Abstract
It has been demonstrated in various animal models that the oral administration of green tea (GT) extracts in drinking water can inhibit tumor growth, but the effects of brewed GT on factors promoting tumor growth, including oxidant damage of DNA and protein, angiogenesis, and DNA methylation, have not been tested in an animal model. To explore these potential mechanisms, brewed GT was administered instead of drinking water to male severe combined immunodeficiency (SCID) mice with androgen-dependent human LAPC4 prostate cancer cell subcutaneous xenografts. Tumor volume was decreased significantly in mice consuming GT, and tumor size was significantly correlated with GT polyphenol (GTP) content in tumor tissue. There was a significant reduction in hypoxia-inducible factor 1-alpha and vascular endothelial growth factor protein expression. GT consumption significantly reduced oxidative DNA and protein damage in tumor tissue as determined by 8-hydroxydeoxyguanosine/deoxyguanosine ratio and protein carbonyl assay, respectively. Methylation is known to inhibit antioxidative enzymes such as glutathione S-transferase pi (GSTp1) to permit reactive oxygen species promotion of tumor growth. GT inhibited tumor 5-cytosine DNA methyltransferase 1 (DNMT1) mRNA and protein expression significantly, which may contribute to the inhibition of tumor growth by reactivation of antioxidative enzymes. This study advances our understanding of tumor growth inhibition by brewed GT in an animal model by demonstrating tissue localization of GTPs in correlation with inhibition of tumor growth. Our results suggest that the inhibition of tumor growth is due to GTP-mediated inhibition of oxidative stress and angiogenesis in the LAPC4 xenograft prostate tumor in SCID mice.
Keywords: Green tea, LAPC4 prostate xenograft tumor, oxidation, angiogenesis, methyltransferases, macrophage invasion
Introduction
Numerous studies in cell culture and in animal models demonstrate that either green tea extract (GTE) or purified (-)-epigallocatechin gallate (EGCG) (1–3) can inhibit tumor cell proliferation and xenograft tumor growth. Meta-analyses of epidemiological studies demonstrate a small but significant reduction in the risk of breast, lung, and stomach cancer in individuals consuming brewed green tea (4–6). Consumption of 600 mg/day of a GTE by men with high-grade prostate intraepithelial neoplasia (PIN) significantly delayed the progression of PIN to prostate cancer (CaP) (7). The active phytochemicals in GT are the green tea polyphenols (GTPs), also known as flavan-3-ols, including (-)-epigallocatechin (EGC), EGCG, (-)-epicatechin (EC), and (-)-epicatechin-3-gallate (ECG). While EGCG is the most active and abundant polyphenol, we have previously demonstrated that natural products exert their beneficial effects based on the sum of the multiple mixed components (8). GTPs can exhibit antioxidant as well as pro-oxidant activity in cell culture. The antioxidant activity of GTPs derive from their direct radical scavenging activity via electron transfer from hydroxyl groups in the polyphenol ring and indirectly through activation of the nuclear antioxidant response element (ARE) via the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription factor (9;10). Pro-oxidant activity in vitro results from the auto-oxidation and dimerization of EGCG and EGC to form homo- and hetero-dimers in an alkaline environment with concurrent formation of hydrogen peroxide (H2O2) (11). Mitochondrial respiratory chain metabolism and a number of enzymatic reactions including those involving NAD(P)H oxidases, xanthine oxidase, myeloperoxidase, cyclooxygenase and lipoxygenase can serve as endogenous sources of reactive oxygen species (ROS) (12;13). Macrophage infiltration in CaP has been identified universally in prostatectomy tissue (14). In animal models, macrophage infiltration has been demonstrated in orthotopically transplanted human prostate tumors (13). Inflammatory macrophages release ROS, cytokines, chemokines and prostaglandins which can lead to tissue remodeling and angiogenesis (14;15).
Prostate tumors are characterized by a downregulation of key antioxidant enzymes such as glutathione S-transferase pi (GSTp1) and manganese superoxide dismutase (MnSOD) through epigenetic silencing of CpG island hypermethylation (16–18) suggesting that tumor cell proliferation is dependent on a minimal level of ROS.
EGCG has been shown to inhibit 5-cytosine DNA methyltransferase (DNMT1) (19) leading to demethylation of the CpG islands in the promoters and the reactivation of methylation-silenced genes such as p16INK4a, retinoic acid receptor beta, O6-methylguanine methyltransferase, human mutL homolog 1, and GSTp1 (20). Since CaP is commonly associated with hypermethylation and silencing of GSTp1 it is possible that GT at a cellular level may reactivate GSTp1 (21;22) resulting in tumor growth inhibition by reducing the concentration of ROS needed to maintain tumor growth.
Most prior investigations of the mechanisms underlying the cancer preventive activities of GT have utilized either EGCG alone or decaffeinated GTEs highly enriched in EGCG (23). The objective of the present study was to administer brewed GT in drinking water to male severe combined immunodeficiency (SCID) mice bearing a human prostate cancer xenograft (LAPC4) to mimic human tea consumption and to determine the effects on tumor growth, intratumoral GTPs, macrophage infiltration, oxidant stress, angiogenesis, and damage to tumor DNA and protein.
