Abstract
Background
The identification of individuals exposed prenatally to alcohol can be challenging, with only those having the characteristic pattern of facial features, CNS abnormality, and growth retardation receiving a clinical diagnosis of fetal alcohol syndrome (FAS).
Methods
17 anthropometric measurements were obtained at 5 and 9 years from 125 Cape Town, South African children, studied since birth. The children were divided into 3 groups: FAS or partial FAS (PFAS), heavily exposed nonsyndromal (HE), and non-alcohol exposed controls (C). Anthropometric measurements were evaluated for mean group differences. Logistic regression models were used to identify the subset of anthropometric measures that best predicted group membership. Anthropometric measurements were examined at the two ages in relation to prenatal alcohol exposure obtained prospectively from the mothers during pregnancy. Correlation of these facial measurements with key neurobehavioral outcomes including WISC-IV IQ and eyeblink conditioning was used to assess their utility as indicators of alcohol-related central nervous system impairment.
Results
Significant group differences were found for the majority of the anthropometric measures, with means of these measures smaller in the FAS/PFAS compared with HE or C. Upper facial widths, ear length, lower facial depth, and eye widths were consistent predictors distinguishing those exposed to alcohol from those who were not. Using longitudinal data, unique measures were identified that predicted facial anomalies at one age but not the other, suggesting the face changes as the individual matures. 41% of the FAS/PFAS group met criteria for microtia at both ages. Three of the predictive anthropometric measures were negatively related to measures of prenatal alcohol consumption, and all were positively related to at least one neurobehavioral outcome.
Conclusions
The analysis of longitudinal data identified a common set of predictors, as well as some that are unique at each age. Prenatal alcohol exposure appears to have its primary effect on brain growth, reflected by smaller forehead widths, and may suppress neural crest migration to the branchial arches, reflected by deficits in ear length and mandibular dimensions. These results may improve diagnostic resolution and enhance our understanding of the relation between the face and the neuropsychological deficits that occur.
Keywords: fetal alcohol syndrome, fetal alcohol spectrum disorders, prenatal alcohol exposure, anthropometrics, neurobehavior, facial image, 3D photography
INTRODUCTION
Fetal alcohol syndrome (FAS) was first described in the literature over 40 years ago and in the intervening years has been recognized as a devastating public health problem. The prevalence of FAS in the U.S. population has been reported to be 0.5 to 2.0 per 1,000 live births (May and Gossage, 2001) and in a more recent study was estimated to be as high as 2.0 to 7.0 per 1,000 individuals in U.S. school age populations (May et al., 2009). When considering the full spectrum of prenatal effects of alcohol, including those individuals who lack the characteristic alteration of facial features, estimates of the prevalence of fetal alcohol spectrum disorders (FASD) range from 1 in 100 births (Sampson et al., 1997) to as high as 2–5% in the U.S. and Western Europe (May et al., 2009). Some of the highest rates of FAS and partial FAS (PFAS) have been reported in the Western Cape Province of South Africa with a prevalence of 68.0 to 89.2 per 1,000 births (May et al., 2007).
Clinical diagnosis of FAS relies on growth retardation, deficiencies in brain growth (reduced head circumference and/or structural brain anomaly), and at least two of the three cardinal facial features of FAS (short palpebral fissures, thin upper lip, smooth philtrum) (Hoyme et al., 2005). PFAS also requires two of the three facial characteristics; however, deficits are only needed in one of the remaining three areas (growth delay, deficiencies in brain growth, or behavioral and/or cognitive abnormalities). In the case of FAS and PFAS, classification can be made with or without confirmed prenatal alcohol exposure. Other individuals may not meet criteria for FAS or PFAS but due to prenatal alcohol exposure still experience neurobehavioral problems, such as attention deficits, impulsivity, and hyperactivity (Crocker et al., 2011) as well as difficulty in executive functioning or spatial processing (Mattson et al., 2010). It is critical that improved methods are developed that can better screen for and accurately identify those who have been injured by prenatal alcohol exposure but do not have the characteristic features or growth retardation required to meet criteria for FAS or PFAS.
Previous studies have sought to identify features that can be used to distinguish individuals who have been impaired prenatally by alcohol exposure. These have included studies in humans focused on facial features (Moore et al, 2001; Moore et al., 2002; Moore et al., 2007; Mutsvangwa and Douglas, 2007; Mutsvangwa et al, 2010; Douglas and Mutsvangwa, 2010; Klingenberg et al, 2010) and measures obtained from brain imaging or neuropsychological profiles. MRI studies have typically found that those with alcohol exposure have smaller total brain volumes including reductions in both cerebral and cerebellar volumes (Archibald et al., 2001). Gray and white matter volumes have also been consistently smaller in those who have been heavily exposed to alcohol prenatally (Lebel et al., 2011). Individuals with prenatal alcohol exposure have exhibited reductions in IQ scores, deficits in executive ability, verbal and nonverbal learning and memory, visual-spatial ability, and attention (Mattson et al., 2011). Some biobehavioral diagnostic markers, such as eyeblink conditioning and number processing deficits, which may reflect a disturbance in neuroanatomic structures that are disrupted by prenatal alcohol exposure, appear promising (Jacobson et al., 2011a). An additional challenge is that the face and the pattern of deficits in some domains change as the individual grows into adolescence and adulthood, making it difficult to identify a single profile that can successfully recognize an older individual who has been exposed to alcohol prenatally.
This paper presents anthropometric measurements and three-dimensional (3D) facial images obtained from a cohort of children in a high-risk community in Cape Town, South Africa, who are participating in a longitudinal study on FASD. Prenatal alcohol exposure data obtained from the mothers during pregnancy indicates that many of the children were exposed to heavy maternal alcohol consumption. The purpose of this report is to present anthropometric measures that identify and best classify groups of children based on their alcohol exposure and accompanying facial dysmorphology. We employed a longitudinal design to test whether the measures that best discriminate alcohol exposure differ across the early lifespan. We also correlated the most predictive anthropometric measurements with maternal alcohol levels and with neurobehavioral outcomes to identify common pathways affected by prenatal alcohol exposure.
METHODS
Sample description
Facial images were obtained from a well-characterized cohort of 125 children followed longitudinally (Jacobson et al., 2008). Mothers were recruited between July 1999 and January 2002 from an antenatal maternity clinic in Cape Town, South Africa that serves an economically disadvantaged, predominantly Cape Coloured (mixed ancestry) population. Drinking histories during pregnancy were obtained prospectively using a timeline follow-back interview (Jacobson et al., 2002), which was administered to the mothers at recruitment, subsequently during pregnancy, and again 6 weeks postpartum to obtain information about drinking during the 3rd trimester. At each of these visits the mothers were also asked how many cigarettes they smoked per day during pregnancy.
