Abstract
Creeping fat has long been recognized as an indicator of Crohn’s disease activity. Although most patients with Crohn’s Disease (CD) have normal or low BMI, the ratio of intra-abdominal fat to total abdominal fat is far greater than that of controls. The obesity epidemic has instructed us on the inflammatory nature of hypertrophic adipose tissue and similarities between mesenteric depots in obese and CD patients can be drawn. However, several important physiological differences exist between these two depots as well. While the molecular basis of the cross-talk between mesenteric adipose and the inflamed intestine in CD is largely unknown, novel evidence implicate neuropeptides along with adipocyte-derived paracrine mediators (adipokines) as potential targets for future investigations and highlight adipose tissue physiology as a potential important determinant in the course of IBD.
Crohn’s Disease and Mesenteric Adipose
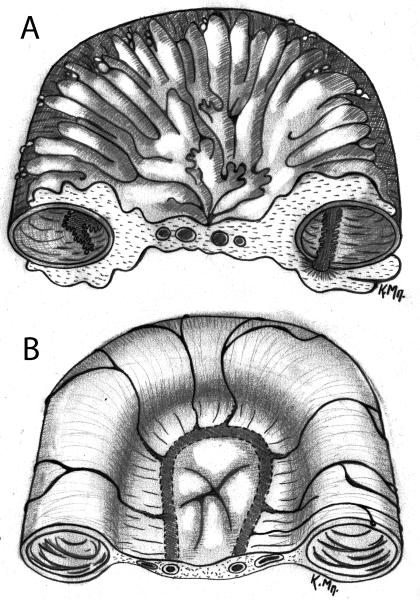
Long before the concept of adipose tissue as an immune organ began to capture the interest of scientists in the early 1990’s, mesenteric fat had been shown to be an important indicator of regional disease activity in CD patients. Fat wrapping or “creeping fat” has been recognized at least since the early 1930’s and used by surgeons to help identify the most diseased regions of the bowel 1. Fat wrapping is defined as fat extending from the mesenteric attachment to partially cover the small or large intestine, resulting in a loss of the bowel-mesentery angle 2 (Figure 1). In general, fat which covers more than 50% of the bowel circumference, is considered to be of significant importance and is a common and specific feature of CD. A retrospective review of 225 small intestinal resections found fat wrapping in more than 53% of CD cases, but not in resections performed for other indications such as intestinal ischemia, Meckel’s diverticulum, carcinoma, lymphoma, peforation from various etiologies, or radiation enteritis 3. Fat wrapping is positively correlated with muscular hypertrophy, fibrosis, transmural inflammation, and stricture formation at the gross level, and macrophage and lymphocyte pervascular infiltration on histology3 .
Figure1.
Intestinal cross sections A. depicting fat wrapping during CD and B. normal comparison.
Increased body mass index (BMI) predicts poorer outcome and earlier time to first surgery in patients with CD 4. Obesity is associated with the development of more active IBD and requirement for hospitalization, and decreases in the time span between diagnosis and surgery 5. This may come as a surprise given the importance of malnutrition in CD patients. Although most patients with CD are underweight, the ratio of intraabdominal fat to total abdominal fat is far greater than in controls when assessed by MRI in these patients 6. In this study fat accumulation in the mesentery of patients with CD is an early event and does not correlate with duration or intensity of disease, raising the question of whether it could be related to IBD pathogenesis.
Adipose Tissue and the Inflammatory Features of Obesity
The importance of adipose tissue in disease pathogenesis has become better appreciated as the prevalence of obesity has reached epidemic proportions. Obesity has now overcome smoking as the leading cause of morbidity and mortality in the US 7, which if left unchecked is predicted to lead to a regression in life expectancy in the US in the 21st century 8. Interest into the mechanisms behind the enormous contribution of obesity to the US disease burden has led to discoveries that suggest that obesity represents a state of low grade inflammation. Adipocytes function much like macrophages, surveying their environment for microbial products 9, and mediating innate immune responses 10, while preadipocytes have direct phagocytic function 11.
