Abstract
Tinnitus perception depends on the presence of its neural correlates within the auditory neuraxis and associated structures. Targeting specific circuits and receptors within the central nervous system in an effort to relieve the perception of tinnitus and its impact on one’s emotional and mental state has become a focus of tinnitus research. One approach is to upregulate endogenous inhibitory neurotransmitter levels (e.g. glycine and GABA) and selectively target inhibitory receptors in key circuits to normalize tinnitus pathophysiology. Thus, the basic functional and molecular properties of two major ligand-gated inhibitory receptor systems, the GABAA receptor (GABAAR) and glycine receptor (GlyR) are described. Also reviewed is the rationale for targeting inhibition which stems from reported tinnitus-related homeostatic plasticity of inhibitory neurotransmitter systems and associated enhanced neuronal excitability throughout most central auditory structures. However, the putative role of the medial geniculate body (MGB) in tinnitus has not been previously addressed, specifically in terms of its inhibitory afferents from inferior colliculus and thalamic reticular nucleus and its GABAAR functional heterogeneity. This heterogeneous population of GABAARs, which may be altered in tinnitus pathology, and its key anatomical position in the auditory CNS make the MGB a compelling structure for tinnitus research. Finally, some selective compounds, which enhance tonic inhibition, have successfully ameliorated tinnitus in animal studies, suggesting that the MGB and, to a lesser degree, the auditory cortex may be their primary locus of action. These pharmacological interventions are examined, in terms of their mechanism of action and why these agents, may be effective in tinnitus treatment.
Keywords: tinnitus, GABAAR, GlyR, inhibition, medial geniculate body, homeostatic plasticity
1. Introduction
A number of studies over the past few years have focused on inhibitory neurotransmitter receptors, their related metabolic enzymes and trafficking/scaffolding proteins in effort to better understand the neurobiological underpinnings of tinnitus. As a result, tinnitus-related changes in inhibitory neurotransmission have been identified (Roberts et al., 2010; Wang et al., 2011). In an effort to counteract such maladaptive homeostatic plasticity of or compensatory changes in inhibitory mechanisms, recent animal studies have targeted GABAergic or glycinergic inhibitory function and have been moderately successful in reducing behavioral evidence of tinnitus (Brozoski et al., 2007; Brozoski et al., 2010; Yang et al., 2011). This review examines findings in these areas, focusing first on recently identified tinnitus-related changes in inhibitory mechanisms and ways in which these changes may impact neuronal activity in the DCN, IC and auditory cortex (see also Wang et al., 2011). Also considered is the putative role of the auditory thalamus (MGB) in tinnitus pathology and how targeting a specific subtype of GABAAR inhibition (tonic) may be advantageous in a therapeutic approach for tinnitus treatment. Finally, some successes of tinnitus pharmacotherapy in animal models and the compounds’ mechanism of action are reviewed.
The idea that aberrations in inhibitory neurotransmission could underlie tinnitus pathology stems from the hypothesis supported by recent data that a partial peripheral deafferentation induced by an acoustic insult could trigger a series of maladaptive homeostatic plastic changes. Liberman and Kujawa (2009) demonstrated how a modest acoustic trauma results in permanent partial cochlear nerve deafferentation (i.e. SGC loss) despite normal or near normal acoustic thresholds. In fact, Bauer and colleagues (2007) showed SGC loss was more predictive of tinnitus than hearing loss in rats. Similarly, in a recent human study using electrophysiologic measures, Schaette and McAlpine (2011) found that individuals experiencing tinnitus displayed partial deafferentation of the cochlear nerve, even when hearing loss was undetected by standard audiologic measures. Since the loss of peripheral input appears to remain uncompensated, without a replacement of lost afferent neurons, pre and postsynaptic central plastic changes in neural inhibition (e.g. down-regulation of GABA/glycine release and altered postsynaptic receptor constructs) may represent an example of homeostatic plasticity, compensating for this loss of input. Such an altered balance between driving excitation and central inhibition may underlie and result in the percept of tinnitus.
2. Inhibitory Neurotransmitters, Receptors and Related Proteins
An accurate representation of the acoustic scene depends not only on the driving excitatory input, but on inhibitory mechanisms that serve to shape spontaneous activity, input/output functions, frequency tuning, and temporal response accuracy (Caspary et al., 2008). Two prominent ways neuronal responses may be inhibited are through activation of GlyRs and GABAARs following release of the amino acid neurotransmitters glycine and GABA, respectively. GlyRs and GABAARs are ligand-gated channels, made up of five separate proteins or subunits that, in adult animals, allow for Cl− influx, resulting in hyperpolarization or clamping of a neuron’s resting membrane potential below threshold. Nineteen GABAAR (α1–6, β1–3, γ1–3, δ, π, ε, θ, and ρ1–3) and five GlyR (α1–4 and β) subunits which assemble into pentamers with a limited number of stoichiometric combinations provide a range of functional receptors with variable levels of expression across cell types and locations in the CNS (Farrant and Nusser, 2005; Lynch, 2009; Walker and Semyanov, 2008).
It has become apparent that the presence of specific subunits in the composition of inhibitory receptors can confer dramatically different properties on these receptors, altering pharmacology, location of insertion in the membrane (synaptic vs. extrasynaptic) and kinetic/temporal properties (Belelli et al., 2009; Farrant and Nusser, 2005; Richardson et al., 2011; Walker and Semyanov, 2008; Figure 1). Moreover, data suggest that a relatively limited number of functional subunit combinations exist and are exclusively expressed in defined brain regions (Pirker et al., 2000; Sieghart and Sperk, 2002; Wisden et al., 1992).
Figure 1.