Two groups of children were included in the analyses reported in this paper: (1) alcohol exposed: born to mothers who reported consuming at least 7 ounces (oz) absolute alcohol (AA) per week (the equivalent of 14 or more standard drinks per week) or binges of 5 drinks or more per occasion during pregnancy; (2) controls: born to mothers who reported drinking no alcohol during pregnancy. Offspring from the resulting pregnancies were followed longitudinally and are the focus of this report. Written informed consent was obtained from the mothers at all visits and oral assent was obtained from the children older than 9 years of age. Approval for human research was obtained from the Wayne State University Human Investigation Committee and the University of Cape Town Ethics Committee. Mothers and children were given a snack or lunch during the clinic and breakfast, snack, and lunch during visits to our University of Cape Town (UCT) Child Development Research Laboratory; the mothers also received a small monetary compensation, and the children were given a small gift. All alcohol-consuming pregnant women were advised to stop drinking or reduce their alcohol intake during pregnancy and referred for help in doing so, if they agreed.
Subject assessment
Each child was examined for growth deficits (including microcephaly) and facial morphology during two study clinic visits, once in 2005 and again in 2009, when the children’s ages averaged 4.8 (SD = 1.0) and 8.7 (SD = 1.0) years, respectively. At both visits, the children were independently evaluated by two dysmorphologists (HEH, LKR) using the standard protocol developed by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) (Hoyme et al., 2005). A case conference was subsequently conducted by HEH, LKR, SWJ, JLJ, and CDM, who then reached consensus regarding the FAS diagnosis. Based on the first evaluation, children who were heavily exposed to alcohol were assigned to one of three mutually exclusive categories based on the revised IOM criteria: FAS, PFAS, or heavy alcohol exposure but not meeting FAS or PFAS criteria (HE) (Jacobson et al., 2008). Offspring of women who reported abstaining from alcohol during pregnancy were assigned to the control (C) group unless their child met criteria for FAS (n = 3), in which case confirmation of maternal drinking is not required (Hoyme et al., 2005). For all analyses, the FAS and PFAS groups were combined, resulting in three comparison groups: FAS/PFAS, HE, and non-alcohol exposed controls (Table 1).
Table 1.
Sample characteristics (N = 125)
| FAS/PFAS (n = 35) | Heavy Alcohol Exposure (HE) (n = 41) | Control (n = 49) | F or χ2 | |
|---|---|---|---|---|
| Mean ± SD or % | Mean ± SD or % | Mean ± SD or % | ||
| Age at first clinic (years) in 2005 | 5.2 ± 0.8 | 4.7 ± 1.1 | 4.6 ± 0.9 | 4.66* |
| Age at second clinic (years) in 2009 | 9.2 ± 0.8 | 8.6 ± 1.0 | 8.6 ± 0.9 | 5.50** |
| Sex (male) | 60.0% | 58.5% | 46.9% | 1.83 |
| JSAIS IQa | 79.5 ± 8.0 | 83.5 ± 11.2 | 85.6 ± 9.5 | 4.03* |
| WISC-IV IQb | 64.7 ± 10.3 (n=28) | 71.5 ± 14.9 (n=20) | 75.9 ± 12.2 (n=23) | 5.39** |
| Prenatal alcohol exposure at conception | ||||
| oz AA/day | 1.5 ± 2.0 | 1.3 ± 1.7 | 0.0 ± 0.0 | 14.61*** |
| oz AA/occasion | 4.1 ± 2.8 | 3.7 ± 3.0 | 0.0 ± 0.0 | 44.00*** |
| Frequency (days/week) | 2.3 ± 1.7 | 2.0 ± 1.7 | 0.0 ± 0.0 | 38.44*** |
| Prenatal alcohol exposure across pregnancy | ||||
| oz AA/day | 1.1 ± 1.3 | 0.8 ± 1.0 | 0.0 ± 0.0 | 16.45*** |
| oz AA/occasion | 3.7 ± 2.3 | 3.5 ± 2.7 | 0.0 ± 0.0 | 49.00*** |
| Frequency (days/week) | 1.7 ± 1.4 | 1.3 ± 1.1 | 0.0 ± 0.0 | 38.53*** |
| Cigarettes/day during pregnancy | 1.7 ± 2.9 | 4.9 ± 5.9 | 2.8 ± 5.2 | 4.29* |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
JSAIS IQ for 124 children at 5 years; IQ missing for 1 child with HE, who did not cooperate with testing procedures.
WISC-IV IQ for 71 children (28 FAS/PFAS, 20 HE, 23 C) at 9 years.
FAS – fetal alcohol syndrome; PFAS – partial fetal alcohol syndrome; HE – heavy exposed; C – healthy control; JSAIS --Junior South African Intelligence Scales; WISC-IV – Wechsler Intelligence Scales for Children; AA – absolute alcohol.
Three-dimensional facial images were collected at each of the two clinic visits by an expert 3D photographer (ESM or LW). At the first visit in 2005, images were acquired using the Minolta system (Konica Minolta, Ramsey, NJ). Details from the first study visit, including the collection and processing of images, were previously reported (Moore et al., 2007). In 2009 the newer 3dMD system (3dMD, Atlanta, Georgia) was utilized. The advantages of the 3dMD capture system include more than twice the geometric accuracy coupled with four times the color texture mapping density. The 3dMD system also offers the advantage of a single versus multiple image capture to acquire 180° “ear-to-ear” frontal facial data. Furthermore, the 3dMD system has a capture time of 1.5 milliseconds (0.0015 seconds), much faster than over half a second (0.6 seconds) per capture on the Minolta 3D capture system. Once the 3D image was securely stored and processed at Indiana University (IU), a trained researcher (ESM, SVB) selected the frontal scans with the best resolution and consistency of patient placement and facial expression. Landmarks were identified on the 3D model and used to obtain the linear measurements. Replication of landmark placement was required, with less than 2 mm difference per linear measurement. If the tolerance criterion was not met, a third measurement was taken and the average of the two closest measurements was chosen for analysis. For bilateral measurements, only the left side was used in analyses.
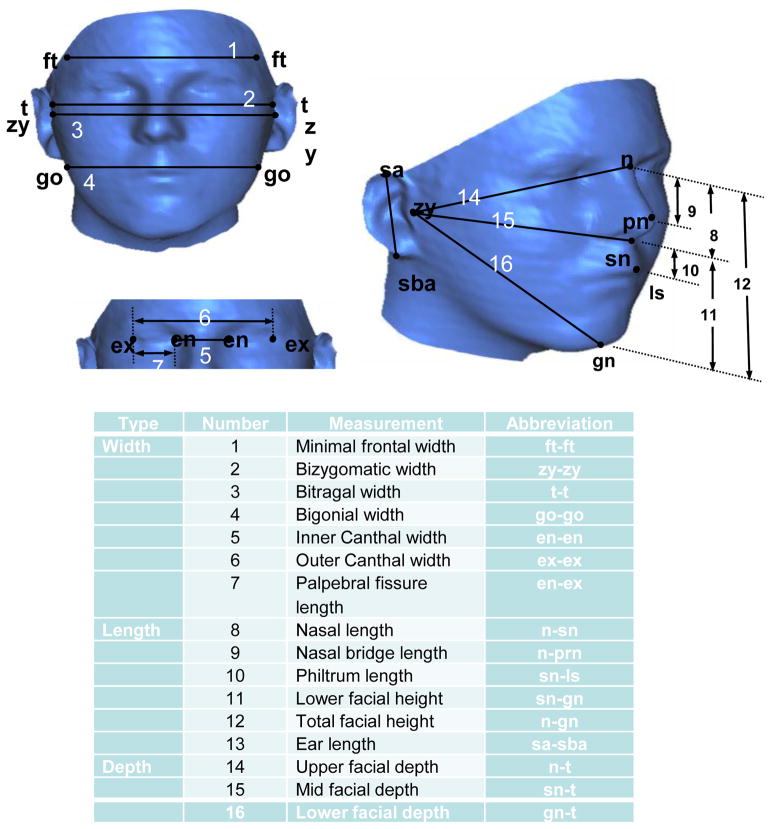
A set of 16 facial measurements were selected for use in studies of prenatal alcohol exposure based on their likely relevance to known craniofacial anomalies associated with FAS (e.g., short palpebral fissures) and selected to include only measurements for which there were published age- and sex- matched reference norms (Moore et al, 2001; Moore et al, 2007). The sixteen standard anthropometric measurements (Moore et al, 2007) included seven width measurements, six height measurements, and three depth measurements (Figure 1) and were obtained from the 3D facial images. Occipital frontal circumference (OFC) was measured directly at each study visit during the dysmorphology evaluation and when facial images were collected because it cannot be obtained from the 3D image, which only captures the front of the head and face. Only children who completed both clinic visits, had high quality images at both time points, and were between 3–6 years of age at the first visit in 2005 were included in statistical analyses. Age at the clinic visit was included as a variable in the regression model since there were significant group differences in the mean age at the time of the visit.