Methods and Materials
Green tea intervention
Green tea was brewed every 3 days by steeping 1 tea bag in 250 mL of boiling water (pH 3) for 5 minutes. Tea bags (Authentic Green Tea) were generously provided by Celestial Seasonings (Boulder, CO). The stability of GTP was tested and was stable for 3 days. The GTP composition of the brewed green tea in mg/L was as follows: EGC 202 ± 11, EGCG 397 ± 28, EC 52 ± 5, ECG 62 ± 5 and catechin 8 ± 2.
Animal studies
All procedures carried out in mice were approved by the UCLA Animal Research Committee. Male SCID mice (Charles River Laboratories) were bred in a pathogen-free colony (24), where they were housed in groups of five per cage and fed a sterilized AIN-93G diet (DYETS, Inc., Bethlehem, PA) and water. The brewed GT was provided in the same manner as drinking water. Androgen-dependent LAPC4 prostate cancer cells mixed in 0.1 mL of matrigel (5 × 105 LAPC4 cells per animal; gift from Dr. Charles Sawyers) were implanted subcutaneously into the flank of 5 week-old SCID mice. A small preliminary experiment (#1) was performed to determine oxidative DNA damage in LAPC4 xenograft tumors in mice drinking water ad lib. Control mice were left intact and intervention mice were surgically castrated when tumors were palpable (about 4 weeks after inoculation) (n=5). When tumors reached a volume of 1 cm3 mice were sacrificed and tumors and the prostate gland (all 4 lobes) were removed and frozen for later oxidative DNA damage determination. In experiment 2, to test the chemopreventive effect of brewed green tea, GT was administered in drinking water for 13 weeks (n=5 per group) orally 7 days per week starting two weeks after LAPC4 tumor cell inoculation. Tumor size was measured with calipers three times a week starting at day 7. Tumor volume was calculated using the formula: length × width × height × 0.5236 (25). Mice in the control group were sacrificed after 8 weeks post inoculation and mice in the tea group 13 weeks after the inoculation to obtain serum samples, liver, lung, kidney and tumor tissue. Tumors were excised and rinsed in cold phosphate buffered saline (PBS). A 2 mm slice was cut in longitudinal direction from the center of the tumor and transferred into a tissue cassette, fixed in 10% neutral buffered formalin for 12h and transferred to 70% alcohol for immunohistochemistry (26). The remaining tumor tissue was cut into 6–8 sections, carefully labeled according to the location in the tumor (outside, inside, center) and immediately frozen in liquid nitrogen together with other tissues for further analyses. The GT intervention study using brewed GT was performed twice with n=5 per group in each experiment. The outcomes were very similar in both experiments.
Immunohistochemistry of microvessel density and macrophage presence
Paraffin-embedded sections were cut at 4 µm thickness and paraffin removed with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval (HIER), 95°C for 25 minutes (microvessel density) and proteolytic induced epitope retrieval (PIER) were carried out with proteinase K (Dako, S3020, Carpinteria, CA) at 37°C for 10 min (F4/80). To quantify microvessel density goat polyclonal Pecam-1 (CD31) (Santa Cruz, sc-1506, Santa Cruz, CA) primary antibody at 1:200 dilution for 1 hr and for F4/80 primary antibody (Serotec MCA497b, Raleigh, NC) 1:50 dilution overnight at 4°C, followed by 30 min with secondary polyclonal rabbit anti-goat immunoglobulins/biotinylated (Dako, E0466, Carpinteria, CA) 1:200 was used. The signal was detected using the mouse DAKO horseradish peroxidase EnVision kit (DAKO) and anti rabbit HRP polymer and visualized with the diaminobenzidine reaction. The sections were counterstained with hematoxylin. Number of vessels was manually counted in 20 fields at 400× magnification using the Ariol SL-50 automated slide scanner (Applied Imaging, San Jose, CA) at the Translational Pathology Core Laboratory, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA. The presence of F4/80-stained macrophages was assessed by semiquantitative scoring of staining intensity of the cells grouped into five grades: 0, lack of expression, 1+, low expression in less than 10% of cells, 2+, low to moderate expression in 11 to 30% and 3+ moderate to strong staining 31% to 50% and 4+ strong expression in 50% or more.
Western blot analysis of protein expression in mouse tumors
50 µg of protein was used for the analysis of HIF1-α, VEGF and DNMT1. Protein was separated on a 4–20% Tris-HCl gel. Separated proteins were electrotransferred to nitrocellulose membranes and blocked in Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk for 1 hour at room temperature. Membranes were incubated with primary antibody against HIF1-α (sc-10790), VEGF (sc-152) or DNMT1 (sc-20701, Santa Cruz) at a dilution of 1:100 overnight at 4°C. Protein was visualized and analyzed using a ChemiDoc XRS (Bio-Rad Laboratories) chemiluminescent detection and imaging system. After stripping the membrane, monoclonal antibody to β-actin or GAPDH was applied as loading control.