Mesenteric adipose tissue from obese animals shows significantly higher density of macrophages compared to lean littermates and recruitment of these cells may by due to the release of monocyte chemoattractant protein-1 (MCP-1) from preadipocytes and endothelial cells 12. In gonadal tissue of immunodeficient mice that received stem cell transplants, macrophages are of bone marrow origin indicating, migration of these cells to this area 13. However, adipocytes may possess the ability to de-differentiate into preadipocytes 14, which possess several of the features of macrophages, including bacterial phagocytosis 11. Preadipocytes are also able to rapidly differentiate into macrophages 11. Thus, the increased number and activity of macrophages in visceral adipose tissue may result not only from macrophage migration but also from local adipocyte plasticity. Indeed, the preadipocyte gene expression profile is closer to macrophages than mature adipocytes 14. Over 50 adipocyte derived mediators termed adipokines have been identified 15. Most notable among them are leptin and adiponectin together with other prominent proinflammatory mediators not specific to adipocytes, such as TNFα, IL-6, and IL-8. Leptin is a pro-inflammatory appetite suppressant secreted in quantities proportional to total fat mass that regulate adipocyte and preadipocyte Toll-like receptors expression in vitro 16. Adiponectin is an anti-inflammatory adipokine that seems to play a protective role against the development of metabolic syndrome 17. Its expression levels and secretion are inversely related to fat mass. An increasing amount of evidence have demonstrated an adiponectin-induced direct inhibition of proinflammatory pathways, including those regulated by TLRs 18, 19, via inhibition of NF-κB, in several cell types including adipocytes 20. In addition, adiponectin inhibits proinflammatory cytokine secretion while it upregulates secretion of the anti-inflammatory cytokine IL-10 18, 21, and reduces leptin-mediated TNFα expression via inhibition of MAPK activation 22. Resistin, another adipokine, increases the expression of TLR-2 and activates JNK1 and 2 in human subcutaneous adipocytes 23. Interestingly resistin levels are significantly increased during obesity 24.
Much of the morbidity associated with obesity is secondary to the associated metabolic syndrome that includes type 2 diabetes, dyslipidemia, and hypertension 25. Intra-abdominal obesity is of particular importance since clinically, waist circumference rather than absolute weight or BMI is more closely correlated with the development of metabolic syndrome 26,27. The involvement of intra-abdominal adipose tissue in metabolic syndrome development correlates well with elevations in cytokine levels observed in obese individuals. In particular, IL-6 is 50% higher in the portal vein of obese individuals compared to the radial artery, underlining the importance of mesenteric fat for these systemic elevations 28.
In addition to elevated circulating cytokines in obese individuals, circulating macrophages are also in an activated state characterized by increased NF-κB nuclear translocation and decreased NF-κB inhibitor IκBβ levels 29. In these individuals the concentration of plasma free fatty acid (FFA) is significantly corelated not only with BMI but also with IL-6 and TNF-α mRNA expression, as well as plasma CRP levels. It is likely that hyperlipidemia induces inflammatory responses via activation of the same signaling pathways as lipopolysacharide 30. This hypothesis is demonstrated in studies using hyperlipidemic myD88 deficient mice, which have deficient signal transduction downstream of Toll like receptors, where these animals show significant reduction in early atheroscerosis despite the increases in their circulating FFAs.
Adipocyte-specific effects on innate immune responses have been well documented and are shown to be affected by obesity 23, 31. In adipocytes, TLR-2 expression was upregulated by LPS in a TLR-4-dependent manner 32. In the same cells, LPS treatment induced IL-6 and TNFa secretion via NF-kB-associated pathways. TLR-2 levels are incresed during obesity and affected by increased resistin levels, the levels of which are also correlated with those of adiposity. Thus, adipocytes demonstrate a strong proinflammatory potential with their ability to induce innate immune responses along with their capacity to produce specific molecules that affect the expression of inflammatory mediators. Interestingly, levels of proinfammatory mediators during obesity are not only affected by the levels of adiposity, but also interact with each other to furhter influence the inflammatory millieu in fat depots in obese states 33, 34.
