Abstract
Background
Severe sepsis is associated with persistent high-levels of morbidity among older survivors. But the impact of severe sepsis on population health—particularly population levels of disability—is unknown.
Objectives
Ascertain the absolute number of patients surviving at least 3 years after severe sepsis in Medicare, and estimate their burden of cognitive dysfunction and disability.
Design
Retrospective cohort analysis of Medicare data.
Setting
All short-stay inpatient hospitals in the United States, 1996–2008.
Participants
Patients aged 65 and older.
Measurements
Severe sepsis was detected using a standard administrative definition. Case-fatality, prevalence and incidence rates were calculated.
Results
There were 637,867 Medicare patients alive at the end of 2008 who survived severe sepsis 3 or more years earlier. An estimated 476,862 (95% CI: 455,026, 498,698) had functional disability, with 106,311 (95% CI: 79,692, 133,930) survivors having moderate-to-severe cognitive impairment. The annual number of new 3-year survivors following severe sepsis rose 119% during 1998–2008. The increase in survivorship resulted from more new diagnoses of severe sepsis rather than a change in case fatality rates; severe sepsis rates rose from 13.0 per 1,000 Medicare beneficiary-years to 25.8 (p<0.001), whereas 3-year case fatality rates changed much less, from 73.5%to 71.3% (p<0.001) for the same cohort. Increasing rates of organ dysfunction among hospitalized patients drove the increase in severe sepsis incidence, with an additional small contribution from population aging.
Conclusions
Sepsis survivorship, which carries with it substantial long-term morbidity, is a common and rapidly growing public health problem for older Americans. There has been little change in long-term case fatality, despite changes in practice. Clinicians should anticipate more frequent sequelae of severe sepsis in their patient populations.
Keywords: Medicare, severe sepsis, disability, population health
INTRODUCTION
The syndrome of severe sepsis occurs when an acute infection leads to organ dysfunction.1 This syndrome encompasses many common causes of hospitalization, such pneumonia with hypoxemia or urinary tract infection complicated by acute renal failure. Severe sepsis is also a common cause of critical illness, as when the organ dysfunctions include acute respiratory failure or shock. Severe sepsis has been recognized to have a high acute risk of death.2 More recent data demonstrate that survivors of severe sepsis—a majority of those with the diagnosis—have poor quality of life,3,4 frequently develop cognitive and functional disability,5 and require substantial ongoing acute and long-term care.6,7 These levels of disability impose a substantial burden on caregivers.8 Severe sepsis has been termed “a quintessential disease of aged.”9
While we better understand the impact of severe sepsis on individual patients, we know little about the impact of severe sepsis on population health—particularly the impact on population levels of disability. This stands in marked contrast to conditions such as cancer and stroke, where the burdens of survivorship are considered core components of patient management and public health impact.10 Physicians caring for patients who have survived cancer are aware of the special physical and emotional sequelae for which they must maintain an elevated index of suspicion.11,12 No such information exists about whether the population burdens of severe sepsis survivorship warrant consideration in discussions of population health, disability and caregiving needs.
Therefore, we sought to measure the incidence and prevalence of long-term survivorship after severe sepsis in Medicare. Our primary objectives were to measure the absolute size of the population of Medicare patients who survive at least 3-years after severe sepsis and to estimate, based on prior work,5 their likely numbers with cognitive dysfunction and disability. We also examined temporal trends in survivorship, and tested several targeted hypotheses for potential changes in sepsis survivorship over time. Specifically, we hypothesized that changes in sepsis survivorship might have resulted from: the aging of the population; changing age-specific rates of hospitalization with infection; changing rates of acute organ dysfunction among those with infections; or changes in the case fatality rate among those with severe sepsis.
METHODS
Definition of Survivorship
Conceptually, a patient is a “survivor” when the acute burdens of an illness have passed, but the sequelae of that illness and its treatment may now become important to their health and functioning.13 Operationalizing this is typically done by defining a time-point—necessarily somewhat arbitrary—and examining those who are alive at least that long after diagnosis.10 Our primary outcome was survival to 3 years from date of admission, which we term 3-year survivorship; this was based on our clinical experience, data on the period of most elevated risk of mortality after severe sepsis, and for comparability with other reports.14,15
Data Source
In this retrospective cohort study, we analyzed all fee-for-service Medicare beneficiaries aged 65 and above in the 1996–2008 Medicare Provider Analysis and Review (MedPAR) files, and the linked Medicare Denominator Files. Medicare provides insurance for over 96% of older Americans.16 Fewer than 15% of Medicare beneficiaries were in Medicare managed care programs that did not file claims; such patients were excluded from these analyses.
