomas KM, Mezey J, Clark AG, Hempstead BL, Lee FS. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164(1):108–20. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586(Pt 23):5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:386–396. [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Coles K, Tomporowski PD. Effects of acute exercise on executive processing short-term and long-term memory. J Sports Sci. 2008;26(3):333–44. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;20(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Allison JD, Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized controlled trial. Health Psychol. 2011;30(1):91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci Biobehav Rev. 2011;35(6):1305–1325. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol. 2003;25(5):643–53. doi: 10.1076/jcen.25.5.643.14583. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24(5):1265–76. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Hutchison KE, Carey G, Craighead WE. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. J Affect Disord. 2009;115(1–2):215–9. doi: 10.1016/j.jad.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Erikson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain derived neurotrophic factor levels and cognitive function. Medicine & Science in Sport & Exercise. 2007;39(4):728–34. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein F, Knecht S. Physical activity and memory functions: Are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49(3):2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–7. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Pozzi G, Gianfagna F, Manzoli L, Boccia S. Meta-analysis of the brain derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3–4):163–70. doi: 10.1159/000182892. [DOI] [PubMed] [Google Scholar]
- García-Capdevila S, Portell-Cortés I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202(2):162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. MolPsychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gauvin L, Rejeski WJ, Norris JL. A naturalistic study of the impact of acute physical activity on feeling states and affect in women. Health Psychol. 1996;15(5):391–7. doi: 10.1037//0278-6133.15.5.391. [DOI] [PubMed] [Google Scholar]
- George JD, Stone WJ, Burkett LN. Non-exercise VO2max estimation for physically active college students. Med Sci Sports Exerc. 1997;29(3):415–23. doi: 10.1097/00005768-199703000-00019. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009 Oct;19(10):973–80. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Castille C, Olvet DM, Dunning JP, Roohi J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain Behav. 2009;8(1):80–5. doi: 10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol. 2001;20(4):267–75. doi: 10.1037//0278-6133.20.4.267. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic Factor in mood disorders. Brain Res Brain Res Rev. 2004;45(2):104–14. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Herring MP, O’Connor PJ, Dishman RK. The effects of exercise training on anxiety symptoms among patients. Archives of Internal Medicine. 2010;170(4):321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–54. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MD, Hoffman DR. Exercisers achieve greater acute exercise-induced mood enhancement than nonexercisers. Arch Phys Med Rehabil. 2008;89(2):358–63. doi: 10.1016/j.apmr.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology of Learning and Memory. 2010;94(2):278–84. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and BDNF expression. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30(7):1353–61. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Kamijo K, Nishihira Y, Hatta A, Kaneda T, Wasaka T, Kida T, Kuroiwa K. Differential influences of exercise intensity on information processing in the central nervous system. Eur J Appl Physiol. 2004;92(3):305–11. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness aging and neurocognitive function. Neurobiol Aging. 2005;26(1):124–127. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–8. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Lambert CP, Flynn MG, Braun WA, Mylona E. Influence of acute submaximal exercise on T-lymphocyte suppressor cell function in healthy young men. Eur J Appl Physiol. 2000;82(1–2):151–154. doi: 10.1007/s004210050665. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, Kunz D, Gallinat J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180(1):95–9. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Larson EB. Physical activity for older adults at risk for Alzheimer’s disease. JAMA. 2008;300(9):1077–1079. doi: 10.1001/jama.300.9.1077. [DOI] [PubMed] [Google Scholar]
- Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;19(3):CD004691. doi: 10.1002/14651858.CD004691.pub2. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, Sankin L, Pine DS, Ernst M. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53(3):952–61. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox SS, Bedell JR, Stone AA. The effect of exercise on normal mood. J Psychosom Res. 1990;34(6):629–36. doi: 10.1016/0022-3999(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010;179(1):86–90. doi: 10.1016/j.psychres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol. 1997;83(1):5–10. doi: 10.1152/jappl.1997.83.1.5. [DOI] [PubMed] [Google Scholar]
- Peyrin L, Pequignot JM, Lacour JR, Fourcade J. Relationships between catecholamine or 3-methoxy 4-hydroxy phenylglycol changes and the mental performance under submaximal exercise in man. Psychopharmacology (Berl) 1987. 1987;93(2):188–92. doi: 10.1007/BF00179932. [DOI] [PubMed] [Google Scholar]
- Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000;94(1):29–42. doi: 10.1016/s0165-1781(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136(4):991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of Short Versus Long Bouts of Aerobic Exercise in Sedentary Women With Fibromyalgia: A Randomized Controlled Trial. Physical Therapy. 2003;83:340–358. [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto CA: Mind Garden; 1983. [Google Scholar]
- Tkacz J, Young-Hyman D, Boyle CA, Davis CL. Aerobic exercise program reduces anger expression among overweight children. Pediatr Exerc Sci. 2008;20(4):390–401. doi: 10.1123/pes.20.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol (Amst) 2003;112(3):297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118(6):1378–90. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis learning and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]