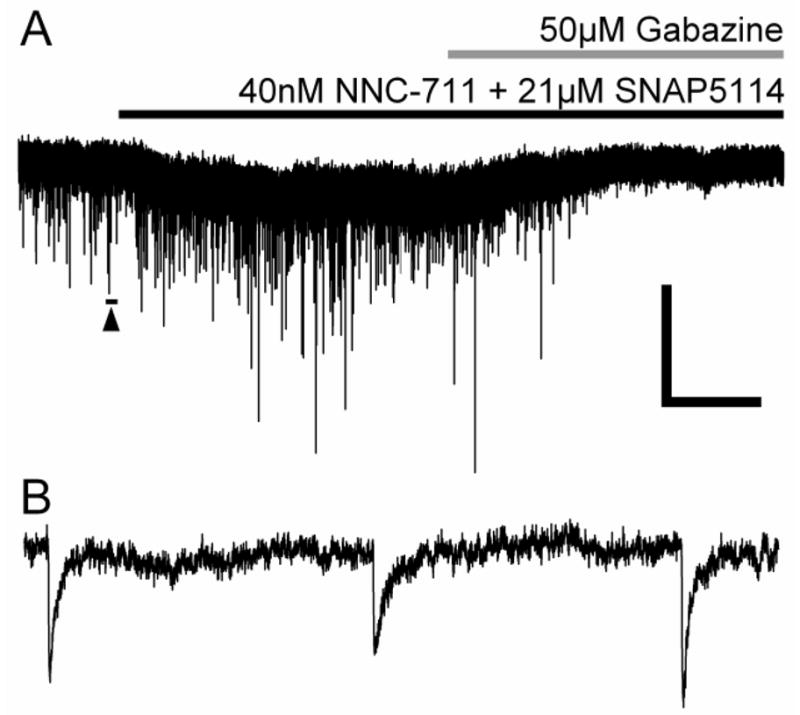
Tonic and Phasic Inhibition in the Rat MGB. A) Voltage-clamp (CsCl based internal solution, Vhold = −60mV, ECl ≈ 0mV) recording from an MGB neuron of a 7 month old FBN rat during the application of GABA uptake blockers (NNC-711 and SNAP5114, blockers of GAT-1,2 &3 mediated GABA uptake) [black bar] and subsequent application of the GABAAR selective antagonist 50μM gabazine (SR95531) [gray bar] reveal the presence of extrasynaptic GABAARs mediating a tonic Cl− current in the MGB which is blocked by gabazine. B) Expanded sIPSCs (underlined region in A with arrowhead) are also blocked by gabazine and therefore GABAAR mediated (A). Scale bar is 50sec and 50pA for A and 100msec and 40pA for B. Modified from Richardson et al., 2011.
One basic distinction in ligand-gated ionotropic inhibitory receptor types is between the tonic/prolonged form of inhibition (Figure 1A) mediated by a high affinity extrasynaptic receptor population and the classic phasic (30–50msec) inhibition (Figure 1B) mediated by a different low affinity synaptic GlyRs or GABAARs population (Belelli et al., 2009; Farrant and Nusser, 2005; Xu and Gong, 2010; Zhang et al., 2008). The expression of receptors with differential time courses of inhibition in specific central auditory regions and cell types may be useful in establishing a specific/targeted tinnitus pharmacotherapy. Targeting receptors mediating tonic non-desensitizing inhibition with selective agonists or positive allosteric modulators would circumvent desensitization of phasic inhibitory receptors and could enhance responses to endogenous neurotransmitter release (see Section 4).
Neurons may regulate the diversity of inhibitory receptor constructs at the level of gene transcription or protein translation, but functionally, only receptors expressed on the cell membrane are relevant. The membrane insertion of functional GlyRs and GABAARs is governed by specific trafficking and anchoring molecules which play an important role in regulating inhibitory synaptic strength/receptor density (for review see Kittler et al., 2002; Kneussel and Loebrich, 2007; Luscher and Keller, 2004; Luscher et al., 2011). Most notably, gephyrin serves two functions; trafficking receptors to and from membrane active zones and anchoring receptors to other scaffolding molecules like GABARAP and Radixin (Fritschy et al., 2008; Jacob et al., 2008; Vithlani et al., 2011). This group of molecules and their interaction with cytoskeletal components have the ability to dynamically regulate plasticity of GABAARs and GlyRs by modulating receptor insertion into the cell membrane and controlling their lateral mobility in addition to their transcriptional/translational regulation (Calamai et al., 2009; Charrier et al., 2006; Danglot et al., 2003; Dumoulin et al., 2009; Jacob et al., 2005). However, regulatory mechanisms of inhibitory receptor plasticity are likely different for synaptic and tonically active extrasynaptic GABAAR types as the two do not appear to interact with the same set of associated trafficking and scaffolding proteins (Peden et al., 2008; Vithlani et al., 2011). Understanding how protein expression and signaling pathways regulate participation of these molecules in normal synaptic function may be key to understanding the plastic inhibitory changes that occur in tinnitus (Wang et al., 2011 for review).
3. Tinnitus-Related Changes in Neuronal Activity and Inhibitory Mechanisms
One consistently noted tinnitus-related change in cellular function throughout the central auditory system is an increase in spontaneous/bursting activity and acoustically driven firing rates subsequent to peripheral insult, which are briefly reviewed here (see Kaltenbach and Godfrey, 2008; Roberts et al., 2010; Wang et al., 2011 for detailed review). In dorsal and/or ventral cochlear nuclei of guinea pigs, hamsters or chinchillas exposed to sounds capable of inducing behavioral evidence of tinnitus, spontaneous and driven activity were elevated (Brozoski et al., 2002; Kaltenbach et al., 2000; Kaltenbach et al., 2004; Vogler et al., 2011). For fusiform cells, the largest increases in tinnitus-related acoustic driven activity appear at higher intensities. The dependence of non-monotonic rate-intensity functions on glyicnergic inhibition points to altered glycinergic function and the GlyR as a candidate in the inhibitory neuropathology that may occur with tinnitus (Brozoski et al., 2002; Caspary et al., 1987). Indeed, immunohistochemistry and strychnine binding indicate that the subunit make-up of GlyRs is altered and density of GlyRs is reduced whereas the trafficking protein, gephyrin, is elevated in the DCN of sound exposed rats (Wang et al., 2009). Other inhibitory mechanisms may also be altered and reflect tinnitus pathology at the level of the DCN. For example, enhanced neural synchrony is seen within the DCN of mice with behavioral evidence of tinnitus, which appears due to a loss of GABAergic inhibition (Middleton et al., 2011). This would also contribute to elevated single unit activity. Pilati and colleagues (2011) identified an increased incidence of burst responses in rat DCN fusiform cells following intense sound exposure, implicating a down-regulation of HVA K+ channels. This is yet another example of homeostatic plasticity following noise exposure may comprise part of what underlies neuronal hyperexcitability in tinnitus, in addition to described losses/changes in ligand-gated inhibition. Significantly, the DCN appears necessary for establishment of tinnitus since ablation of the DCN prior to sound exposure prevents the establishment of behavioral evidence of tinnitus in a rat model (Brozoski et al., 2011).