Adipose Tissue and IBD Pathogenesis
The earliest and most important IBD susceptibility loci have been mapped in nucleaotide-binding oligomerization domains (NOD) now called N-terminal caspase recruitment domain (CARD) 35. Recent studies employing genome-wide association studies have now strengthened this association identifying novel risk factors as well as protective splice variants linking IBD with these domains 36. Mutations especially within the NOD2/CARD15 region increase suseptability to CD 20 to 40 fold 37, 38 and predict earlier onset of the disease, ileal location, increased risk for the requirement of resection, and an increased risk of post-operative relapse after resection 39. Similar to the Toll-like receptors also expressed by adipocytes and preadipocytes 16, NOD/CARD receptors are microbial pattern recognition molecules of the innate immune system that detect bacterial products such as peptidoglycans in the cytoplasm and activate transcription factors and intracellular signaling kinases. NOD/CARD domains code for receptors present on antigen presenting cells such as macrophages and are constitutively expressed by preadipocytes 40. Intererstingly, NOD1/2−/− deficient mice demonstrate less insulin intolerance and adipose tissue inflammation in response to high fat diet while direct activation of NOD1 in adipocytes is associated with similar metabolic changes 41. Given the magnitude of these effects and the abundanse of the receptors and their signaling mediators in adipocytes, understanding the mechanism that makes these receptors so relevant to the pathogenesis of IBD is likely to shed light into the role of mesenteric fat in the pathogenesis of these diseases.
A better understanding of the molecular mechanism of the association between the function of the adipose tissue and the development of intestinal inflammatory pathological conditions can be achieved via the investigation of the potential involvement of adipocyte-specific mediators (adipokines) in the generation of these responses. Studies employing IBD models have demonstrated an important proinflammatory role for leptin during colitis 10, 42, while increased leptin expression is found in the colonic lumen of IBD patients compared to controls 9. In addition, increased levels of the leptin receptor in T lymphocytes are associated with increased severity of colitis in mice 43. Leptin also regulates CD4+ T-cell polarization in vitro and in vivo 44. Together, these results implicate leptin in the development of chronic intestinal inflammation. Interestingly, despite the ability of other cell types to produce and secrete leptin the effects of leptin in colitis are strongly associated with adipocyte-derived leptin 45.
Several studies indicate a potential role for adiponectin in regulating IBD-associated inflammatory responses. Significantly increased levels of adiponectin within the newly developed “hypertrophic” mesenteric fat mass are found during CD 46. Interestingly, adiponectin expression from hypertrophic adipocytes has been shown to decrease dramatically during obesity, while this is not the case for “hypertrophic” CD-associated fat 47. In another study, female and male CD patients had lower adiponectin levels compared to UC, and adiponectin was lower in female CD patients compared to female control subjects 48. Adiponectin was higher in UC with inactive disease, while corticosteroid treatment is associated to elevated adiponectin in male CD patients 48. Valentini et al showed that, compared to controls, adiponectin levels were decreased in both active and inactive IBD disease 49. Interestingly, infliximab therapy in IBD patients did not alter levels of leptin or adiponectin, but significantly reduced serum levels of the adipokine resistin 50.
Additional evidence for a potential crosstalk between adipose and intestinal tissue as it relates to the development of intestinal inflammation is provided by calprotectin, a biomarker of intestinal inflammation and increased risk for the development of colorectal cancer, the levels of which also increase significantly with obesity 51. Intervention to reduce C-reactive protein serum levels, also increased during obesity, is able to correct these changes 52, 53.
Finally, recent studies using the DSS colitis mouse model, demonstrated that adipose tissue-derived stem cells ameliorate the clinical and histopathological severity of colitis, abrogate weight loss, diarrhea and inflammation, and increase survival 54. This is accomplished, at least in part via the induction of IL-10 secreting T regulatory cells and the impairment of Th1 cell activation. Overall, adipose tissue-derived stem cells decreased inflammatory responses, including the down-regulation of proinflammatory cytokine expression from macrophages.