Figure 1. Anthropometric measurements (from Moore et al., 2007).
A: Sixteen standard anthropometric measurements obtained from each of the 3D facial images;
B. Table of Correspondence
A small pilot study was conducted in which images were processed by the same person for the same 10 subjects using both camera systems. There was a high correlation between the measurements obtained using the two cameras (Spearman’s rho > 0.90; four measures yielded 0.76–0.87). Anthropometric measurements describing facial breadth (e.g., minimal frontal breadth, inner and outer canthal, palepbral fissure) were consistently smaller using the 3dMD camera, although only two were significantly different (bitragal width and inner canthal width; p < 0.01). Measurements describing facial length were also primarily smaller using the 3dMD camera, although only one measurement was significantly so (nasal bridge length; p = 0.04). Measurements pertaining to depth were consistently larger on the 3dMD camera, although none of these differences were significant. Differences in standard deviations were minimal for all measurements. Because the differences between camera systems were small and consistent, it is unlikely that they contributed to any longitudinal between-groups differences observed.
As part of the larger longitudinal cohort study, each mother and child were transported to the UCT Child Development Research Laboratory where the child completed a battery of neurobehavioral assessments at the 5- and 9-year visits (Jacobson et al., 2008). Delay eyeblink conditioning (EBC), which involves contingent temporal pairing of a conditioned stimulus (a tone) with an unconditioned stimulus (an air puff), has been found to be impaired in alcohol-exposed children (Coffin et al., 2005; Jacobson et al., 2011b; Jacobson et al., 2008) and animals (Green et al., 2000; Stanton and Goodlett, 1998; Thomas et al., 1998). The EBC assessment was administered during the 5-year visit. While watching a video (Finding Nemo) on a monitor, the child wore a lightweight headgear, which supports a flexible plastic tube that delivers an air puff to the right eye and uses a photodiode to record eye blinks. Two small speakers at either side of the child’s head delivered the auditory conditioned stimulus, a 1-kHz, 80-dB tone. Each session consisted of 50 trials, in which the air puff was administered during the last 100 ms of 750-ms presentation of the tone. In this paradigm the child must learn to adjust the timing of the anticipatory blink, which occurs optimally between 300–650 ms after the onset of the tone in the delay condition. Eyeblinks within 350 ms prior to the air puff onset were considered conditioned responses (CRs). Two sessions consisting of 50 trials each were administered on the same day about 2.5 hr apart; a third session was conducted the following day for those children who did not meet criterion for delay conditioning of 40% CRs.
The California Verbal Learning Test-Children’s Version (CVLT-C)(Delis et al., 1994), a list learning task which has also been shown to be affected by prenatal alcohol exposure (Mattson and Roebuck, 2002; O’Leary et al., 2011), was administered at the 9-year visit. The CVLT-C generates a measure of the total number of correctly recalled words learned over five consecutive trials as well as a measure of the number of words recalled after a short delay involving a distracter list. IQ was assessed on the Junior South African Intelligence Scales (JSAIS; Madge et al., 1981) at the 5-year visit and on the Wechsler Intelligence Scales for Children-IV (WISC-IV) at the 9-year visit (Jacobson et al., 2011b; O’Leary et al., 2011). Scores on the WISC-IV and JSAIS were highly correlated, r = .79, for this cohort. Testing was conducted by examiners who were blind with respect to prenatal alcohol exposure and FASD diagnosis, except in the most severe cases of FAS. The CVLT-C and WISC-IV were translated into Afrikaans and back-translated by researchers for whom Afrikaans was their first language. The testing was conducted in Afrikaans or English, depending on the primary language used in the child’s home and school.
Statistical analysis
All analyses were performed in SAS (v 9.1.3, Cary, NC) or SPSS (v 18.0, Chicago, IL). Analyses were performed separately for each of the two clinic visits. Demographic differences between the three groups were estimated using analysis of variance (ANOVA) models for age at first clinic visit, age at second clinic visit, WISC-IV IQ scores, and all alcohol and cigarette exposure during pregnancy and at conception. A chi-square test was employed to assess the association between gender and alcohol exposure group.
We initially performed an ANOVA for each visit using group (FAS/PFAS, HE, C) and age of the child at the relevant clinic visit as between-subject factors to identify measures for which group was a significant factor. A Bonferroni correction was employed for the 17 (16 from the 3-D image plus OFC from direct measurement,) variables evaluated at each time point (0.05/17 = 0.003). Post-hoc pair-wise comparisons using a Tukey-corrected t-test were performed for any measure for which the group variable met the threshold, in order to identify which groups differed for that particular measure.
Relative growth between the two clinic visits was also computed. This was defined for each anthropometric measurement as: the measurement taken at the first time point, subtracted from the measurement taken at the second time point, divided by the value of the measurement at the first time point. The same ANOVA models were then used to test for group differences in the relative growth of the anthropometric measures.
A stepwise logistic regression analysis was employed to identify variables that best predicted group membership (FAS/PFAS, HE, C). The significance level for entering a variable was 0.40, and the significance level for keeping a variable was 0.15. Variables included in the model were the anthropometric measurements acquired from the images as well as OFC, obtained at each dysmorphology evaluation. Age of the child at the relevant clinic visit was included as a covariate. The estimated probability of being in a group was obtained from the measurements selected in each respective stepwise solution, and a cutoff value of ≥ 0.40 was used for classification.