In the mammalian IC, increases in spontaneous and evoked firing rates are found up to three months following sound exposure (Longenecker and Galazyuk, 2011; Ma et al., 2006; Mulders et al., 2011; Mulders and Robertson, 2011). Increases in specific patterns of neural firing (e.g. bursting) may be a necessary hallmark of the nervous system’s physiological tinnitus signature, not simply increases in rate, and could be enhanced by a loss of inhibitory tone. In support of this hypothesis, Bauer and colleagues showed an increased intraburst rate in addition to increased spontaneous firing rates 8–9 months after noise exposure in the IC of chinchillas with behavioral evidence of tinnitus (Bauer et al., 2008). As in the DCN, changes in inhibitory neurotransmitter related mechanisms are also present in the IC following sound exposure. Levels of the presynaptic inhibitory synthetic protein, GAD, remain decreased up to a month following noise exposure suggesting that the level of GABA available for vesicular storage and release is likely decreased (Milbrandt et al., 2000). The work of Dong, Robertson and their colleagues (2010), used qRT-PCR to correlate plasticity of genes related to inhibitory neurotransmission with neuronal activity in the guinea pig IC. Following sound exposure, observed elevations in spontaneous firing rate were accompanied by a down-regulation of mRNA levels for the α1 subunits of the GlyR and GABAAR. These findings, in part, exemplify how homeostatic plasticity alters IC inhibitory receptors in tinnitus. However, as discussed previously, posttranslational modification of mature receptor constructs and receptor insertion and mobility in the plasma membrane may also contribute a significant component to plastic responses to an acoustic insult and will require further study at multiple levels of the auditory pathway.
Changes in neural activity and inhibitory mechanisms have also been characterized for auditory cortex. In addition to increased firing rates, re-organization of tonotopically aligned receptive fields of auditory cortical neurons seem characteristic of tinnitus (for reviews see Eggermont and Roberts, 2004; Roberts et al., 2010). Recently, Yang and colleagues (2011) addressed the role of GABAergic inhibition in cortical reorganization following sound exposure. Animals with behavioral evidence of tinnitus showed a loss of high frequency responding neurons (cortical remapping), elevations in firing rate, reductions in tonic and phasic GABAAR current amplitudes and frequency along with decreases in presynaptic GAD levels in high frequency regions (Yang et al., 2011). This direct, frequency specific loss of inhibition and increase in excitability, persisting for at least two weeks after exposure, convincingly demonstrating how tinnitus-related plasticity impacts not only molecular markers of inhibition, but inhibitory function as well. Similar approaches will be beneficial in further characterizing the nature of tinnitus related inhibitory plasticity that occurs throughout the auditory neuraxis.
In summary, evidence for decreased inhibitory neurotransmitter release may be accompanied by decreases in postsynaptic receptor density or the replacement of wild-type receptors by other receptor subtypes with different subunit combinations and functional/pharmacologic characteristics. One goal for pharmacotherapeutic treatment of tinnitus would be to enhance a specific system’s remaining endogenous inhibitory mechanisms that may remain intact, but deficient.
4. The Auditory Thalamus and Tinnitus
While tinnitus and sound-exposure related brainstem, midbrain and cortical changes have received significant attention, few studies have examined the impact of tinnitus and sound exposure on auditory thalamic neurons (MGB). Traditionally thought of as only a conduit for neural signals representing the auditory scene, arising from midbrain ascending to the cortex, it is now apparent that additional processing occurs in the MGB (Antunes et al., 2010; Bartlett and Wang, 2007; Bartlett and Wang, 2011). The MGB is an obligatory nucleus of the auditory system, thus regardless of the site of genesis for the tinnitus signal; it is likely that the MGB is involved in tinnitus pathology. Leaver, Rauschecker and colleagues suggested that due to the prime position of the MGB in the ascending and descending auditory neuraxis and unique inhibitory mechanisms (see below), the MGB is a promising target for tinnitus research (Leaver et al., 2011; Rauschecker et al., 2010). The fact that many tinnitus sufferers have a severe emotional component to their tinnitus further points to the involvement of MGB in tinnitus pathology (Malouff et al., 2011). The MGB projection to the amygdala demonstrates LTP and is necessary for auditory fear conditioning (McKernan and Shinnick-Gallagher, 1997; Quirk et al., 1995; Rogan et al., 1997; Weinberger, 2011). This suggests that the MGB might be an important link in understanding the emotional aspect of tinnitus.
Inhibitory MGB inputs have been well characterized. In rodents, primary sources of inhibition are from GABAergic projections from the TRN and IC, with the addition of a substantial yet poorly characterized GABAergic interneuron population in the MGB of higher order species (Rouiller and de Ribaupierre, 1985; Shosaku and Sumitomo, 1983; Villa, 1990; Winer and Larue, 1996; Winer et al., 1996). Thus, GABAergic shaping of MGB neuron output is primarily through ascending and descending inhibition from the IC and TRN, respectively. Certain tinnitus sufferers are particularly affected by their tinnitus and others may pay little attention to the phantom sound. Evidence suggests that mechanisms which regulate attention may be impaired in tinnitus sufferers more bothered by their tinnitus (Cuny et al., 2004; Dornhoffer et al., 2006; Husain et al., 2011). One well characterized subcortical mechanism involved in the regulation of attention is the tonotopically aligned inhibitory (GABAergic) projection from the TRN to the MGB (Cotillon-Williams et al., 2008; Crick, 1984; Guillery et al., 1998; McAlonan et al., 2000; Weese et al., 1999; Yu et al., 2009). This interaction may also be important in understanding gating of the tinnitus signal that occurs at the level of the MGB (Rauschecker et al., 2010).
Opposite defined GABAergic inputs to the MGB are postsynaptic GABAARs that are notably heterogeneous in MGB and other sensory thalamic regions. In addition to wild-type/classical synaptic GABAARs (containing α1 and γ2 subunits) that are widely distributed throughout the brain, MGB neurons also express extrasynaptic receptors, defined by GABAAR constructs containing α4 and δ subunits which mediate tonic inhibition (Figure 1 & 2A; Section 2; Richardson et al., 2011). Thalamic extrasynaptic GABAARs are activated by low level increases in ambient GABA levels and mediate a tonic, long-lasting inhibitory current which has been suggested to account for up to 90% of a thalamocortical neuron’s inhibitory conductance (Belelli et al., 2009; Cope et al., 2005; Farrant and Nusser, 2005; Walker and Semyanov, 2008). Gaboxadol, a “superagonist” at GABAARs, is capable of binding and activating extrasynaptic GABAARs selectively, when present at low (nM-μM) concentrations (Mortensen et al., 2010). Indeed, selective activation of extrasynaptic GABAARs by low concentrations of gaboxadol or taurine is capable of reducing spontaneous and evoked firing rates of sensory thalamic neurons (Figure 3; Herd et al., 2009; Jia et al., 2008b). Such reductions in excitability of MGB neurons following activation of extrasynaptic GABAARs, as exemplified in Figure 3, could prove advantageous in reducing the transmission of hyperexcitability to the auditory cortex.