Oxidative DNA and protein damage
DNA was extracted from 80–100 mg of tumor tissue by the phenol/chloroform/isoamyl alcohol extraction method described by Hofer et al. (27). 100 µg of DNA was digested according to Huang et al. (28) and the concentrations of 8-hydroxydeoxyguanosine (8-oxodG) and total deoxyguanosine (dG) were determined by high performance liquid chromatography (HPLC) using coulochem II electrochemical detection (ESA, Chelmsford, MA) as described previously (29). Protein oxidation was determined by concentration of protein carbonyl using the Cayman protein carbonyl assay kit (Cayman Chemical Company, Ann Harbor, MI) according to manufacturer’s instructions.
Tea polyphenol analysis
150 mg of the tumor tissue was homogenized in 200 µL of 2% ascorbic acid solution on ice and transferred to 2 mL tubes. The homogenate was incubated with 1,000 units of β-glucuronidase (G7896, Sigma Chemicals, St Louis, MO) and 40 units of sulfatase (S-9754, Sigma Chemicals) buffered in 300 µL of 0.5 M phosphate buffer (pH 5.0) at 37°C for 45min to digest the conjugated forms into their free forms. After incubation, 4x extracts with 1ml of ethyl acetate were combined with 20 µL of 2% ascorbic acid in methanol and dried in vacuum and reconstituted for HPLC-CoulArray detection (ESA, Chelmsford, MA) (24). The compounds were separated on an Alltima C18 reverse-phase column (3µm, 53 × 7mm) (Alltech Associates Inc, Deerfield, IL) as described by Henning et al (24). The 8 channels of the CoulArray detector (ESA, Chelmsford, MA) were sequentially set at −60 mV, 20 mV, 100 mV, 180 mV, 260 mV, 340 mV, 420 mV, and 500 mV potentials.
Quantitative real-time PCR of DNMT1
PCR primers and fluorogenic probes were provided by TaqMan(R) Gene Expression Assay kit (ID: Hs00154749_m1 for DNMT1) (Applied Biosystems, Foster City, CA). The final volume of 20 µL PCR mixture contained 2 µL of cDNA template, 1 µL of 20x primer and probe mixture, 10 µL of 2x TaqMan Universal PCR MasterMix (Applied Biosystems), and 7 µL of nuclease-free water. PCR amplification was performed by a 7900HT Fast Real-Time System (Applied Biosystems) with the following thermal cycling conditions: 50°C for 2 minutes, followed by 95°C for 10 minutes, and 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. Each sample was in triplicate. In addition, each run included no-template negative controls. The 2−(ΔΔCt) method was used to normalize the expression of DNMT1 in each sample to GAPDH expression and to compare to the average ΔCt value.
Statistical Methods
SPSS (Version 17.0, Chicago, IL) and Graph Pad Prism 4.0 (Graph Pad Software Inc., San Diego, CA) were used for statistical analyses. Mean value, median, and standard deviation (SD) were calculated using descriptive statistics. Comparison of means was performed by two independent samples t-test. Differences were considered significant if P < 0.05.
Results
Tumor volume, tissue GTP content and GTP metabolism
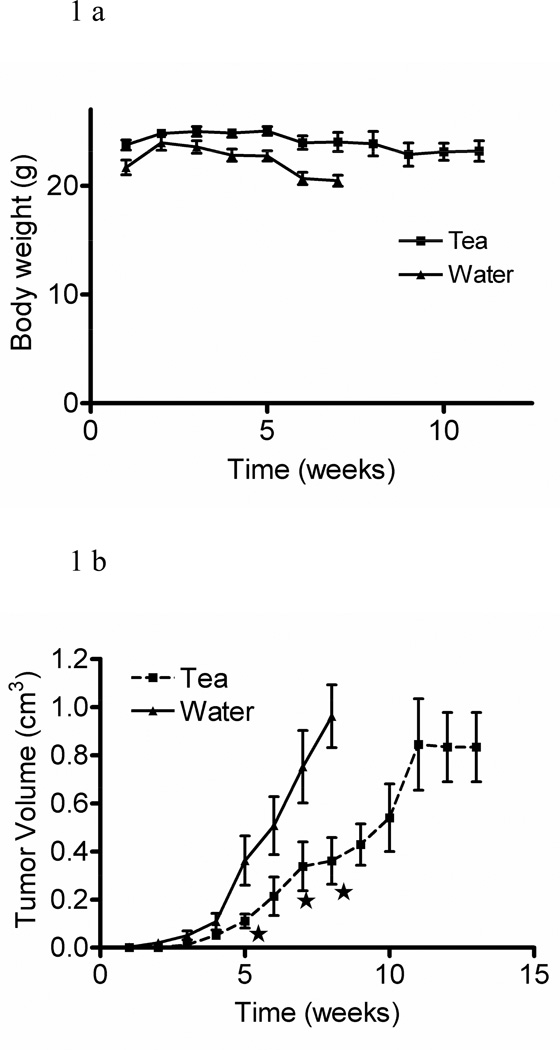
GT prepared from commercially available tea bags contained a mixture of GTPs as found in GT leaves. To evaluate whether brewed GT, as consumed in the general population, inhibits prostate tumor growth in SCID mice implanted with LAPC4 human androgen-dependent prostate tumor cells, we replaced drinking water with brewed GT. There was no difference in body weight between GT-consuming and control mice (Figure 1a). After 5 weeks tumor volume was inhibited significantly in GT-consuming mice. After 11 weeks tumor growth reached a plateau (Figure 1b).