Four comparisons that tested the hypothesis that different anthropometric measurements would be required to predict group membership were made. These comparisons were based on the extent of facial dysmorphology. The most extreme comparison, FAS/PFAS versus C, would be expected to identify facial features included in the criteria for FAS and PFAS. A broader comparison of all alcohol-exposed children (FAS/PFAS + HE) versus C would be expected to identify more subtle facial features because it would focus on traits shared by the HE and FAS/PFAS groups. The comparison of HE and C would identify novel features not traditionally included in the FAS/PFAS criteria but which are likely affected by prenatal alcohol exposure. Finally, features classifying FAS/PFAS from HE were best interpreted in light of the other comparisons and allowed us to identify features that were uniquely affected in FAS/PFAS and were unaffected, or significantly less affected, in those with heavy alcohol exposure who do not meet criteria for either FAS or PFAS. In such cases, HE individuals may be less susceptible to damage from intrauterine exposure to alcohol and, therefore, show none or only minimal effects, making them difficult to distinguish from the healthy controls.
Incidence of microtia (or abnormally small ears) was determined using Caucasian age- and gender-adjusted normative z-scores (Farkas et al, 1981) because no population specific standards exist for the Cape Colored population. The values were computed using the left ear length, or right ear length when the left ear length was unavailable. An individual with a z-score < −2.0 was determined to exhibit microtia. A chi-square test was used to test whether microtia was associated with group classification (FAS/PFAS, HE, C) at each time point.
Pearson correlations were calculated to determine the relation between prenatal alcohol exposure and the most consistent facial measurements. Mean ounce AA/day and per drinking occasion as well as frequency of drinking (days/week) were used to quantify prenatal alcohol exposure at the time of conception and across pregnancy. To limit the number of comparisons, only anthropometric measurements consistently identified as significant predictors in multiple models at both time points were included in the analysis. Smoking was included as a potential confounding variable because it usually co-occurs with maternal alcohol consumption and is known to affect fetal growth (Carter et al., 2010; Jacobson et al., 1994a; Jacobson et al., 1994b). In addition, child sex and age at clinic visit were also tested as potential confounders to prenatal alcohol exposure using simple linear regression models. Pearson correlations were also used to examine the relation between the selected anthropometric measures and EBC, CVLT-C, and JSAIS and WISC-IV IQ scores.
RESULTS
Data from 125 children were analyzed at the two time points. Sample characteristics for the children by diagnostic group are shown in Table 1. Children in the FAS/PFAS group were slightly older than those in the HE and control groups at both time points, p < 0.05, but no significant differences were found between the HE and controls at either age. There were no significant between-group gender differences. As expected, the children with FAS/PFAS had the lowest IQ scores on the JSAIS and WISC-IV IQ. Alcohol exposure both at time of conception and across pregnancy was higher for the two alcohol-exposed groups than controls, all ps < 0.001. By contrast, cigarettes smoked per day was highest among the HE mothers, both ps < 0.05, but not significantly different between FAS/PFAS and control women during pregnancy.
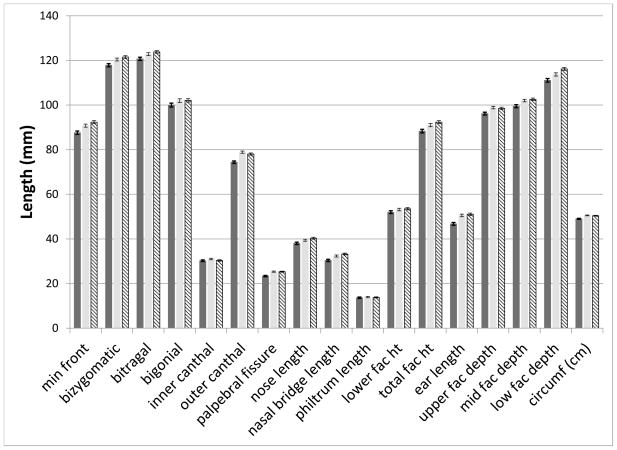
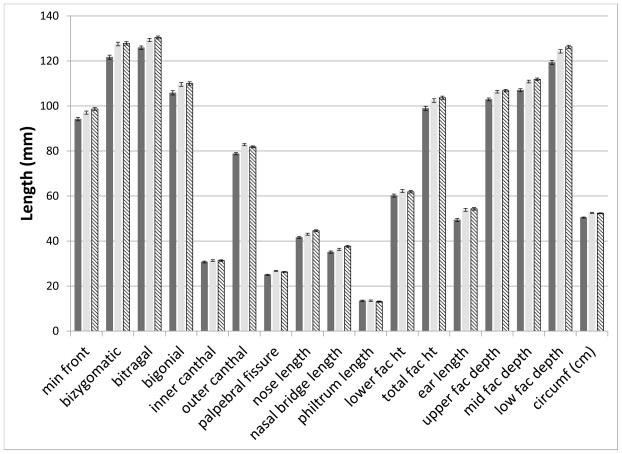
We initially tested for group differences among the 16 anthropometric measurements and OFC. Significant effects of group (p < 0.003) were found for 10 measures (minimal frontal width, bizygomatic width, outer canthal width, palpebral fissure width, mid and lower facial depths, nasal bridge length, total facial height, ear length and OFC) at the younger age. At the second time point, all these measures were still significant but the variables bitragal width, bigonial width, upper facial depth, and nasal length were also added. Post-hoc pair-wise comparisons were then performed among the groups (FAS/PFAS, HE, C) for measures with significant group effects. At the first time point, means for all measures in the FAS/PFAS group were significantly (p < 0.05) smaller than either the HE or C groups. For lower facial depth, the means of the HE were also significantly smaller (p=0.01) than the C group. At the second visit, the means of the FAS/PFAS group were significantly smaller than either the HE or C groups (p < 0.05), with the exception of nasal bridge length (FAS/PFAS vs HE p = 0.08). In addition, the HE group had a smaller nasal length (p = 0.009) and nasal bridge length (p = 0.03) than the C group. All other comparisons of the HE and C groups were not significant (p > 0.06). Figures 2A and B display the means for all measures, by group, for the two time points.
Figure 2. Significant differences in anthropometric measures across the groups at both ages.
A. First Visit: Mean and standard error bars for all measures at the first time point. Solid dark bar: FAS/PFAS; Solid light bar: HE; Hatched bar: C.
B. Second Visit: Mean and standard error bars for all measures at the second time point. Solid dark bar: FAS/PFAS; Solid light bar: HE; Hatched bar: C.
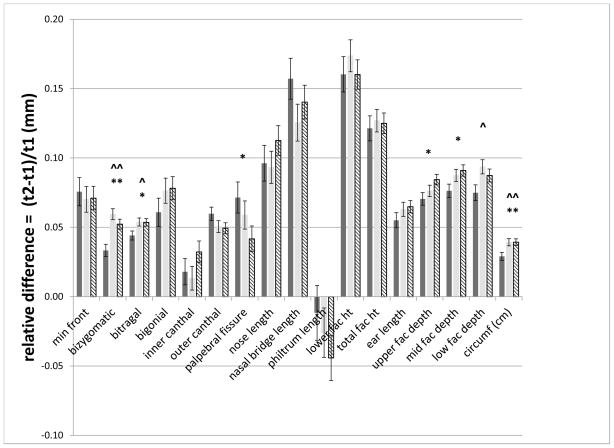
We computed the relative growth in each measure over the 4-year interval between the two clinic assessments and tested whether there were significant group differences in average relative growth (Figure 3). A significant (p < 0.003) effect of group (FAS/PFAS, HE, C) was found for the relative growth in bizygomatic width and OFC. Post-hoc pair-wise comparisons revealed that the HE and C groups grew at approximately equal rates (all p>0.14), and both groups grew more than the FAS/PFAS group for bizygomatic width (p < 0.001), and OFC (p < 0.0001).