We relied on a previously validated and widely used claims-based definition of severe sepsis.2,6,7,17–21 Only inpatient hospitalizations in short-stay hospitals (as opposed to long-term acute care or skilled nursing facilities) were eligible. This definition requires evidence of both an infection and new-onset organ dysfunction during a single hospitalization, in accordance with the internationally accepted consensus definition of severe sepsis1,22 and in validation studies identifies a similar population of patients to those identified through chart review.23 This definition has prognostic validity, identifying patients with substantial short-term2 and long-term mortality,5 high health care-related spending,6,7 and increased risks of long-term cognitive and physical disability.5 This definition is distinct from other epidemiologic approaches to the study of sepsis (rather than severe sepsis) that use primarily codes for explicit septicemia, bacteremia and disseminated fungal infections, rather than for all infections.24 We used the components of the severe sepsis definition to define hospitalizations with infection as well as organ dysfunctions. A code for “severe sepsis” or “septic shock” was considered evidence of at least one organ dysfunction, as per the consensus definition.
As a secondary outcome, spending was defined as the amount Medicare reimbursed the hospital for the inpatient stay. To provide a point of comparison for the sepsis findings, we also examined the incidence and costs for acute myocardial infarction as a common cause of critical illness of established importance to population health. We defined hospitalizations with AMI as those with a primary International Classification of Diseases, 9th Revision—Clinical Modification (ICD-9-CM) diagnostic code of 410.xx (excluding 410.x2).25,26 We excluded cases with a length of stay of 1 day—unless that patient died, left against medical advice, or was transferred to another hospital—since such a short length of stay likely represented “rule-out” admissions and not true AMI.27
Disability and Cognitive Impairment Definitions
We estimated the burdens of moderate-to-severe cognitive impairment and functional disability using previously published data for patients surviving 3-years after severe sepsis, estimated using the same definition of severe sepsis and drawn from a similar population of Medicare beneficiaries.5 In that study, patients or their proxies were asked if, as a result of a health problem, they required assistance with any of 6 Activities of Daily Living (ADLs: walking, dressing, bathing, eating, getting into and out of bed, and toileting) or 5 Instrumental Activities of Daily Living (IADLs: preparing a hot meal, shopping for groceries, making telephone calls, taking medicines, and managing money). Disability was defined as a limitation in any ADL or IADL that was due to a health problem.
Cognitive impairment was defined as poor performance on the modified Telephone Interview for Cognitive Status(m-TICS) or cognitive problems as reported by a proxy informant, similar to a level of impairment associated with dementia. The m-TICS was 35-point scale that included tests of memory, serial seven subtractions, naming, and orientation, with 0 to 7 on this scale defining moderate-to-severe cognitive impairment.28,29 For patients who were unable to be interviewed themselves, the validated Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)30 was administered to proxies, and a score of 4.59 to 5.00 defined moderate-to-serve cognitive impairment.
Using these definitions in the population of patients alive 3 years after severe sepsis in the Health and Retirement Study,5 it was shown that the prevalence of moderate-to-severe cognitive impairment was 16.7% (95% CI: 12.3%, 21.0%), and 74.8% (95% CI: 71.3%, 78.2%) of survivors had at least one limitation in an activity of daily living or instrumental activity of daily living. This disability includes both that which existed before severe sepsis and those associated with severe sepsis.
Analysis
We calculated our primary outcomes, the incidence and prevalence of severe sepsis survivorship among patients in fee-for-service Medicare.
In order to examine the temporal trends in the primary outcome, we use standard demographic methods to measure the relative contributions when many contributing rates are each changing. The number of survivors of severe sepsis is the product of the number of incident cases of severe sepsis multiplied by one minus the case fatality rate. The number of incident cases of severe sepsis is the product of the number hospitalizations with infection multiplied by the organ failure rate per hospitalization with infection. The number of hospitalizations with infection is the product of the number of people at risk in each age group, multiplied by the age-group-specific infection rate. A worked example of this approach is provided in Appendix 1.