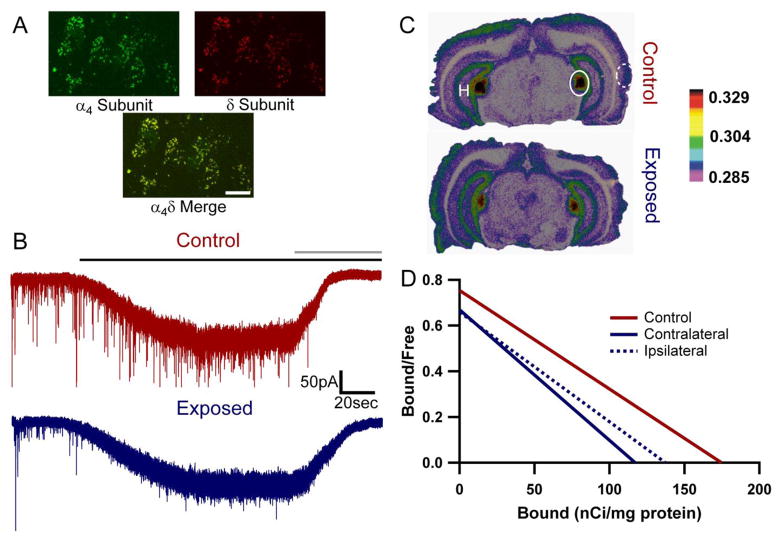
Figure 2.
Extrasynaptic GABAARs and Tonic Inhibition in MGB. A) Confocal image of fluorescent immunolabeling of α4 (green, top left), δ (red, top right) and colocalized α4δ (yellow, bottom) GABAAR subunits in the MGB demonstrate the presence of the subunits necessary for functional tonically active extrasynaptic GABAAR constructs. Scale bar = 10μm. B) Voltage-clamp recording (CsCl based internal solution, Vhold = −60mV, ECl ≈ 0mV) of tonic inhibitory current mediated by extrasynaptic GABAARs in the MGB of a control 19 month old FBN rat (red, top) and from an MGB neuron contralateral to the sound exposed ear in a 24 month old FBN rat with behavioral evidence of tinnitus (blue, bottom) in response to 1μM gaboxadol (black bar) and blocked by 50μM gabazine (gray bar). C) Autoradiographs of 250nM [3H]gaboxadol binding in adult FBN rats with and without behavioral evidence of tinnitus (modified from Richardson et al., 2011). Relative optical density spectrum is positioned at right. At these concentrations, [3H]gaboxadol binds preferentially to extrasynaptic GABAARs. Binding density is highest in the MGB (outlined with solid line) and lower in auditory cortex (dashed line) and hippocampus (H). D) Binding scatchards and saturation curves (not shown) indicate a significant difference in [3H]gaboxadol binding between the MGB of control and animals with tinnitus and a 20–30% decrease in Bmax (x-axis), corresponding to a decrease in extrasynaptic GABAAR density in the MGB, while Kd (slope) (Kd=231.7±89.6 (control), 175.0±77.9 (contra), 208.3±87,95 (ipsi)) is relatively unaffected (n=5 control, 5 tinnitus).
Figure 3.
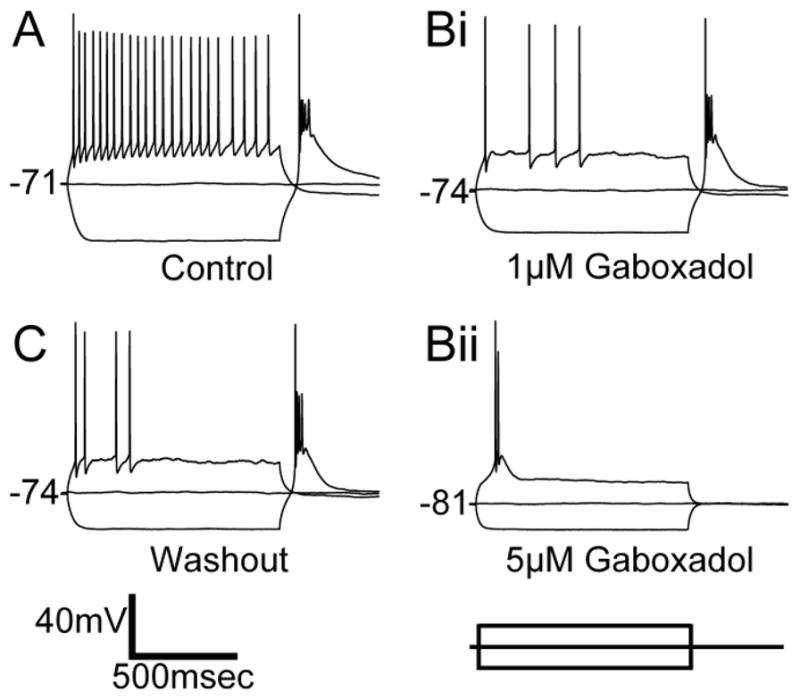
Regulation of MGB Neuron Excitability by Activation of Extrasynaptic GABAARs. Whole cell recording of response from a MGB neuron of a 4 month FBN rat to a 1sec, ±100pA current injection in normal ACSF (A), the presence of 1μM gaboxadol (Bi), 5μM gaboxadol (Bii) and following a 10min washout period (C). As the concentration of gaboxadol is increased, the firing rate in response to the depolarizing pulse decreases and the resting membrane potential becomes more negative. The cell eventually responds with a single t-type Ca2+ dependent burst upon depolarization and remains inactive, consistent with increasing tonic inhibition and hyperpolarization. Internal solution is K-gluconate based.