Figure 3. Significant differences in relative growth of anthropometric measures across the groups.
Mean and standard error bars for relative differences of all measures. Solid dark bar: FAS/PFAS; Solid light bar: HE; Hatched bar: C. ^^ = FAS/PFAS vs HE p ≤ 0.0001; ^ = FAS/PFAS vs HE p ≤ 0.05; ** = FAS/PFAS vs C p ≤ 0.0001; * = FAS/PFAS vs C p ≤ 0.0
Since many of these anthropometric measures are correlated, stepwise logistic regression analysis was performed to develop models that best predicted group membership. Results are shown in Table 2. OFC was a significant predictor in the comparisons of FAS/PFAS with either HE or C; this is to be expected since a small OFC (<10th percentile) or structural brain damage is a required criterion for FAS and a conditional criterion for PFAS. Similarly, the HE and C were slightly younger (6–8 months) than the FAS and PFAS children; therefore, age was a significant predictor in all comparisons except those of HE and C.
Table 2.
Results of the four comparisons at the first and second visits
| Clinic visit | Measure | FAS + PFAS vs. C | FAS + PFAS + HE vs. C | HE vs. C | FAS + PFAS vs. HE | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| Width | Minimal frontal | --- | --- | --- | --- | --- | |||
| Bizygomatic | |||||||||
| Bitragal | |||||||||
| Bigonial | |||||||||
| Inner canthal | |||||||||
| Outer canthal | +++ | +++ | --- | ||||||
| Palpebral fissure | --- | +++ | --- | --- | |||||
| Length | Nasal | --- | --- | +++ | |||||
| Nasal bridge | --- | --- | --- | ||||||
| Philtrum | |||||||||
| Lower facial height | +++ | ||||||||
| Total facial height | +++ | ||||||||
| Ear length | --- | --- | --- | --- | |||||
| Depth | Upper facial | +++ | +++ | ||||||
| Mid facial | |||||||||
| Lower facial | --- | --- | --- | --- | +++ | ||||
| OFC | --- | --- | --- | ||||||
| Age | +++ | +++ | +++ | +++ | +++ | +++ | |||
| % Sensitivity | 82.9 | 91.2 | 86.8 | 88.2 | 75.6 | 73.2 | 85.7 | 91.2 | |
| % Specificity | 87.8 | 79.6 | 36.7 | 32.7 | 59.2 | 49.0 | 78.0 | 76.3 | |
| % Correct | 85.7 | 84.3 | 67.2 | 66.4 | 66.7 | 60.0 | 81.6 | 83.3 | |
+++ indicates the measure is larger in the first group listed in the comparison as compared with the second.
--- indicates the measure is smaller in the first group listed in the comparison as compared with the second.
FAS – fetal alcohol syndrome; PFAS – partial fetal alcohol syndrome; HE – heavy exposed; C – healthy control; OFC occipital frontal circumference.
Several anthropometric measurements were consistent predictors across both time points and also across multiple group comparisons. Minimal frontal width, palpebral fissure width, ear length, and lower facial depth were all included in four or five of the 8 models. Outer canthal width, nasal length, and nasal bridge length were included in three comparisons. There are two measures that were only predictive among the comparisons at the older age: lower and total facial height, and two measures that were only predictive at the younger age: nasal bridge and upper facial depth.
Smaller ear length, or microtia, has not typically been reported as a feature in fetal alcohol syndrome. At age 5, 15 of 35 FAS/PFAS subjects met criteria for microtia using norms from Caucasian populations (43%); in contrast, at the same visit, only 4 of 41 (10%) HE and 3 of 49 (6%) C had microtia. This was a highly significant difference in the frequency of small ears among the groups (X2(2) = 21.6, p < 0.0001) and resulted in a 9 times greater likelihood of FAS among those with this feature. Similar results were observed at the age 9 visit. 41% of the subjects classified as FAS/PFAS met criteria for microtia at both visits.
The rate of correct classification was relatively similar at both time points. Comparisons of the FAS/PFAS group with either HE or C provided very good sensitivity and specificity at both time points (> 75%). Interestingly, when all alcohol-exposed subjects were compared as a group to the C (FAS/PFAS + HE vs. C), the sensitivity (accurate prediction of the alcohol exposed group) was more than two times greater than the specificity (accurate prediction of healthy controls) (~85% vs. ~35%). When comparing the HE and C groups, there was modest sensitivity at both time points in correctly classifying the HE (73–76%) and C (49–59%).
The correlation between several measures of prenatal alcohol exposure (oz AA per day, oz AA per occasion, and frequency of drinking) obtained at conception and throughout pregnancy and the four most predictive anthropometric measures (ear length, minimal frontal width, lower facial depth, palpebral fissure length) was calculated. There was a significant negative correlation of all three measures of prenatal alcohol exposure at time of conception and during pregnancy with ear length and lower facial depth at times 1 and 2 and with minimal frontal at time 1 (Table 3). Palpebral fissure length was not significantly correlated with any alcohol exposure measure at either time point.
Table 3.
Relation of prenatal alcohol exposure to facial measurements controlling for confounding influences of age and smoking
| At conception | Across pregnancy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA/day | AA/occasion | Frequency | AA/day | AA/occasion | Frequency | |||||||
| r | β | r | β | r | β | r | β | r | β | r | β | |
| Ear length | ||||||||||||
| Time 1 | −.25** | −.27** | −.17† | −.19* | −.23** | −.25** | −.24** | −.27** | −.22* | −.25** | −.26** | −.30*** |
| Time 2 | −.28** | −.28** | −.19* | −.19* | −.28** | −.28** | −.27** | −.27** | −.26** | −.26** | −.30*** | −.30*** |
| Lower facial depth | ||||||||||||
| Time 1 | −.20* | −.24** | −.22* | −.26** | −.13 | −.17* | −.18* | −.24** | −.19* | −.27*** | −.15† | −.23** |
| Time 2 | −.26** | −.27** | −.29*** | −.30*** | −.18* | −.18* | −.25** | −.28** | −.27** | −.31*** | −.22* | −.24** |
| Minimal frontal | ||||||||||||
| Time 1 | −.25** | −.25** | −.28** | −.28** | −.20* | −.20* | −.26** | −.26** | −.27** | −.27** | −.25** | −.25** |
| Time 2 | −.11 | −.11 | −.16† | −.16† | −.09 | −.09 | −.13 | −.13 | −.21* | −.21* | −.14 | −.14 |
| Palpebral fissure | ||||||||||||
| Time 1 | −.11 | −.12 | −.10 | −.12 | −.09 | −.11 | −.09 | −.12 | −.08 | −.12 | −.11 | −.14 |
| Time 2 | −.08 | −.07 | −.09 | −.07 | −.08 | −.06 | −.09 | −.06 | −.08 | −.05 | −.12 | −.10 |
Age at visit and maternal smoking during pregnancy were included in the regression models for those outcomes to which they were related at p < .10. Age at visit was controlled for ear length at time 1, lower face depth and palpebral fissure length at both time points; smoking was controlled for lower face depth at both time points.