We calculated all rates on a monthly basis. For numerators and denominators, we classified individuals by their age on the first of each month. All months in which individuals were in fee-for-service Medicare were analyzed.
To test the sensitivity of our conclusions to changes in specific coding practices for the organ dysfunctions used in our definition, we replicated all of our analyses, sequentially excluding each organ system. Detailed results are in Appendix 2.
For statistical comparisons, we tested for annual differences in incidence rate ratios using Stata 10.1. A p value of <0.05 was considered statistically significant. Given the large numbers of cases, statistical significance should not be equated with clinical significance. This work was approved by the University of Michigan Institutional Review Board.
RESULTS
In 1996, 34,782,442 Medicare beneficiaries aged 65 and above were examined for a total of 357,662,059 beneficiary-months. Their median age was 73 (IQR: 68 to 79); 59% were female. In 2008, 39,337,348 Medicare beneficiaries were examined for a total of 350,267,105 beneficiary-months in 2008. Their median age was 73 (IQR: 68 to 80); 57% were female.
Absolute Number of Survivors and Estimated Population-Level Burden of Disability
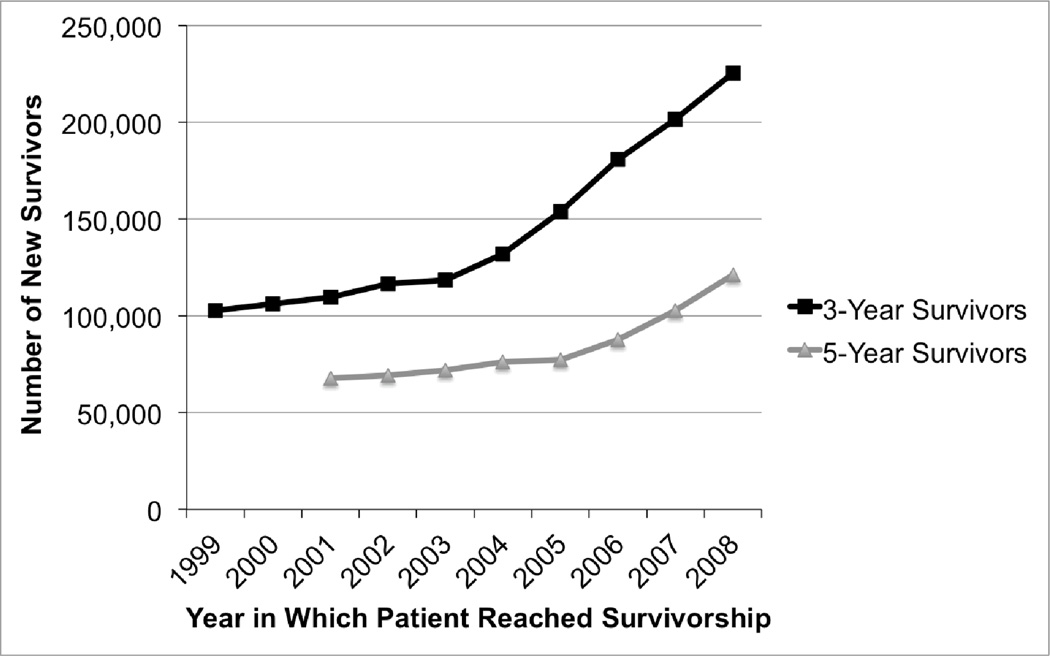
By our definition, the incidence of 3-year survivorship in a given year is the number of patients who were still alive exactly 3 years after severe sepsis in Medicare. Using this definition there were 225,251 new 3-year survivors in Medicare in 2008 (who were hospitalized for severe sepsis in 2005), up from 102,767 new 3-year survivors in 1999 (who were hospitalized in 1996), an increase of 119% in a decade. (Figure 1) Repeating the analysis for 5-year survival, the numbers of new survivors rose from 67,799 in 2001 (who were hospitalized in 1996) to 121,029 in 2008, an increase of 79%.
Figure 1.
New 3-Year and 5-Year Survivors After Severe Sepsis. Figure 4 shows the relative contributions of increasing rates of organ dysfunction per hospitalization with infection, increasing rates of hospitalization with infection, population aging and changes in the 3-year case fatality to these trends.