Fig. 1.
Body weight (a) and tumor volume (b) of SCID mice inoculated with LAPC4 prostate tumor cells and treated with brewed green tea or water (means ± SD, n = 5, *p ≤ 0.05).
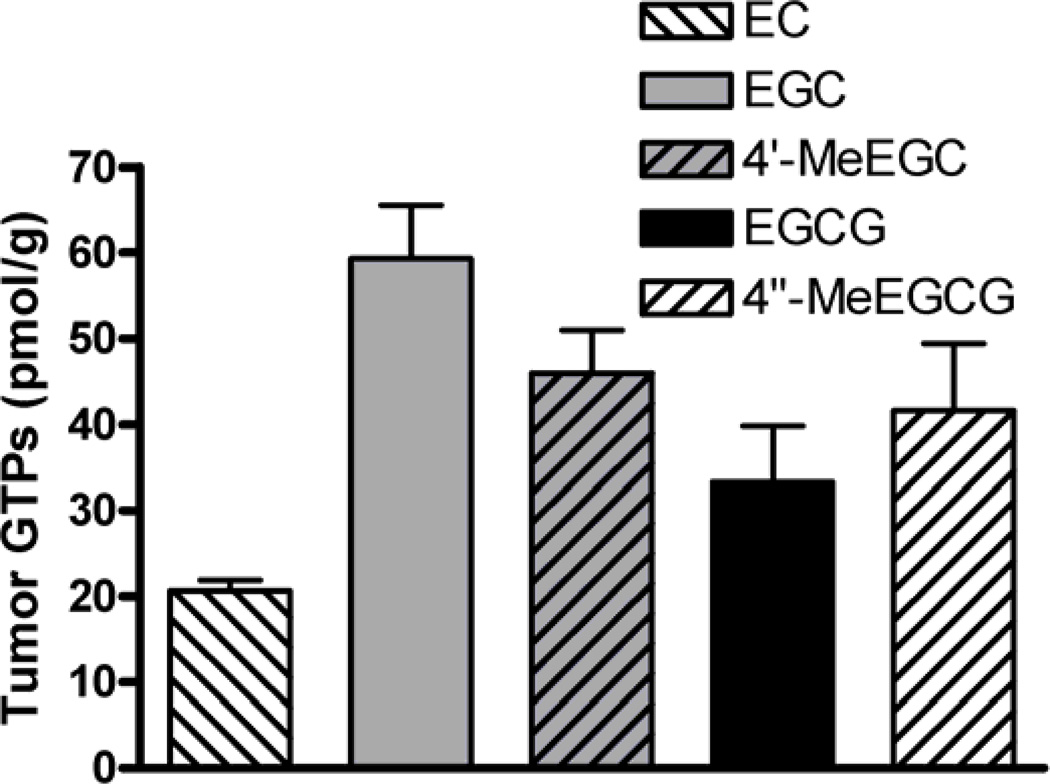
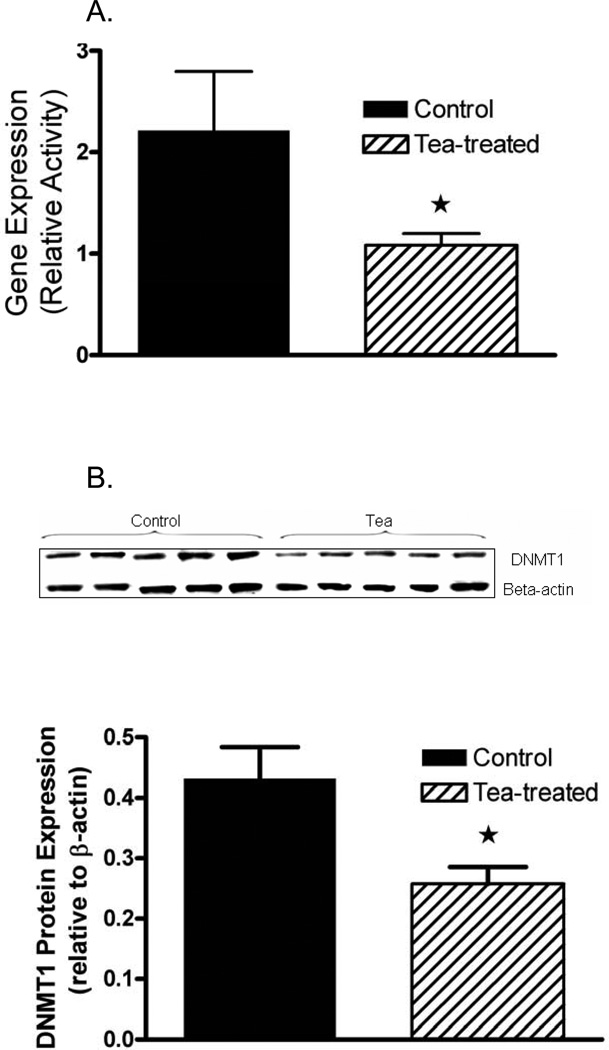
EGCG, 4″-O-methyl-EGCG (4″-MeEGCG), EGC, 4′-O-methyl-EGC (4′-MeEGC) and EC were found in the tumor tissue of mice consuming GT (Figure 2). The identity of the 4″-MeEGCG and 4′-MeEGC standards was confirmed by LC-MS/MS analysis and excellent correlation with the HPLC analysis with coularray electrochemical detection was established. EC, EGC and 4′-MeEGC were found mostly in the conjugated (glucuronidated + sulfated) form (86 ± 4, 75 ± 4 and 83 ± 4% of total, respectively). EGCG and 4″-MeEGCG were present in the free form at 68 ± 5 and 50 ± 5% of total, respectively. The total concentration of GTPs and methyl-metabolites was significantly correlated to tumor volume (r= 0.94, p<0.007). The administration of brewed GT inhibited the gene and protein expression of DNMT1 significantly 55 %, and 40 % respectively (Figure 3a,b).
Fig. 2.
Concentration of total (free + conjugated) GTPs and their methyl-metabolites in tumor tissue of mice treated with green tea (means ± SD, n = 5, *p ≤ 0.05).
Fig. 3.