AA – absolute alcohol.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
It is possible that the significant negative correlation of prenatal alcohol exposure measures with ear length, lower facial depth and minimal frontal width was attributable to other confounding variables (sex, age at visit, and prenatal exposure to cigarette smoking). Therefore, multivariate analyses were performed including these potential confounders. Cigarette smoking was a potential confounder of the effect of prenatal alcohol exposure on lower facial depth at both time points. Age at clinic visit was related to lower face depth at both time points, but there were no sex differences for these outcomes. Regression analyses indicated that neither age at visit nor prenatal exposure to smoking accounted for the association between prenatal alcohol exposure and any of these features (Table 3).
To validate the utility of the facial features as markers of alcohol exposure, correlations between the four key facial features and three sets of neurobehavioral outcomes were assessed on a subset of this sample (Table 4). Our data showed that greater lower face depth and longer palpebral fissures were associated with more optimal delay eyeblink conditioning performance at both ages. Larger ear length, lower face depth, and palpebral fissures were all correlated with better performance during acquisition across the five trials of the CVLT-C (List A total correct). These features were also associated with better short delay-free recall on the CVLT-C, particularly at time 2. Larger values for all four anthropometric measures were associated with higher IQ scores on the WISC-IV, except for ear length at time 1.
Table 4.
Correlation of facial measurements to neurobehavioral outcomes
| Ear length
|
Lower face depth
|
Minimal front
|
Palpebral fissures
|
|||||
|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2a | Time 1 | Time 2 | Time 1 | Time 2 | Time 1 | Time 2 | |
| Delay eyeblink conditioning (n= 70)b,c | .21† | .08 | .36** | .35** | .24* | .15 | .26* | .25* |
| CVLT-C d (n = 45) | ||||||||
| List A total correct | .39** | .50*** | .30* | .44** | .09 | .29† | .34* | .32* |
| Short delay-free recall | .25† | .35* | .27† | .38* | .08 | .24 | .39** | .36* |
| JSAISc (n = 124) | .09 | .16† | .17* | .34*** | .19* | .21* | .09* | .18* |
| WISC-IVd (n = 71) | .15 | .25* | .31** | .45*** | .39*** | .16 | .29* | .22† |
p < 0.10
p < 0.05
p < 0.01
p < 0.001. Values are Pearson rs.
Missing for four cases.
Met criterion for conditioning within three sessions.
Collected at 5 years.
Collected at 9 years.
CVLT-C – California Verbal Learning Test-Children’s Version; JSAIS – Junior South African Intelligence Scales; WISC-IV – Wechsler Intelligence Scales for Children.
DISCUSSION
We utilized longitudinal analyses to explore which facial measurements best predict prenatal alcohol exposure and whether these predictive facial measurements change with age and are correlated with maternal drinking behavior. These comparisons are critical if we are to develop improved methods to identify those who have been exposed to alcohol in utero but do not meet diagnostic criteria for FAS or PFAS.
Our data show that measures of craniofacial width (minimal frontal), orbital width (palpebral fissure width) and ear and mandibular measures (ear length and lower facial depth) were consistently found to be predictive of group membership across both age groups and multiple comparisons. The reduction in the minimal frontal width is likely a reflection of the underlying size of the frontal cortex (DeMyer et al, 1964). The minimal frontal measurement is made on the skull, whose expansion is due to brain growth. The reduction in the size of the lower facial depth likely reflects a small, retruded or rotated mandible. The shorter ear length found in 41% of the FAS children met criteria for microtia in Caucasian populations (Farkas, 1981), a finding that has not previously been reported in FASD. Interestingly, both the mandible and external ear are derived from branchial arch mesenchyme (Moore KL, 1998) and, therefore, their reduction in alcohol-exposed children may reflect a generalized reduction in neural crest proliferation and/or migration. The mandible typically grows through late adolescence, completing growth much later than the forehead or orbital regions (Enlow and Hans, 1996); therefore, it might be expected that a reduction in lower facial depth would be a feature of alcohol exposure that would be expressed later in adolescence, contributing to a differing diagnostic profile FAS for older individuals
All measures, except palpebral fissure width, were negatively correlated with the amount of alcohol consumed by the mother both around the time of conception and across pregnancy. These correlations remained significant even after adjusting statistically for potential confounding by cigarette smoking, indicating that the findings are specific to prenatal alcohol exposure and not attributable to smoking, which is often found to be related to alcohol use or was associated with the outcomes in this study. Further studies are needed to replicate this association, which may assist in predicting the spectrum of deficits that may be expected due to the timing of prenatal alcohol exposure.
While some measures appear predictive at both the younger and older time points, in each comparison there were unique measures that predicted at one age but not the other. To explore whether these differences were due to age effects, we computed the relative growth of each anthropometric measurement across the two visits. Interestingly, only two measures, bizygomatic width and OFC, demonstrated significantly different rates of growth among the three groups. In both cases, the rate of growth was significantly slower in the FAS/PFAS as compared with both HE and C groups. These data suggest that while most measurements remain significantly smaller in the FAS/PFAS group at the older age, the actual rate of growth is similar to that of the HE and C groups. This would imply that if these rates continue, those individuals currently classified with FAS/PFAS may have some features which are less distinctive and other features (OFC and bizygomatic width) that become even more apparent as they grow into adulthood. In a comparison of 5- and 12-year-old children from South Africa, Mutsvangwa and colleagues (2010) found that the differences in facial shape at these two ages was greater among those with FAS as compared to controls – supporting the concept that FAS facial anomalies become less pronounced as the individuals mature. Of note, unlike our study, which used the same children at both ages, the Mutsvangwa study used different individuals at the two ages. Thus, while these initial results from two studies are consistent, additional longitudinal testing will be necessary to confirm this hypothesis and to also compare the heavily exposed group who do not meet criteria for FAS or PFAS with unexposed controls.
This study identified measures of facial width, length, and depth with high sensitivity and specificity that discriminated children with FAS and PFAS from controls. Sensitivity and specificity were also high for discriminating children with FAS and PFAS from the heavily exposed (HE) children who did not meet criteria for FAS or PFAS. Although these measures also showed high sensitivity for discriminating all the exposed children (FAS, PFAS, and HE) from controls, specificity for that discrimination was poor, presumably because these altered features are not characteristic of the HE group. We also identified a group of measures that provided moderate sensitivity (73–75%) in distinguishing between children in the HE and C groups. Both minimal frontal and outer canthal were included in the model at each timepoint. Minimal frontal width was smaller in those with heavy prenatal alcohol exposure as compared with controls, while outer canthal width was larger in HE as compared with C. Interestingly, at each time point, only one anthropometric measure from the model was significantly different between the two groups (lower facial depth at first visit; nasal length at second visit). While the mean of several of the variables included in the models were not significantly different in the two groups, when included jointly in the analysis they were able to identify 75% of those who were heavily exposed. These variables included measures of both the upper and lower face.