Mesenteric Adipocytes in CD patients as a Source of Adipokines
TNF-α is a crucial mediator involved in the pathopysiology of IBD, and therapy with anti TNF-α monoclonal antibodies along with corticosteroids, are the only agents shown to induce remission during active CD 55, 56. As discussed above and as is also the case with obesity, mesenteric adipose tissue in patients with CD is a source of many cytokines in significant concentrations that are released directly adjacent to the bowel and are differentially regulated. Adipose tissue is composed of mature adipocytes, preadipocytes, endothelial cells, fibroblasts, macrophages, and lymphocytes. A study in mouse gonadal adipose tissue showed that macrophages and endothelial cells were responsible for greater production of TNF-α and IL-6 compared to adipocytes 13. When mesenteric adipose tissue in patients with CD was studied, however, it was found that adipocytes themselves were a major mesenteric source of TNFα 6. No positive immunohistochemical staining for TNFα was observed in the subcutaneous fat of patients with CD or the mesenteric or subcutaneous fat of healthy controls, however mesenteric adipose from patients with CD did stain positive 6. In-situ hybridization of a TNF-α antisense probe was used to further localize the source of TNF-α. The probe detected no TNF-α in mesenteric adipose tissue from controls whereas all specimens from CD patients were positive. In addition, only some of the mononuclear cells stained positive while all of the adipocytes were positive with the TNF-α signal present in the cytoplasmic and perinuclear regions 6.
Stool calprotectin is a marker of inflammation of the bowel and levels are increased in obesity. Intervention to reduce serum C-reactive protein (CRP) levels abolishes this association 51, while weight loss also reduces CRP levels 53. Interestingly, CRP levels are significantly increased in the mesenteric fat depots of CD patients while bacterial products and proinflammatory cytokines are shown to induce CRP mRNA expression in 3T3-L1 preadipocytes 57.
Growth hormone deficiency is associated with increased central adiposity and has been consistently observed in patients with CD 58, 59. Clinical trials of human growth hormone as a therapy for CD have shown it to be effective for the treatment of clinical disease as well as growth failure 60, 61. In addition over production of the hormone was shown to be beneficial in animal models of colitis 62. These results are interesting in that mitigation of the deleterious effect of intra-abdominal fat may explain the therapeutic effect of growth hormone in CD. Further MRI analysis of patients pre and post growth hormone treatment to determine the effect of this agent on mesenteric fat depot size may add information on the potential use of growth hormone as a therapeutic agent.
Neuropeptides are a link between adipose tissue and IBD
The potential involvement of neuropeptides in IBD has been demonstrated by several lines of evidence (reviewed in 63, 64). Studies from our group have also demonstrated proinflammatory effects of neuropeptides on human adipocytes as well as the presence of the receptors for substance P (SP) and neurotensin (NT) on the surface of these cells 65, 66. SP and NT treatment of preadipocytes induced the expression of IL-8 and IL-6, respectively in an NF-κB-dependent manner. Interestingly, the levels of these receptors increased in adipose tissue after the induction of experimental colitis in mice. Such proinflammatory effects may create a cycle of events that contributes to inflammatory cell recruitment and activation observed within the “creeping” fat depots of IBD patients, especially via the chemo attraction of neutrophils (potential model reviewed in 64).
Another potential avenue by which SP may affect the adipose-intestinal interactions during IBD is via direct effects on mesenteric fat depot physiology and the development of “creeping” fat around the inflamed areas of the intestine during CD. It has been suggested that autocrine signals as well as secreted factors acting in a paracrine fashion may affect preadipocyte proliferation and differentiation 67, 68. SP is present in fat depots 68-70, and is thought to participate in brown adipose tissue trophic responses 71, 72. In a recent study we have also demonstrated that SP can affect fat depot size via effects on preadipocyte replication and apoptosis. In particular, treatment of preadipocytes with SP increased their proliferative potential while it decreased FasL-induced apoptosis 73. In the same study, we showed that SP-induced increased proliferation was likely caused via activation of Akt and PKCθ along with the activation of the translational promoters p70 S6 kinase and 4E-BP1. Protection from apoptosis was likely due to induction of PARP and caspase-7 cleavage as well as reduction of caspase-3 activation.