One way to determine the presence of extrasynaptic GABAARs and quantify conditional differences in receptor density is through quantitative receptor binding using [3H]gaboxadol, as described previously (Friemel et al., 2007; Richardson et al., 2011). In a small cohort of animals, a nonlinear regression analysis and comparison of [3H]gaboxadol binding between the MGB of FBN control rats and the contralateral MGB of rats with tinnitus was significantly different [F=4.709 (2,81), p=0.011], but not between control and the ipsilateral MGB of animals with behavioral evidence of tinnitus [F=2.526 (2,81), p=0.086] (Figure 2C&D). These preliminary findings suggest there may be a slight decrease (~20–30%) in extrasynaptic GABAAR density as indicated by the tinnitus-related change in Bmax for [3H]gaboxadol in the MGB of exposed animals (Bmax=174.4±36.5 (control), 117.1±25.6(contra), 137.2±30.2(ipsi). Despite significant differences, these changes are limited in magnitude, suggesting that while GABAARs might be altered in tinnitus, extrasynaptic GABAARs are still present and capable of mediating tonic inhibition in animals with tinnitus (Figure 2B). [3H]Gaboxadol binding density is higher in the MGB relative to AI (Figure 2C). This increase corresponds to elevated GABAAR extrasynaptic subunit protein concentrations and reflect the larger tonic current amplitudes (in response to 1μM gaboxadol using similar whole cell recording configurations) observed in sensory thalamocortical neurons (100–320pA) relative to sensory cortical neurons (50–60pA) (Chandra et al., 2006; Cope et al., 2005; Drasbek and Jensen, 2006; Herd et al., 2009; Peng et al., 2002; Pirker et al., 2000; Yang et al., 2011).
Pharmacologic targeting specific extrasynaptic GABAAR constructs could prove more useful than targeting GABAARs nonspecifically (Yang et al., 2011). In tinnitus treatment, the efficacy of agents targeting extrasynaptic GABAARs would likely involve the MGB as the magnitude of tonic current and histological characterization of receptor density strongly indicates that extrasynaptic GABAAR expression may be two or more fold greater than in the auditory cortex. In addition to gaboxadol, other agents capable of inducing GABAAR mediated tonic inhibition have been effective at attenuating behavioral evidence of tinnitus (see Section 5).
5. Tinnitus Pharmacotherapy Targeting Inhibition and Underlying Mechanisms
A number of therapeutic strategies have been applied to tinnitus treatment, but presently there is no universally effective treatment or established standard of care. Non-pharmacologic interventions that combine acoustic stimulation and counseling reduce the intrusiveness of tinnitus, without altering the physical properties of the sensation, such as loudness or pitch. Cochlear implantation for aural rehabilitation of severe hearing loss significantly improves and in some cases eliminates tinnitus in many people (Baguley and Atlas, 2007; Kompis et al., 2012; Van de Heyning et al., 2008). Transcranial magnetic stimulation (TMS) is reported to provide relief to tinnitus sufferers and is proposed to do so through suppression of cortical activity by enhancing intracortical inhibition and inhibitory input from GABAergic TRN neurons onto MGB neurons (Langguth et al., 2007). Case reports and clinical trials suggest that limited success has been obtained with a variety of drug classes, including benzodiazepine derivatives, such as alprazolam (Johnson et al., 1993), clonazapam (Bahmad et al., 2006), and the GABA homolog, gabapentin (Bauer and Brozoski, 2006); anti-epileptics, such as carbamazepine (Levine, 2006); and antidepressants (for detailed review see Salvi et al., 2009; Shulman et al., 2002; Sullivan et al., 1989). Due to the tinnitus-related changes observed with drugs which enhance inhibition and the importance of inhibition in regulating neuronal function, inhibitory receptors remain a primary target in this line of research.
Multiple compounds are now available as selective agonists or modulators of inhibitory receptors. With more refined pharmacological profiles, these compounds take advantage of the pharmacologic diversity, specificity and natural distribution of inhibitory receptor subunit combinations. One interesting class of agents acts to positively or negatively regulate/modulate activity of specific GABAAR subtypes (i.e. with specific subunit configurations) which may be beneficial in treating tinnitus. For example, similar to the way in which benzodiazepines selectively allosterically modulate certain synaptic GABAAR subtypes, other compounds can selectively activate or modulate the activity of extrasynaptic GABAAR constructs like gaboxadol (Mortensen et al., 2010), taurine (Jia et al., 2008b), isoflurane (Jia et al., 2008a) and endogenous neurosteroids (Belelli et al., 2006; Herd et al., 2007; Lambert et al., 2009).
Since extrasynaptic GABAARs are highly sensitive to changes in ambient GABA concentration., Artificial elevation of these levels, induced by blockade of GABA uptake transporters can enhance tonic inhibitory current amplitude (Figure 1; Cope et al., 2005). Recent studies using compounds that affect inhibitory neurotransmission have proven effective in alleviating or attenuating tinnitus in animal models. As suggested by Yang (Yang et al., 2011), based on their mechanism of action determined in vitro, the tinnitus-related benefits of these compounds may be via activation or amplification of a tonic inhibitory conductance mediated by extrasynaptic GABAARs.
A recent study by Brozoski and colleagues (2010) used the partially selective GABAAR agonist, taurine, to alleviate tinnitus in a rat model of tinnitus (Bauer and Brozoski, 2001; Jia et al., 2008b). Taurine is an endogenous sulfur containing β-amino acid with agonist activity at GlyRs (in IC) and extrasynaptic GABAARs in the thalamus (Jia et al., 2008b; Xu et al., 2004; Xu et al., 2006). In this design, Long-Evans rats exposed to 116dB-16kHz narrowband noise for 60 minutes resulted in psychophysical evidence of tinnitus at 10, 16, and 20kHz in an operant conditioning paradigm (Brozoski et al., 2010). Following daily oral dosing of 4mg/ml of taurine dissolved in the animals’ drinking water, evidence of tinnitus was significantly attenuated as indicated by the shift in the task suppression ratio function. This effect was most dramatic at the 16kHz test frequency, but also evident at 10 and 20kHz. The therapeutic administration of taurine in the drinking water over the course of weeks likely leads to a continuous up-regulation of taurine concentrations in the brain. Although CSF taurine increases are likely marginal, prolonged increases in taurine may achieve its physiological effect in the central nervous systems by mechanisms that fit this temporal profile. Synaptic GlyRs and GABAAR demonstrate faster desensitization rates and require higher concentrations of taurine for activation than thalamic extrasynaptic GABAARs agonists [and possibly uncharacterized tonically active extrasynaptic GlyRs (Wang et al., 2005; Xu et al., 2004; Xu et al., 2006)]. These extrasynaptic constructs would be sensitive to low level ambient increases in GABA [or glycine] and other selective agonists. Therefore, extrasynaptic GABAARs in the MGB are likely to be a primary source of inhibition activated in systemic dosing of taurine responsible for the therapeutic effect on the animals’ perception of tinnitus (Jia et al., 2008b; Richardson et al., 2011). As described in section 4, the elevated density of extrasynaptic GABAARs in the thalamus most likely mediate taurine’s therapeutic effect on the tinnitus percept through its ability to increase tonic inhibition of MGB neurons and to a lesser degree, auditory cortical neurons. Taurine’s activation of extrasynaptic GABAARs in MGB which are sensitive to small changes in ambient agonist concentrations in the CSF (and to some degree GlyRs in IC) may reduce neuronal excitability and transmission of the neuronal tinnitus signal to cortex, suggesting a role for these ligand-gated ionotropic receptors in the future of tinnitus pharmacotherapy.