Regression analyses indicated that continuous measures of prenatal alcohol exposure were predictive of these facial features after controlling for potential confounders. Most impressive were the correlations of ear length, lower facial depth, minimal frontal width, and palpebral fissure length with three important neurobehavioral outcomes: eyeblink conditioning, verbal learning, and IQ at the two ages. Poorer verbal learning has been linked to smaller hippocampal volumes (Willoughby et al., 2008) and reduced medial temporal activation (Sowell et al., 2007) in children with FASD, and the fetal alcohol-related deficits in eyeblink conditioning seen in Cape Coloured children have recently been shown to be mediated, in part, by microstructural deficits in the left middle cerebellar peduncle, a white matter structure that links the brain stem to the cerebellum (Spottiswoode et al., 2011).
Yang et al. (2011) have recently shown that reduced palpebral fissure length is correlated with increased inferior frontal cortical thickness in children with fetal alcohol exposure. The association of alcohol-related facial features with the IQ and other neurobehavioral outcomes seen here lends further support to the premise that these facial measurements may be biomarkers of impairment in cognitive function. Our findings are consistent with a recent report by Rousette et al. (2011) showing that smoother philtrum and shorter palpebral fissures in alcohol-exposed group were associated with lower IQ scores. Thus, our neurobehavioral findings as well as the correlation of the continuous prenatal alcohol data with the facial measurements provide validation for the assessment of these facial features using the 3D techniques described here.
Low IQ scores were seen in both the alcohol-exposed and the control children in this Cape Town cohort (Jacobson et al., 2008, 2011b). The validity of the WISC-IV IQ assessment was supported by the strong correlation (r = 0.79) reported above between WISC-IV IQ scores obtained at 9 years and those based on the 5-year JSAIS, which is normed for South African children. Although the IQ scores were low, presumably due to socioeconomic disadvantage and limited educational opportunities, the mean for the control group was comparable to IQ scores reported for U.S. inner-city children, whose scores are consistently lower than suburban, nonminority children (e.g., Burden et al., 2010). Nor were the lower IQ scores in this Cape Town cohort attributable to malnutrition. As expected, the children with heavy prenatal alcohol exposure were smaller in weight and height by about 0.4 standard deviations than controls (Carter et al., 2010). However, in both the alcohol-exposed children and controls, moderate and severe thinness, an indicator of malnutrition, was rare, and only one child, a control, met WHO criteria for thinness at 9 years. Nor were any differences seen in percent body fat at 9 years (Carter et al., 2010). Thus, malnutrition was unlikely responsible for the alcohol-related IQ and neurobehavioral deficits seen in this cohort.
Although there were significant between-group differences for prenatal alcohol exposure between the exposed and control groups, post-hoc tests indicated that alcohol exposure in this sample was not significantly different for the FAS/PFAS vs. HE groups. This lack of difference appears to be due in part to inclusion of the alcohol interview data for the three mothers of children with FAS who denied alcohol use. When these women’s alcohol use data were removed from the comparisons, the FAS/PFAS and HE women did not differ in amount of alcohol consumed per occasion, but the FAS/PFAS group drank more frequently than the HE group, M = 1.8 vs. 1.3 days/week, respectively, p = 0.02. Moreover, exclusion of these three children with FAS from the analyses relating facial features to the neurobehavioral outcomes did not alter the significant associations presented here.
We have previously reported results of analyses of anthropometric measures in the Cape Town sample at the first time point (Moore et al., 2007). In those analyses, we included children with a wider initial age range; whereas here we have focused on a narrower age range to allow for a more homogeneous group to reduce the confounding effect of age when interpreting results. The current study is also the first study utilizing 3D facial imaging to examine continuous measures of alcohol exposure and to include heavily exposed children who did not meet criteria for either FAS or PFAS; the inclusion of data for the HE group allows us to make comparisons across a potentially wider range of prenatal alcohol exposure. Since we specifically sought to test for differences in the same individual across time, in the current analyses we only included children evaluated at both ages. Finally, the subject classification in both the earlier analysis of Moore et al. and the present study differs in nomenclature but not in substance. Moore et al. utilized the CIFASD criteria FAS, no FAS, or deferred (Jones et al., 2006), whereas in the present study we explicitly classified children using the revised IOM criteria (Hoyme et al., 2005) but still analyzed the FAS and PFAS together. Interestingly, the specificity and sensitivity is quite similar in both studies; however, the measures predictive of group classification were not identical. Two measures were the same (minimal frontal width, ear length), and both studies had a measure of smaller eye width (inner canthal or palpebral fissure). In the present study, OFC was a significant predictor for several comparisons, whereas in the previous study, bizygomatic width, which is another measure of decreased facial width was a significant predictor.
Philtrum length was predictive in the earlier study but not in any of our comparisons in the new and expanded sample from Cape Town. Our data suggest that in exposed children, the philtrum does not continue to grow at the same rate as most of the other areas of the face. This may be due to the fact that philtrum length does not appear to be as affected by prenatal alcohol exposure. Our study, as well as others, have found that the philtrum length in exposed children is rarely abnormal (< 2 SD below the mean), and it does not differ significantly between exposed and non-exposed children (Moore et al., 2002; Moore et al., 2007).
Comparison of our results with those from other studies of facial morphometry identifies some consistent findings, but also some unique features. Mutsvangwa and colleagues (2010) evaluated children at ages 5 and 12. They found that the features that best distinguished children with FAS from controls were small palpebral fissures, a thin upper lip, and midfacial hypoplasia. We also found small palpebral fissures to be predictive of group membership. The geometric morphometric approach Mutsvangwa and colleagues employed did not include evaluation of the ears or lower facial depth. Thus, it is not surprising that the two studies did not identify all of the same features. Their study was also limited to children with FAS and controls and did not include children who were heavily exposed to alcohol prenatally but did not meet criteria of FAS.
We have focused our analyses on commonly used craniofacial anthropometric measurements. These linear or angular measurements are derived from physical landmarks that can be easily obtained in a clinical setting or from 3D images. As detailed above and in previous studies, they have proven to be reasonably effective in differentiating affected from control individuals (Moore et al. 2001 and Moore et al. 2002). However, a known limitation of this approach is that it is restricted to only those regions of the face where landmarks can be placed reliably at biologically meaningful and identifiable locations. Alternative approaches which employ shape analysis – either through analysis of distances and angles or more recently using Cartesian coordinates (Slice, 2007) - can fully utilize the extensive surface data captured through a 3D facial image and may, therefore, provide further improvements in the rate of classification. Furthermore, these methods may also yield new insights regarding the biological effects of prenatal alcohol exposure,
Extensive research has been performed in mouse models in which alcohol exposure can be carefully controlled. Exposure to alcohol on gestational day 7 results in a range of median facial and forebrain deficiencies, which are typically within the holoprosencephaly spectrum. In addition, micrognathia has also been observed on this day of alcohol exposure (Godin et al., 2010). Interestingly, exposure to alcohol on day 8 results in craniofacial features that are reminiscent of DiGeorge syndrome and continue to include micrognathia but also cleft palate, hypertelorism, inner ear and ocular abnormalities, and central nervous system defects (Sulik, 2005). In fact, some of the most prominent features which distinguish alcohol-exposed individuals from unexposed controls in the current study are also found in the mouse models in which alcohol exposure is limited to a single day.