Thus neuropeptides may link adipose and intestinal responses during IBD by either affecting the formation of the “creeping” fat mass (yet of unknown contribution) in CD, or by their capacity to induce proinflammatory responses in adipocytes which may in turn trigger recruitment of immune cells around the area of the inflamed intestine.
Comparing Obesity and CD Mesenteric Fat Depots
Although obesity research has shed light on the role of adipose tissue in the pathogenesis of IBD there are important differences between the mesenteric fat depots in these two disease states. Adipose tissue in obese individuals, whether subcutaneous or mesenteric, grows by hypertrophy. Increased adipocyte size in obese individuals correlates with overproduction of adipokines 74 and increased macrophage infiltration 75. In contrast, mesenteric fat accumulation in CD appears to occur as a result of hyperplasia. There are approximately four times as many adipocytes per unit area than adipocytes from controls 76. While both diseases involve the release of adipokines as part of a chronic inflammatory condition, obese individuals rarely develop symptoms of IBD, and metabolic syndrome is rare in IBD patients, unless related to chronic corticosteroid use. As described above, adiponectin production is decreased during central obesity but increased during IBD77, 46. Although serum TNFα appears to be a product of both obese and CD mesenteric fat depots, the cell types producing TNFα in these two disease states may be different. TNFα in obese mesenteric adipose may be released from stromal vascular cells, whereas in CD adipocytes may represent the main source 13, 6. At this point it is not evident which of these distinctions, if any, can explain the pathophysiologic difference seen between the obesity and CD disease states. Indeed, not yet identified differences in these two “inflammatory” fat depots may account for the disparity of symptomatology between obese and IBD patients.
Hence, it is clear that the term “hypertrophic” is not appropriate when adipocytes and not whole mesenteric adipose tissue are described in the case of CD. Indeed, a recent study 78 has also confirmed that creeping fat adipocytes isolated from CD patients are smaller in size (due to reduced lipid metabolism pathway activation) and not hypertrophic. Thus, changes in fat tissue size and distribution under different pathological conditions should be characterized separately, while adiposity alone should not be the sole criterion for the prediction of fat tissue function. They conclude that “creeping fat” may play a protective role during CD and this conclusion is mainly based on observations that adipocytes close to the involved intestine produce more anti-inflammatory cytokines compared to those isolated from distal sites which had a cytokine profile similar to adipocytes isolated from obese patients 78.
Common Features between Intestinal and Mesenteric Inflammation
A key question when considering mesenteric adipose tissue role in IBD pathophysiology is how inflammation in the intestine is related to inflammation in the adipose tissue. Some authors have hypothesized that the first event in CD may not be mucosal damage but translocation of bacteria into the mesenteric tissue leading to chronic inflammation that eventually breaks through as mucosal damage and inflammation 79. Genetically defective immune responses to commensal bacteria may lead to translocation into the mesenteric fat or inability to clear it, exposing mesenteric adipocytes and preadipocytes to bacteria derived molecules 80 . Genetically predisposed individuals in this model may be individuals with primary macrophage immunodeficiency 81. The relationship of defective bacterial sensing through NOD1 and 2 to defective definsin production underscores the importance of the mucosal barrier to IBD pathopysiology. In experimental colitis, such as with TNBS or DSS, mucosal damage leads to bacterial translocation to the mesentery precipitating an inflammatory response. PPAR-gamma, realeased in response to bacterial stimuli, up regulates proliferation and differentiation of adipocytes 82, and it is over expressed in the mesenteric adipose of patients with CD 83. Additionally, if the disease is starting in the mesentery and moving to the mucosa, this would help explain the longitudinal linear mucosal ulcerations along the mesenteric border that have been a morphologic feature distinguishing CD from other forms of intestinal inflammation such as infectious or ischemic causes 84, 85.