(a) Gene expression of DNMT1 and (b) protein expression of DNMT1 in tumor tissue of mice treated with tea or water determined by quantitative real time PCR (means ± SD, n = 5, *p ≤ 0.05).
GT effects on oxidative DNA and protein damage and macrophage presence
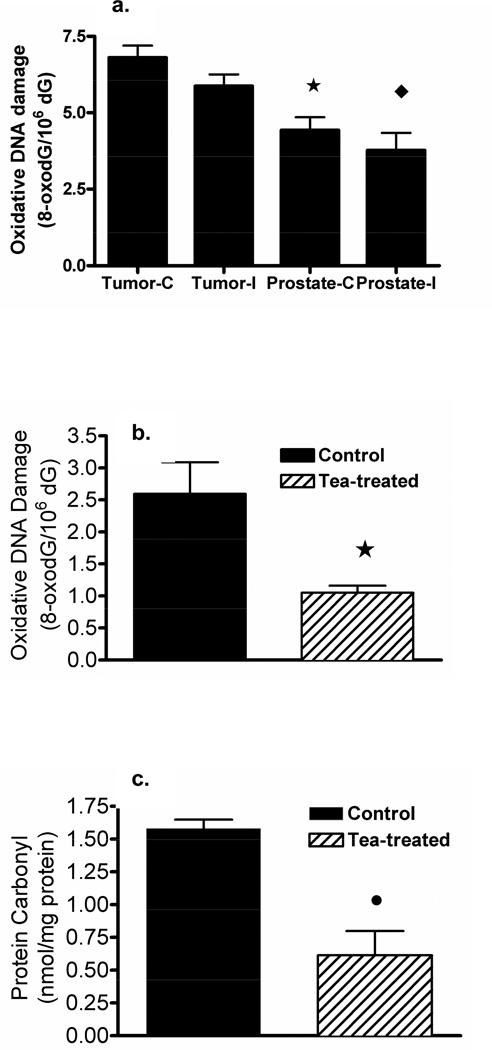
In a preliminary study (experiment 1) we compared the level of oxidative DNA damage in xenograft tumor tissue to prostate gland tissue including prostate tissues from intact mice and from castrated mice (Figure 4a). In this experiment we established that the ratio of 8-oxo-dG to dG was significantly increased in LAPC4 xenograft tumor tissue compared to normal prostate tissue. There was no significant difference in oxidative DNA damage in normal tissues from castrated mice compared to intact mice. In xenograft tumors from SCID mice consuming brewed GT, the ratio of 8-oxo-dG to dG was significantly decreased by 60 percent compared to tumors from control tumor-bearing mice consuming plain water (Figure 4b). In addition, we demonstrated that the concentration of carbonyl-protein as an indicator of oxidized protein was significantly decreased by 60 percent in GT drinking mice compared to the tumor-bearing controls consuming water alone (Figure 4c).
Fig. 4.