In addition to using selective agonists, blockers of endogenous neurotransmitter uptake and catabolism, which can enhance tonic inhibition and amplify inhibitory synaptic responses, have also been effective in ameliorating the tinnitus percept. Tonic inhibition mediated by extrasynaptic GABAARs can be induced by elevation of ambient GABA levels following the blockade of GABA uptake by GAT-1 selective blockers (NNC-711 or NO-711) and blockade of GABA catabolism by a GABA-T inhibitor (vigabatrin) (Figure 1; Chandra et al., 2006; Jia et al., 2008b; Larsson et al., 1986; Wu et al., 2003; Yang et al., 2011). In animal models, systemic administration of NO-711 and vigabatrin have been shown to reduce behavioral evidence of tinnitus, presumably due to their induction of elevated ambient GABA levels in vivo (Brozoski et al., 2007; Richards and Bowery, 1996; Yang et al., 2011). Activation of extrasynaptic GABAARs by this ambient increase in GABA could tonically hyperpolarize MGB and auditory cortical neurons further below threshold, reducing their probability of responding to lower level excitatory synaptic input with an action potential. Unfortunately, vigabatrin treatment has been shown to cause a restriction of the visual field which has only been attenuated following the up-regulation of taurine (Jammoul et al., 2009; Krauss et al., 1998).
6. Conclusions
An acoustic insult to the auditory periphery (i.e. loud sound-exposure) induces partial deafferentation and may alter the balance between driving excitation and plastic essential inhibitory mechanisms through the down-regulation or altering of ligand gated inhibitory receptors, their associated proteins and neurotransmitter metabolic enzymes. As a result, the central auditory system can become hyperexcitable, leading to the perception of tinnitus. Certain types of hyperactivity or altered temporal firing patterns (i.e. bursting) specific to regions of the central auditory system can result from 1) what is passed up from lower structures, 2) increases in intrinsic neuronal excitability or 3) unopposed excitatory drive that would normally be attenuated by inhibitory mechanisms. In order to develop tinnitus pharmacotherapy, specifically targeting inhibitory mechanisms may prove to be more effective than less-specific inhibitory mimetics or modulators due to the intricacy and heterogeneity of inhibitory neurotransmission. Some compounds have been found to be efficacious in attenuating the tinnitus percept and are linked to the activation of tonic inhibition through extrasynaptic GABAARs or GlyRs which may also enhance synaptic inhibition as well. Due to the high expression of tonically active receptors in the auditory thalamus and its key position in the auditory neuraxis, targeting these receptors and the role of the thalamus in attention and sensory gating may result in more successful treatment. A tightly titred combination therapy which includes different classes of selective GABAergic compounds like benzodiazepines, vigabatrin and taurine may be beneficial in treating tinnitus with reduced negative side effects.
7. Experimental Procedure
7.1 Confocal Fluorescent Imaging
Fischer Brown Norway (FBN) rats were anesthetized with an intramuscular injection mixture of ketamine (105mg/kg, Aveco, Fort Dodge, IA, USA) and xylazine (7mg/kg, Lloyd Laboratories, Shennandoah, IA, USA) followed by transcardial perfusion with 100ml of saline and 750ml of fixative containing 4% paraformaldehyde in phosphate buffer saline (PBS, pH7.4). Sections (30μm) were cut through MGB and collected in PBS. Free-floating sections were processed in specially designed tissue capsules and incubated 30min in blocking solution containing PBS with 1.5% of normal serum and 3% bovine serum albumin. Sections were then transferred to primary antibody solution (goat anti-GABAAR α4 and rabbit anti-GABAAR δ (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), incubated for 1hr at room temperature and then overnight at 4°C on the shaker. After washing with PBS, sections were incubated with donkey anti-goat IgG (Dylight 488, 1:100, Jackson ImmunoResearch, West Grove, PA, USA) and donkey anti-rabbit IgG (Rhodamine Red-X, 1:100; Jackson ImmunoReseach Laboratory, West Grove, PA, USA) for 1hr at room temperature. Following PBS washing, the sections were mounted onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA, USA), cover-slipped with VectorShield (Vector Laboratories, Burlingame, CA, USA). Fluorescent imaging was performed using a Leica TCS SP5 II Confocal Laser Microscope (Leica Microsystems CMS GmbH, Mannheim, Germany).
7.2 Acoustic Exposure and Tinnitus Induction
Methods are similar to Richardson et al. (2011) and Wang et al. (2009). For radioligand binding experiments, FBN rats (3–4 months old) from two groups (unexposed control [n=5] and exposed to acoustic trauma with behavioral evidence of tinnitus [n=5]) were prepared identically to those in Wang et al., 2009. Briefly, to induce behavioral evidence of tinnitus, rats were anesthetized with a mixture of ketamine (50mg/kg) and xylazine (9mg/kg) and exposed to 116dB SPL octave-band noise, centered at 17kHz peak intensity for 1hr and behavioral evidence was determined using the gap/startle method of Turner and colleagues (2006) (see Wang et al., 2009 for behavioral results). For whole cell recording experiments (see Richardson et al., 2011 for detailed methods), rats (19–24 months) were similarly exposed to those described above except under isoflurane anesthesia and exposure was adjusted to 122dB SPL octave-band noise, centered at 16kHz peak intensity for 2hrs and resulted in the expression of behavioral evidence of tinnitus as determined by the gap/startle method of Turner and colleagues (2006).