Conclusions
Over the last decade the macroscopic, histological and molecular evidence for a potential involvement of mesenteric adipose tissue in IBD pathophysiology are mounting. Although far from a consensus, it is now becoming evident that, at least in the case of “creeping fat” we are faced with a different type of adiposity, with different biochemical properties. There is also evidence to suggest anti-inflammatory and anti-bacterial roles for this tissue during the different stages of CD. The plasticity of adipose tissue, as well as the plurality of the responses that adipocytes have been shown to produce during different pathological conditions, make the aforementioned hypotheses very plausible. Additionally, an important, indirect, obesity-associated, effect of adipose tissue in the outcome of IBD should merit significant consideration due to the inflammatory state and increased levels of circulating risk factors in obese individuals described in this review. Early clinical evidence certainly point to this direction but better controlled studies on animal models are still lacking. As data on adipose tissue physiology and function continue to surface, it is imperative that the adipose tissue question is approached carefully with regard to depot location (it is now well accepted that different fat depots are virtually different mini-organs 86), size and physiological condition before any conclusions on its potential effects on disease are drawn. Furthermore, the IBD example teaches us that characterization of adipocyte state and size within a depot should precede, and indeed offer a guide for the direction of our studies as they relate to fat tissue contribution in disease pathophysiology. Collectively, these studies underline the importance of adipose tissue for the identification of targets for future, novel therapeutic approaches for IBD. The generation of adipocyte specific study systems is still a work in progress but as more tools (adipocyte-specific vectors, cell lines, animal models) become available that allow for the greater experimental focus on the adipocyte level, important insight on adipose-associated effects on IBD etiology should increase exponentially.
Table 1.
Similarities and differences between mesenteric adipose depots in obesity versus CD
| Central Obesity | CD | |
|---|---|---|
| Adiopse growth by | Hypertrophy87 | Hyperplasia84 |
| Increased BMI | Associated | Not associated |
| Dyslipidemia | Associated | Not associated |
| Insulin resistance | Associated | Not associated |
| Serum CRP | Increased88 | Increased6 |
| Serum TNFα | Increased, produced by stromal vascular cells13 |
Increased, produced by adipocytes6 |
| Serum IL-8 | Increased89 | Increased6 |
| Serum IL-6 | Increased89 | Increased6 |
| Serum leptin | Increased77 | Increased47 |
| Serum adiponectin | Decreased77 | Increased46 |
Acknowledgments
Grant Support: Supported by National Institutes of Health training grant T32 DK07180 (CF), DK60729, DK47343, RC1 DK086150, 1P50 DK64539 (CP), The Broad Medical Research Program (IK), the Blinder Research Foundation for Crohn’s Disease (KB), and the Eli and Edythe Broad Chair (CP).
Reference
- 1.Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA : the journal of the American Medical Association. 1984;251(1):73–9. doi: 10.1001/jama.251.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Weakley FL, Turnbull RB. Recognition of regional ileitis in the operating room. Dis Colon Rectum. 1971;14(1):17–23. doi: 10.1007/BF02553169. [DOI] [PubMed] [Google Scholar]
- 3.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79(9):955–8. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 4.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4(4):482–8. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Saxena NK, Titus MA, Ding X, et al. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004;18(13):1612–4. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117(1):73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 7.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010;38(2):138–44. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 9.Sitaraman S, Liu X, Charrier L, et al. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004;18(6):696–8. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 10.Hoda MR, Scharl M, Keely SJ, McCole DF, Barrett KE. Apical leptin induces chloride secretion by intestinal epithelial cells and in a rat model of acute chemotherapy-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G714–21. doi: 10.1152/ajpgi.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charriere G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278(11):9850–5. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 12.Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22(Suppl 2):3–10. doi: 10.1111/j.1365-2036.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ron D, Brasier AR, McGehee RE, Jr., Habener JF. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP) J Clin Invest. 1992;89(1):223–33. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 16.Batra A, Pietsch J, Fedke I, et al. Leptin-dependent toll-like receptor expression and responsiveness in preadipocytes and adipocytes. Am J Pathol. 2007;170(6):1931–41. doi: 10.2353/ajpath.2007.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 16(17):1896–901. doi: 10.2174/138161210791208893. [DOI] [PubMed] [Google Scholar]