Oxidative DNA damage (a) in xenograft LAPC4 tumor or normal prostate tissue in intact (I) or castrated (C) mice; significant difference between tumor and prostate in intact (♦) and castrated mice (*); (b) oxidative damage in tumor tissue and (c) oxidative protein damage in tumor tissue of mice treated with green tea or water (means ± SD, n = 5, *p ≤ 0.05).
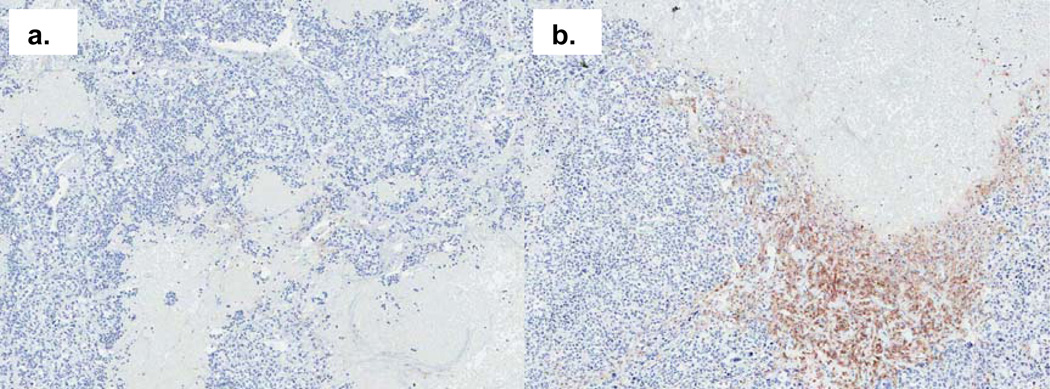
F4/80 staining of tumor sections also indicated the presence of macrophages. Macrophages were distributed throughout the tumor section in an irregular manner, mainly accumulating in areas bordering the invasive zone. Figure 6 (a) demonstrates an example of a tumor from a tea-treated mouse with reduced macrophage infiltration. However, overall there was no significant difference in F4/80 positive macrophage marker staining demonstrated in tumors from tea-treated and control mice.
Fig. 6.
Macrophage presence in tumor tissue of mice treated with green tea (a) or water (b) by F4/80 immunohistochemistry staining (×50 magnification).
GT effects on markers of angiogenesis
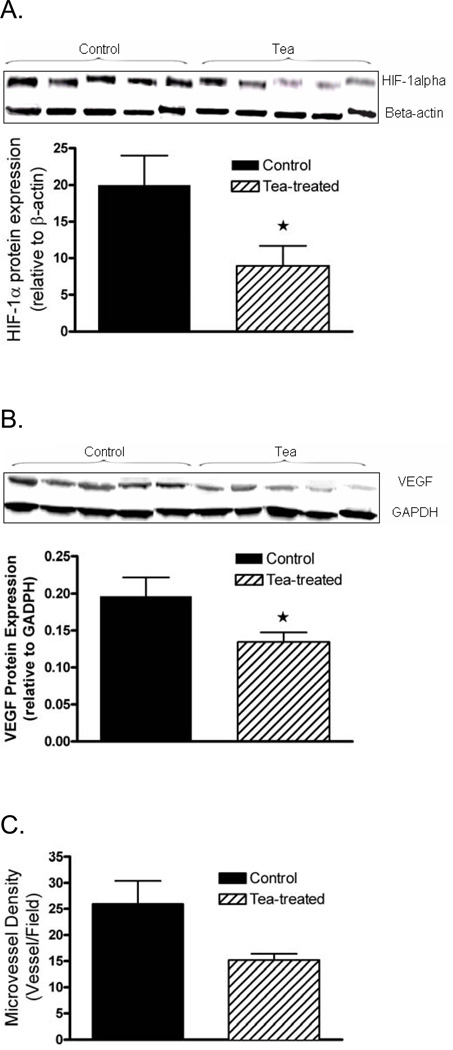
In addition to the antioxidant effects, we determined the effect of consumption of brewed GT on markers of angiogenesis. Protein expression of HIF-1α and VEGF in tumor tissue was significantly decreased by 60% and 35% in mice drinking brewed GT (Figure 5a, b). Vessel density showed a nearly significant trend to decrease by 42% from 26±8.9 to 15.2±2.5 microvessels per field (p = 0.058) (Figure 5c).
Fig. 5.
Protein expression of (a) HIF-1α and (b) VEGF in tumor tissue of mice treated with green tea or water by Western blot and (c) microvessel density by immunohistochemistry (means ± SD, n = 5, *p ≤0.05).
Discussion
Polyphenolic botanical extracts such as GT exert their effects on tumor growth through multiple concurrent direct and indirect mechanisms captured under the term of antioxidant effects (30). In this study, we demonstrated that brewed GT inhibited markers of oxidative damage in the prostate cancer xenograft tumor tissue. In addition many effects are beyond those that can be characterized as simply antioxidant effects. Pathways involved in antioxidant defense, redox-status, inflammation and methylation are closely interrelated and interact with the multi-step process of carcinogenesis (31).