7.3 Radioligand Binding
Following the classification of rats into groups based on behavioral performance following sound exposure, they were decapitated and the brains were rapidly removed, rinsed in ice-cold phosphate buffer at 4°C (pH 7.4), frozen in powdered dry ice and stored at −80°C. Serial transverse sections were cut at 16μm using a Leica CM1850 cryostat at −18°C. Selected sections were thaw-mounted onto Superfrost/Plus slides and stored at −20°C. Anatomical locations of the MGB were verified to match neural structures with those previously described (Paxinos and Watson, 1998).
[3H]Gaboxadol (courtesy Merck & Co. Inc., Rahway, NJ, USA) was used with modified protocols from Milbrandt and Caspary (1995) and Dr. Bjarke Ebert (personal communication). In brief, tissue sections were subjected to pre-wash twice for 5 minutes in buffers, followed by incubating with [3H]gaboxadol: 10–400nM and post-wash with buffers for 4 quick dips. Buffer solutions used were 50mM Tris-citrate (pH7.1). Non-specific binding was determined in adjacent sections by the addition of cold excessive GABA to the ligand buffer and was subtracted from total binding.
Dried slides were apposed to [3H]-hypersensitive phosphor screens for 3–5 days at room temperature. The phosphor screens were scanned using a Cyclone Storage Phosphor System (PerkinElmer, Waltham, MA, USA). The MGB was outlined and analyzed using OptiQuant Image Analysis software (Canberra-Packard, Schwadorf, Austria) which provided tools for gray-scale quantification in digital light units (DLU). DLUs were then converted to nCi/mg protein using a standard curve generated from co-exposed 3H-embedded plastic standards (ARC, St. Louis, MO, USA)(Pan et al., 1983). Values from the left and right MGB of control animals were averaged for each animal and treated as one group (n=5) while values from the MGB contralateral and ipsilateral to the exposed ear were treated as two separate groups (ipsilateral: n=5, contralateral: n=5) in statistical analysis. Statistical comparison of [3H]gaboxadol binding saturation curves between groups was completed using non-linear regression analysis with a one-site binding model.
Acknowledgments
The authors would like to thank K.A. Bartosiak for assistance in collecting data in Figure 2, Dr. Pete Hutson for his help and support and Merck & Co., Inc. (Rahway, NJ) for supplying the [3H]gaboxadol used in Figure 2C&D as well as Dr. Bjarke Ebert of H. Lundbeck A/S (Copenhagen, DK) for his advice and Dr. Jeremy Turner of Southern Illinois University School of Medicine (Springfield, IL) and Illinois College (Jacksonville, IL) for supplying animals with behavioral evidence of tinnitus. This work was supported by NIH DC008532.
Abbreviations
- CSF
cerebrospinal fluid
- DCN
dorsal cochlear nucleus
- FBN
Fischer Brown Norway
- GABA
γ-aminobutyric acid
- GABARAP
GABAA receptor associated protein
- GAD
glutamic acid decarboxylase
- GlyR
glycine receptor
- GABAAR
GABAA receptor
- GABA-T
GABA transaminase
- GAT
GABA transporter
- HVA
high voltage activated
- IC
inferior colliculus
- MGB
medial geniculate body
- qRT-PCR
quantitative real time – polymerase chain reaction
- SGC
spiral ganglion cell
- TRN
thalamic reticular nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ben D. Richardson, Email: brichardson@siumed.edu.
Thomas J. Brozoski, Email: tbrozoski@siumed.edu.
Lynne L. Ling, Email: lling@siumed.edu.
Donald M. Caspary, Email: dcaspary@siumed.edu.
References
- Antunes FM, Nelken I, Covey E, Malmierca MS. Stimulus-Specific Adaptation in the Auditory Thalamus of the Anesthetized Rat. PLoS ONE. 2010;5:e14071. doi: 10.1371/journal.pone.0014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM, Atlas MD. Cochlear implants and tinnitus. Prog Brain Res. 2007;166:347–55. doi: 10.1016/S0079-6123(07)66033-6. [DOI] [PubMed] [Google Scholar]
- Bahmad FM, Jr, Venosa AR, Oliveira CA. Benzodiazepines and GABAergics in treating severe disabling tinnitus of predominantly cochlear origin. Int Tinnitus J. 2006;12:140–4. [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol. 2007;97:1005–17. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. J Neurophysiol. 2011;105:2647–67. doi: 10.1152/jn.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Effect of gabapentin on the sensation and impact of tinnitus. Laryngoscope. 2006;116:675–81. doi: 10.1097/01.MLG.0000216812.65206.CD. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–98. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–9. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8:105–118. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Caspary DM, Bauer CA, Richardson BD. The effect of supplemental dietary taurine on tinnitus and auditory discrimination in an animal model. Hear Res. 2010;270:71–80. doi: 10.1016/j.heares.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral Dorsal Cochlear Nucleus Lesions Prevent Acoustic-Trauma Induced Tinnitus in an Animal Model. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamai M, Specht CG, Heller J, Alcor D, Machado P, Vannier C, Triller A. Gephyrin oligomerization controls GlyR mobility and synaptic clustering. J Neurosci. 2009;29:7639–48. doi: 10.1523/JNEUROSCI.5711-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kossl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417:273–82. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C, Ehrensperger MV, Dahan M, Levi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J Neurosci. 2006;26:8502–11. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. Journal of Neuroscience. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillon-Williams N, Huetz C, Hennevin E, Edeline JM. Tonotopic control of auditory thalamus frequency tuning by reticular thalamic neurons. J Neurophysiol. 2008;99:1137–1151. doi: 10.1152/jn.01159.2007. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Norena A, El Massioui F, Chery-Croze S. Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurootol. 2004;9:294–302. doi: 10.1159/000080267. [DOI] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci. 2003;23:264–78. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010;31:1616–28. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dornhoffer J, Danner C, Mennemeier M, Blake D, Garcia-Rill E. Arousal and attention deficits in patients with tinnitus. Int Tinnitus J. 2006;12:9–16. [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–41. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Kneussel M. Cellular transport and membrane dynamics of the glycine receptor. Front Mol Neurosci. 2009;2:28. doi: 10.3389/neuro.02.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Friemel A, Ebert B, Hutson PH, Brust P, Nieber K, Deuther-Conrad W. Postnatal development and kinetics of [3H]gaboxadol binding in rat brain: in vitro homogenate binding and quantitative autoradiography. Brain Res. 2007;1170:39–47. doi: 10.1016/j.brainres.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–64. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for delta-GABA(A) receptors. Eur J Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, Pajor NM, Smith JF, Kim HJ, Rudy S, Zalewski C, Brewer C, Horwitz B. Discrimination task reveals differences in neural bases of tinnitus and hearing impairment. PLoS ONE. 2011;6:e26639. doi: 10.1371/journal.pone.0026639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–78. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammoul F, Wang Q, Nabbout R, Coriat C, Duboc A, Simonutti M, Dubus E, Craft CM, Ye W, Collins SD, Dulac O, Chiron C, Sahel JA, Picaud S. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann Neurol. 2009;65:98–107. doi: 10.1002/ana.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABA(A) receptors in the thalamus. J Pharmacol Exp Ther. 2008a;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine Is a Potent Activator of Extrasynaptic GABAA Receptors in the Thalamus. Journal of Neuroscience. 2008b;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, Brummett R, Schleuning A. Use of alprazolam for relief of tinnitus. A double-blind study. Arch Otolaryngol Head Neck Surg. 1993;119:842–5. doi: 10.1001/archotol.1993.01880200042006. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–92. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–5. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol. 2008;17:S148–61. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–68. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99:297–309. doi: 10.1042/BC20060120. [DOI] [PubMed] [Google Scholar]