In summary, multidisciplinary studies of this high risk population from South Africa combining longitudinal data from clinical observations, maternal drinking behavior, neurobehavioral studies, and 3D anthropometric analysis of the face indicate that a correlation exists between amount of alcohol consumed during pregnancy and neurobehavioral outcomes and the facial phenotype. Specifically, prenatal alcohol exposure seems to have primary effects on brain growth, as reflected in smaller forehead widths (minimal frontal widths) and orbit dimensions. In addition, there appears to be a global reduction in size of the head and face. Deficits in ear length and mandibular dimensions may indicate a suppression of neural crest migration to the branchial arches and other tissues in the developing face. Longitudinal analyses of relative growth suggest that the rate of growth in OFC and facial width (bizygomatic width) fall further behind normal with age, while other dimensions grow at comparable rates to those seen in normal individuals. These findings support the contention that the facial features associated with intrauterine exposure to alcohol consist of both a neural component and a primary microsomia. The latter evidences a more normal pattern of growth than the former, resulting in a changed alcohol-related phenotype with age. Thus, features associated with smaller brain (forehead, orbits) will appear more pronounced with age. Likewise the apparent rotation of a congenitally small mandible will contribute an apparently greater facial height in older affected individuals. In spite of the changing phenotype with age, the present study indicates that craniofacial measurements derived from 3D images can accurately separate individuals with FAS and PFAS and suggests that minimal frontal width, palpebral fissure length, lower facial height, and ear length may be predictive of the degree of neural cognitive disruption. To this end it would be important to collect population specific normative data for these features. Ongoing studies suggest that the development of complex facial profiles from 3D images may improve diagnostic resolution and enhance our understanding of the relation between the face and the neuropsychological deficits that occur.
Acknowledgments
This international collaborative study is supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): U01AA014809 (to T.F.), U01AA014790 (to S.J.), U24AA014815 (to K.J.), U24AA014828 (to E.R.), U24AA014830 (to E.R.), R01AA016781 (to S.J.), and administrative supplements to RO1AA09524 (to S.J.) Additional funds were provided by the National Institutes of Health Office of Research on Minority Health, and the Joseph Young, Sr., Fund from the State of Michigan (to S.J.) This study was conducted in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the NIAAA. Additional information about CIFASD can be found at www.cifasd.org. We thank Sr. Maggie September, for her major contribution in locating all of the participants and helping organize the two clinics at which 3D photographs were taken, and the UCT research staff and R. Colin Carter, Children’s Hospital Boston Harvard Medical School, for their help in collecting the data at the clinics and in conducting the neurobehavioral assessments. We also thank the mothers and children in the UCT longitudinal cohort for their continuing participation and contribution to the study.
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflicts of interest related to this study.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54. [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund AJ, Lundahl LH, Klorman R, Nelson CA, Avison MJ, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Carter RC, Duggan CP, Molteno CD, September M, Pienaar M, Jiang H, Jacobson JL, Jacobson SW. The relation of fetal alcohol exposure to growth and body composition in Cape Town, South African children. Alcohol Clin Exp Res. 2010;34(Suppl s2):102A. [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41(3):389–98. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2011;35(6):1114–21. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Children's Version. The Psychological Corporation; New York: 1994. [Google Scholar]
- DeMyer W, Zeman W, Palmer CG. The face predicts the brain: Diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly) Pediatrics. 1964;34 (2):256–263. [PubMed] [Google Scholar]
- Douglas TS, Mutsvangwa TEM. A review of facial image analysis for delineation of the facial phenotype associated with fetal alcohol syndrome. Am J Med Genet Part A. 2010;152A:528–36. doi: 10.1002/ajmg.a.33276. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Hans MG. Essential of Facial Growth. Philadelphia: WB Saunders; 1996. [Google Scholar]
- Farkas LG. Anthropometry of the head and face in medicine. New York: Elsevier; 1981. [Google Scholar]
- Green JT, Rogers RF, Goodlett CR, Steinmetz JE. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcohol Clin Exp Res. 2000;24(4):438–47. [PubMed] [Google Scholar]
- Godin EA, O’Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johson G, Styner MA, Sulik KK. Magnetic-resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res. 2010;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994a;18(2):317–23. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. J Pediatr. 1994b;124(5 Pt 1):757–64. doi: 10.1016/s0022-3476(05)81371-x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–25. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev. 2011a;21(2):148–66. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011b;35(2):250–64. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EF, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6):e1734–8. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Wetherill L, Rogers J, Moore E, Ward R, Autti-Ramo I, Fagerlund A, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, Li TK, Riley EP, Foroud T the CIFASD Consortium. Prenatal alcohol exposure alters the patterns of facial asymmetry. Alcohol. 2011;44:649–57. doi: 10.1016/j.alcohol.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21(2):102–18. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge EM, van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Human Sciences Research Council; Pretoria: 1981. [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26(6):875–82. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(9):1640–50. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25(3):159–67. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88(2–3):259–71. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Jamison PL, Morris CA, Bader PI, Hall BD. The subtle facial signs of prenatal exposure to alcohol: an anthropometric approach. J Pediatr. 2001;139(2):215–9. doi: 10.1067/mpd.2001.115313. [DOI] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Jamison PL, Morris CA, Bader PI, Hall BD. New perspectives on the face in fetal alcohol syndrome: what anthropometry tells us. Am J Med Genet. 2002;109(4):249–60. doi: 10.1002/ajmg.10197. [DOI] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Wetherill LF, Rogers JL, Autti-Ramo I, Fagerlund A, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, Foroud T. Unique facial features distinguish fetal alcohol syndrome patients and controls in diverse ethnic populations. Alcohol Clin Exp Res. 2007;31(10):1707–13. doi: 10.1111/j.1530-0277.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- Moore KL. The Developing Human: Clinically Oriented Embryology. 6. W.B. Saunders Company; 1998. [Google Scholar]
- Mutsvangwa T, Douglas TS. Morphometric analysis of facial landmark data to characterize the facial phenotype associated with fetal alcohol syndrome. J Anat. 2007;210:209–20. doi: 10.1111/j.1469-7580.2006.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsvangwa TEM, Meintjes EM, Viljoen DL, Douglas TS. Morphometric analysis and classification of the facial phentoype associated with fetal alcohol syndrome in 5- and 12-year-old children. Am J Med Genet Part A. 2010;152A:32–41. doi: 10.1002/ajmg.a.33137. [DOI] [PubMed] [Google Scholar]
- O’Leary CE, Thomas KGF, Molteno CD, Jacobson JL, Jacobson SW. Verbal learning and memory in fetal alcohol spectrum disorder: Findings from Cape Town and Detroit. Alcohol Clin Exp Res. 2011;35(s1):111A. [Google Scholar]
- Roussette FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. doi: 10.1002/hbm.21260. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Slice DE. Geometric Morphometrics. Annu Rev Anthropol. 2007;36:261–81. [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18(7):635–9. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW. Diffusion Tensor Imaging of the Cerebellum and Eyeblink Conditioning in Fetal Alcohol Spectrum Disorder. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01566.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22(1):270–5. [PubMed] [Google Scholar]
- Sulik KK. Genesis of Alcohol-Induced Craniofacial Dysmorphism. Experimental Biology and Medicine. 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105(2):159–66. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14(6):1022–33. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Yang Y, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]