Angiogenesis is a component of the inflammatory pathway involved in tumor promotion and tumors cannot grow without stimulating new blood vessel formation (32). New vessel formation is triggered by hypoxia via stimulation of VEGF and the HIF-1α pathways (33). We demonstrated a significant decrease in tumor tissue angiogenesis-related HIF-1α and VEGF protein expression and an almost significant trend (p=0.058) of decrease in microvessel density. A possible mechanism of HIF-1α inhibition has been described in PC-3 prostate cancer cells where 20–40 µmol of EGCG inhibited the prolyl hydroxylation of HIF-1α, thus preventing the interaction of HIF-1α with von Hippel–Lindau protein (pVHL) necessary for angiogenesis stimulation (34). The inhibition of VEGF protein expression has been demonstrated before by other investigators in a xenograft prostate cancer model and TRAMP mice treated with a GTE enriched for EGCG (35;36). However, our results are unique to demonstrate the inhibition of these angiogenesis factors in mice consuming brewed GT instead of drinking water.
The xenograft model is not a perfect representation of human prostate cancer. In SCID mice inflammatory processes, driven by mechanisms inherent to the host-tumor immune reaction, may be preserved but may be non-responsive to GT treatment. Although the GT intervention did not affect macrophage density in the present study, this does not rule out an inhibitory effect in human prostate tissue where the variety of cells infiltrating may be different, including different subtypes of macrophages (37). Tumor-associated macrophages also have the capacity to generate ROS through NADPH oxidase (Nox) activity and stimulate angiogenesis (38;39). Decreasing the macrophage infiltration or modulating the tumor-associated macrophage subpopulations could be another potential mechanism through which GT could lower oxidative stress and inhibit angiogenesis in tumor tissues indirectly. This area clearly deserves more investigation, but the design of experiments using the xenograft model will be critical in advancing our understanding of intratumoral immune effects of GT.
The antioxidant activity of GTPs in human and animal studies has been reviewed extensively (40;41) and there is evidence from human intervention studies that brewed GT and GT extract supplements exert an antioxidant activity (24;42;43). In the present study, we observed a significant inhibition of oxidative DNA and protein damage in tumor tissue from mice exposed to brewed GT. While it is possible that some of these effects were the result of a direct chemical antioxidant activity of GTPs via electron transfer and radical scavenging of metal ions (44), the low plasma and tissue concentrations of GTPs and the presence of many other stable antioxidant defense systems in the tissues make this unlikely (45). In our view it is more likely that GTPs act through upregulation of antioxidant defense mechanisms via activation of the Nrf-2 axis (10) and reactivation of silenced antioxidant enzyme genes through a decrease in DNA methylation (20). Stage-specific alterations of DNA methyltransferase expression, gene specific DNA hypermethylation and also global DNA hypomethylation have been defined in different stages of prostate carcinogenesis in transgenic (TRAMP) mice (46). Recently published work by Pandey et al. showed that treatment with GTPs in vitro caused promoter demethylation and chromatin remodeling leading to expression of GSTP1 in human prostate cancer cells, which had been suppressed (47).
Our results demonstrate for the first time in vivo that the consumption of brewed GT is associated with significant inhibition of gene and protein expression of DNMT1 in xenograft tumors. A study by Kinney et al. in transgenic TRAMP mice did not demonstrate a decrease in DNA methylation (48), but also failed to demonstrate inhibition of tumor progression in TRAMP mice. Therefore, there is no conflict in the findings from the above transgenic study and our findings. Nonetheless, further studies are needed to investigate whether the inhibition of DNMT1 is sufficient to reverse gene specific DNA hypermethylation in human prostate tissues following GT administration.
A related effect of decreasing the methylation of GTPs is the increase in antiproliferative activity. In previous work we established that methylation of EGCG was associated with a decrease of antiproliferative activity, decreased inhibition of NF-κB activation and decreased apoptosis (49). In the present study 56 ± 6% of EGCG and 45 ± 5% of EGC were found in methylated form, which may have significantly limited their potential to inhibit tumor growth. Similar amounts of methyl-EGCG were found in prostate tissue as part of an ongoing phase II human intervention study in men consuming green tea prior to prostatectomy (49). We are currently investigating the possibility that combinations of agents that inhibit methylation might enhance the chemopreventive activity of green tea in prostate carcinogenesis.