- 18.Neumeier M, Weigert J, Schaffler A, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79(4):803–8. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi N, Argueta JG, Masuhiro Y, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579(30):6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Lira FbS, Rosa JC, Pimentel GD, et al. Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-ΰB pathway in 3T3-L1 adipocytes. Cytokine. doi: 10.1016/j.cyto.2011.10.001. (0) [DOI] [PubMed] [Google Scholar]
- 21.Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005;334(4):1180–3. doi: 10.1016/j.bbrc.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Hou M, Xia M, et al. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238(1):19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Kusminski CM, da Silva NF, Creely SJ, et al. The in vitro effects of resistin on the innate immune signaling pathway in isolated human subcutaneous adipocytes. J Clin Endocrinol Metab. 2007;92(1):270–6. doi: 10.1210/jc.2006-1151. [DOI] [PubMed] [Google Scholar]
- 24.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology. 316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 26.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30(6):1647–52. doi: 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 27.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 28.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 29.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110(12):1564–71. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10(4):416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 31.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275(32):24255–63. doi: 10.1074/jbc.M002137200. [DOI] [PubMed] [Google Scholar]
- 33.Kopp A, Buechler C, Neumeier M, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17(4):648–56. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 34.Kopp A, Buechler C, Bala M, Neumeier M, Scholmerich J, Schaffler A. Toll-like receptor ligands cause proinflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology. 2010;151(3):1097–108. doi: 10.1210/en.2009-1140. [DOI] [PubMed] [Google Scholar]
- 35.Hugot J-P, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 36.Rivas MA, Beaudoin M, Gardet A, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 43(11):1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 38.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 39.Büning C, Genschel J, Bühner S, et al. Mutations in the NOD2/CARD15 gene in Crohn’s disease are associated with ileocecal resection and are a risk factor for reoperation. Alimentary Pharmacology & Therapeutics. 2004;19(10):1073–1078. doi: 10.1111/j.1365-2036.2004.01967.x. [DOI] [PubMed] [Google Scholar]
- 40.Stroh T, Batra A, Glauben R, et al. Nucleotide oligomerization domains 1 and 2: regulation of expression and function in preadipocytes. J Immunol. 2008;181(5):3620–7. doi: 10.4049/jimmunol.181.5.3620. [DOI] [PubMed] [Google Scholar]
- 41.Grossberg AJ, Scarlett JM, Zhu X, et al. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology. 2010;151(2):606–16. doi: 10.1210/en.2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122(7):2011–25. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 43.Siegmund B, Sennello JA, Jones-Carson J, et al. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53(7):965–72. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batra A, Okur B, Glauben R, et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151(1):56–62. doi: 10.1210/en.2009-0565. [DOI] [PubMed] [Google Scholar]
- 45.Sennello JA, Fayad R, Pini M, Gove ME, Fantuzzi G. Transplantation of wild-type white adipose tissue normalizes metabolic, immune and inflammatory alterations in leptin-deficient ob/ob mice. Cytokine. 2007 doi: 10.1016/j.cyto.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54(6):789–96. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(2):100–5. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 48.Weigert J, Obermeier F, Neumeier M, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm Bowel Dis. 2010;16(4):630–7. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- 49.Karmiris K, Koutroubakis IE. Resistin: another rising biomarker in inflammatory bowel disease? Eur J Gastroenterol Hepatol. 2007;19(12):1035–7. doi: 10.1097/MEG.0b013e3282f16449. [DOI] [PubMed] [Google Scholar]
- 50.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Kouroumalis EA. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2007;19(9):789–94. doi: 10.1097/MEG.0b013e3282202bca. [DOI] [PubMed] [Google Scholar]
- 51.Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13(2):279–84. doi: 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