- Kompis M, Pelizzone M, Dillier N, Allum J, Demin N, Senn P. Tinnitus before and 6 Months after Cochlear Implantation. Audiol Neurootol. 2012;17:161–168. doi: 10.1159/000335126. [DOI] [PubMed] [Google Scholar]
- Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–8. doi: 10.1212/wnl.50.3.614. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Langguth B, Kleinjung T, Marienhagen J, Binder H, Sand PG, Hajak G, Eichhammer P. Transcranial magnetic stimulation for the treatment of tinnitus: effects on cortical excitability. BMC Neurosci. 2007;8:45. doi: 10.1186/1471-2202-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson OM, Gram L, Schousboe I, Schousboe A. Differential effect of gamma-vinyl GABA and valproate on GABA-transaminase from cultured neurones and astrocytes. Neuropharmacology. 1986;25:617–25. doi: 10.1016/0028-3908(86)90214-5. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA. Typewriter tinnitus: a carbamazepine-responsive syndrome related to auditory nerve vascular compression. ORL J Otorhinolaryngol Relat Spec. 2006;68:43–6. doi: 10.1159/000090490. discussion 46–7. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–58. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–9. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Schutte NS, Zucker LA. Tinnitus-related distress: A review of recent findings. Curr Psychiatry Rep. 2011;13:31–6. doi: 10.1007/s11920-010-0163-1. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. J Neurosci. 2000;20:8897–901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–11. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–6. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neuroscience. 1995;67:713–719. doi: 10.1016/0306-4522(95)00082-t. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–60. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Ding D, Salvi R, Robertson D. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol. 2011;519:2637–47. doi: 10.1002/cne.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience. 2011;192:753–60. doi: 10.1016/j.neuroscience.2011.06.046. [DOI] [PubMed] [Google Scholar]
- Pan HS, Frey KA, Young AB, Penney JB., Jr Changes in [3H]muscimol binding in substantia nigra, entopeduncular nucleus, globus pallidus, and thalamus after striatal lesions as demonstrated by quantitative receptor autoradiography. Journal of Neuroscience. 1983;3:1189–1198. doi: 10.1523/JNEUROSCI.03-06-01189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos W, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–87. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–97. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Pilati N, Large C, Forsythe ID, Hamann M. Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hear Res. 2011 doi: 10.1016/j.heares.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–26. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Bowery NG. Comparative effects of the GABA uptake inhibitors, tiagabine and NNC-711, on extracellular GABA levels in the rat ventrolateral thalamus. Neurochem Res. 1996;21:135–40. doi: 10.1007/BF02529130. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS ONE. 2011;6:e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre F. Origin of afferents to physiologically defined regions of the medial geniculate body of the cat: ventral and dorsal divisions. Hear Res. 1985;19:97–114. doi: 10.1016/0378-5955(85)90114-5. [DOI] [PubMed] [Google Scholar]
- Salvi R, Lobarinas E, Sun W. Pharmacological Treatments for Tinnitus: New and Old. Drugs Future. 2009;34:381–400. doi: 10.1358/dof.2009.034.05.1362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–7. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shosaku A, Sumitomo I. Auditory neurons in the rat thalamic reticular nucleus. Exp Brain Res. 1983;49:432–42. doi: 10.1007/BF00238784. [DOI] [PubMed] [Google Scholar]
- Shulman A, Strashun AM, Goldstein BA. GABAA-benzodiazepine-chloride receptor-targeted therapy for tinnitus control: preliminary report. Int Tinnitus J. 2002;8:30–6. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Dobie RA, Sakai CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Ann Otol Rhinol Laryngol. 1989;98:867–72. doi: 10.1177/000348948909801107. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–95. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117:645–52. doi: 10.1177/000348940811700903. [DOI] [PubMed] [Google Scholar]
- Villa AE. Physiological differentiation within the auditory part of the thalamic reticular nucleus of the cat. Brain Res Brain Res Rev. 1990;15:25–40. doi: 10.1016/0165-0173(90)90010-l. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–22. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci. 2011;31:6639–45. doi: 10.1523/JNEUROSCI.6538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results Probl Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Wang F, Xiao C, Ye JH. Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol. 2005;565:503–16. doi: 10.1113/jphysiol.2005.085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–59. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–7. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. J Neurosci. 1999;19:10135–9. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res. 2011 doi: 10.1016/j.heares.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci USA. 1996;93:3083–3087. doi: 10.1073/pnas.93.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci USA. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–62. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–34. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhou KQ, Huang YN, Chen L, Xu TL. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004;1021:232–240. doi: 10.1016/j.brainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Xu H, Wang W, Tang ZQ, Xu TL, Chen L. Taurine acts as a glycine receptor agonist in slices of rat inferior colliculus. Hear Res. 2006;220:95–105. doi: 10.1016/j.heares.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Xu TL, Gong N. Glycine and glycine receptor signaling in hippocampal neurons: diversity, function and regulation. Prog Neurobiol. 2010;91:349–61. doi: 10.1016/j.pneurobio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011;108:14974–9. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, He S, He J. Change detection by thalamic reticular neurons. Nat Neurosci. 2009;12:1165–1170. doi: 10.1038/nn.2373. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Gong N, Fei D, Xu L, Xu TL. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology. 2008;33:701–11. doi: 10.1038/sj.npp.1301449. [DOI] [PubMed] [Google Scholar]