In summary, our study has shed light on potential mechanisms through which GTPs from brewed green tea as opposed to purified or concentrated EGCG might affect prostate carcinogenesis. In this regard, our research attempts to recapitulate in animal models and human studies the effects observed in epidemiological studies. It is hoped that this translational research will lead to new approaches to prostate cancer prevention.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health [RO1 CA116242, P50 CA92131] and [RO3 CA150047-01A1]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang CS, Ju J, Lu G, et al. Cancer prevention by tea and tea polyphenols. Asia Pac J Clin Nutr. 2008;(17 Suppl 1):245–248. [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui IA, Afaq F, Adhami VM, Ahmad N, Mukhtar H. Antioxidants of the beverage tea in promotion of human health. Antioxid Redox Signal. 2004;6:571–582. doi: 10.1089/152308604773934323. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–484. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- 5.Tang N, Wu Y, Zhou B, Wang B, Yu R. Green tea, black tea consumption and risk of lung cancer: A meta-analysis. Lung Cancer. 2009;65:274–283. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Myung SK, Bae WK, Oh SM, et al. Green tea consumption and risk of stomach cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2009;124:670–677. doi: 10.1002/ijc.23880. [DOI] [PubMed] [Google Scholar]
- 7.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 8.Sartippour MR, Heber D, Ma J, Lu Q, Go VL, Nguyen M. Green tea and its catechins inhibit breast cancer xenografts. Nutrition and Cancer. 2001;40:149–156. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Neilson AP, Hopf AS, Cooper BR, Pereira MA, Bomser JA, Ferruzzi MG. Catechin Degradation with Concurrent Formation of Homo- and Heterocatechin Dimers during in Vitro Digestion. J Agric Food Chem. 2007;55:8941–8949. doi: 10.1021/jf071645m. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Halin S, Rudolfsson SH, Van RN, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonomura N, Takayama H, Nakayama M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107(12):1918–1922. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 15.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 16.Nelson WG, Yegnasubramanian S, Agoston AT, et al. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–4266. doi: 10.2741/2385. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Khor TO, Cheung KL, et al. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitchler MJ, Wikainapakul K, Yu L, Powers K, Attatippaholkun W, Domann FE. Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics. 2006;1:163–171. doi: 10.4161/epi.1.4.3401. [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69:1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 21.Yegnasubramanian S, Kowalski J, Gonzalgo ML, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Gonzalgo ML, Yegnasubramanian S, Lin X, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer. J Cell Biochem. 2004;91:540–552. doi: 10.1002/jcb.10740. [DOI] [PubMed] [Google Scholar]
- 23.Adhami VM, Siddiqui IA, Sarfaraz S, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res. 2009;15:1947–1953. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henning SM, Niu Y, Lee NH, et al. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 25.Seeram NP, Aronson WJ, Zhang Y, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 26.Sartippour MR, Seeram NP, Rao JY, et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int J Oncol. 2008;32:475–480. [PubMed] [Google Scholar]
- 27.Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Powell J, Mooney LA, Li C, Frenkel K. Importance of complete DNA digestion in minimizing variability of 8-oxo- dG analyses. Free Radic Biol Med. 2001;31:1341–1351. doi: 10.1016/s0891-5849(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 29.Gao K, Henning SM, Niu Y, et al. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. J Nutr Biochem. 2005;17:89–95. doi: 10.1016/j.jnutbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 31.Hitchler MJ, Domann FE. Metabolic defects provide a spark for the epigenetic switch in cancer. Free Radic Biol Med. 2009;47:115–127. doi: 10.1016/j.freeradbiomed.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boddy JL, Fox SB, Han C, et al. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clin Cancer Res. 2005;11:7658–7663. doi: 10.1158/1078-0432.CCR-05-0460. [DOI] [PubMed] [Google Scholar]
- 34.Thomas R, Kim MH. Epigallocatechin gallate inhibits HIF-1alpha degradation in prostate cancer cells. Biochem Biophys Res Commun. 2005;334:543–548. doi: 10.1016/j.bbrc.2005.06.114. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui IA, Zaman N, Aziz MH, et al. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 36.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 37.Mizutani K, Sud S, McGregor NA, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li HL, Huang Y, Zhang CN, et al. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Burckhardt IC, Gozal D, Dayyat E, et al. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med. 2008;177:1135–1141. doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr. 2003;133:3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- 41.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 42.Hakim IA, Harris RB, Chow HH, Dean M, Brown S, Ali IU. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: role of glutathione S-transferase genotypes. Cancer Epidemiol Biomarkers Prev. 2004;13:242–249. doi: 10.1158/1055-9965.epi-03-0193. [DOI] [PubMed] [Google Scholar]
- 43.Luo H, Tang L, Tang M, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 44.Rice-Evans C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc Soc Exp Biol Med. 1999;220:262–266. doi: 10.1046/j.1525-1373.1999.d01-45.x. [DOI] [PubMed] [Google Scholar]
- 45.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 46.Morey K, Sr, Smiraglia DJ, James SR, Moser MT, Foster BA, Karpf AR. Stage-specific alterations of DNA methyltransferase expression, DNA hypermethylation, and DNA hypomethylation during prostate cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Res. 2008;6:1365–1374. doi: 10.1158/1541-7786.MCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–2533. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morey K, Sr, Zhang W, Pascual M, et al. Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate. Cancer Prev Res (Phila Pa) 2009;2:1065–1075. doi: 10.1158/1940-6207.CAPR-09-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang P, Aronson WJ, Huang M, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3:985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.