- 52.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27(7):1699–705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 53.Yesilbursa D, Serdar A, Heper Y, et al. The effect of orlistat-induced weight loss on interleukin-6 and C-reactive protein levels in obese subjects. Acta Cardiol. 2005;60(3):265–9. doi: 10.2143/AC.60.3.2005002. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 55.Fiorino G, Peyrin-Biroulet L, Repici A, Malesci A, Danese S. Adalimumab in ulcerative colitis: hypes and hopes. Expert Opin Biol Ther. 11(1):109–16. doi: 10.1517/14712598.2011.541435. [DOI] [PubMed] [Google Scholar]
- 56.Magro F, Portela F. Management of Inflammatory Bowel Disease with Infliximab and Other Anti-Tumor Necrosis Factor Alpha Therapies. BioDrugs. 24:3–14. doi: 10.2165/11586290-000000000-00000. 10.2165/11586290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: Beyond the adipocyte. Growth Hormone & IGF Research. 21(3):113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenore A, Berman WF, Parks JS, Bongiovanni AM. Basal and Stimulated Serum Growth Hormone Concentrations in Inflammatory Bowel Disease. Journal of Clinical Endocrinology & Metabolism. 1977;44(4):622–628. doi: 10.1210/jcem-44-4-622. [DOI] [PubMed] [Google Scholar]
- 60.Krishnan K, Arnone B, Buchman A. Intestinal growth factors: Potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflammatory Bowel Diseases. 17(1):410–422. doi: 10.1002/ibd.21316. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed SF, Wong JS, McGrogan P. Improving growth in children with inflammatory bowel disease. Horm Res. 2007;68(Suppl 5):117–21. doi: 10.1159/000110604. [DOI] [PubMed] [Google Scholar]
- 62.Williams KL, Fuller C Randall, Dieleman LA, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120(4):925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 63.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007 doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 64.Karagiannides I, Pothoulakis C. Neuropeptides, mesenteric fat, and intestinal inflammation. Ann N Y Acad Sci. 2008;1144:127–35. doi: 10.1196/annals.1418.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karagiannides I, Kokkotou E, Tansky M, et al. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci U S A. 2006;103(13):5207–12. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koon HW, Kim YS, Xu H, et al. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc Natl Acad Sci U S A. 2009;106(21):8766–71. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92(1):3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 68.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2(4):239–54. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 69.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25(2):125–36. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 70.Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci. 1998;111(Pt 17):2587–94. doi: 10.1242/jcs.111.17.2587. [DOI] [PubMed] [Google Scholar]
- 71.De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27(12):877–86. doi: 10.1023/a:1006996922657. [DOI] [PubMed] [Google Scholar]
- 72.Cui J, Zaror-Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol. 1990;259(2 Pt 2):R324–32. doi: 10.1152/ajpregu.1990.259.2.R324. [DOI] [PubMed] [Google Scholar]
- 73.Gross K, Karagiannides I, Thomou T, et al. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1012–9. doi: 10.1152/ajpgi.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 75.Harman-Boehm I, Bluher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92(6):2240–7. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 76.Bertin B, Desreumaux P, Dubuquoy L. Obesity, visceral fat and Crohn’s disease. Curr Opin Clin Nutr Metab Care. 2010;13(5):574–80. doi: 10.1097/MCO.0b013e32833cf0f4. [DOI] [PubMed] [Google Scholar]
- 77.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147(2):173–80. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 78.Zulian A, Cancello R, Micheletto G, et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- 79.Behr MA. The path to Crohn’s disease: is mucosal pathology a secondary event? Inflamm Bowel Dis. 2010;16(5):896–902. doi: 10.1002/ibd.21171. [DOI] [PubMed] [Google Scholar]
- 80.Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23(6):661–6. doi: 10.1097/MOG.0b013e3282c8c8d3. [DOI] [PubMed] [Google Scholar]
- 81.Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206(9):1883–97. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor Î3. Current Opinion in Genetics & Development. 1995;5(5):571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 83.Schaffler A, Herfarth H. Creeping fat in Crohn’s disease: travelling in a creeper lane of research? Gut. 2005;54(6):742–4. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56(4):577–83. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA. 1984;251(1):73–9. doi: 10.1001/jama.251.1.73. [DOI] [PubMed] [Google Scholar]
- 86.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292(1):E298–307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 87.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 88.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–9. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148(5):535–42. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]