By our definition, the prevalence of 3-year severe sepsis survivorship in a given year in Medicare is the number of patients who were hospitalized for severe sepsis at least 3 years earlier and were still alive. Using this definition, there were 637,867 patients who had survived severe sepsis by at least 3 years, as of the end of 2008. (There were 344,111 who had survived by at least 5 years). This 3-year survivorship number implies that there were at least 106,311 (95% CI: 79,692, 133,930) survivors with moderate-to-severe cognitive impairment at the end of 2008 in Medicare. In that same population were 476,862 (95% CI: 455,026, 498,698) survivors with functional disability, requiring assistance with at least 1 activity of daily living (ADL) or instrumental activity of daily living (IADL).
Changes in Number of Cases of Severe Sepsis
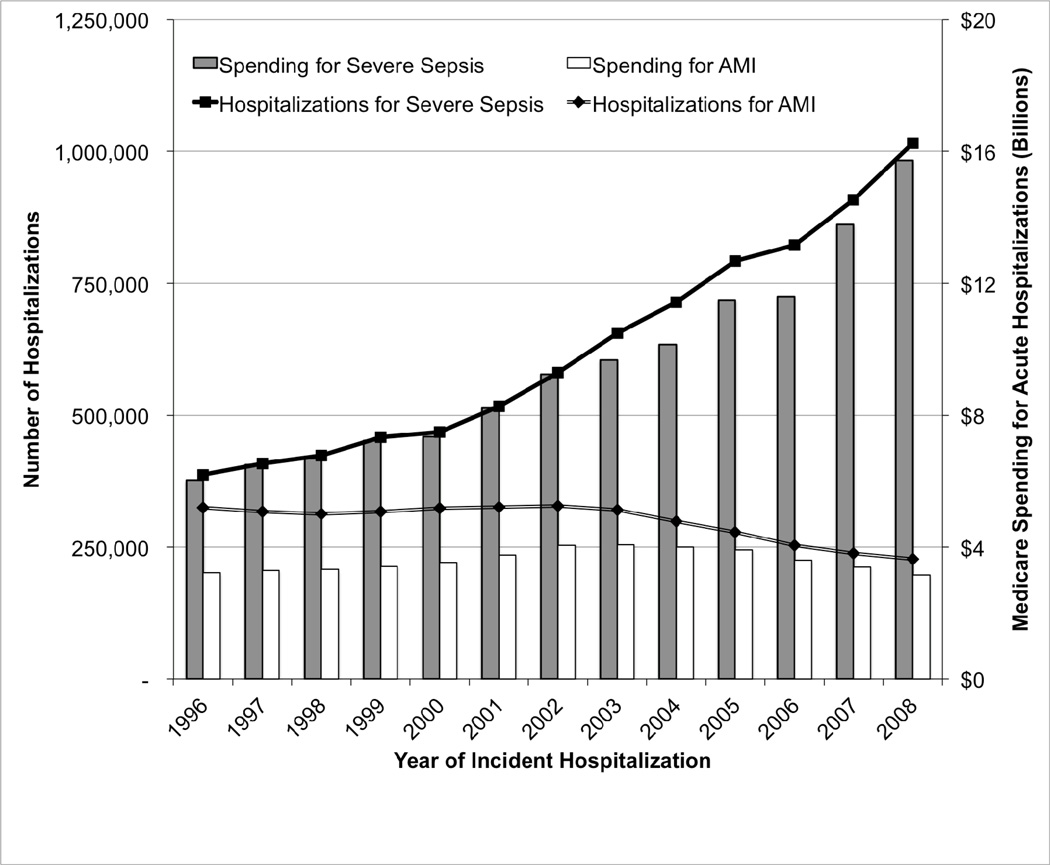
There was a significant increase in the number of hospitalizations for severe sepsis in Medicare. (Figure 2) Whereas, in 1996, 387,330 patients were hospitalized with severe sepsis, by 2008, 1,015,432 patients were hospitalized. The rates of severe sepsis hospitalizations increased from 13.0 per 1,000 Medicare beneficiary-years in 1996 to 34.8 in 2008 (p<0.001). These rising rates of hospitalization were accompanied by increase in direct Medicare spending on severe sepsis hospitalizations from $6.03 billion to $15.73 billion. To contextualize these numbers for severe sepsis, we contrasted them with those seen for acute myocardial infarction (AMI, Figure 2). In 1996 there were 325,108 hospitalizations for AMI, an incidence of 10.91 per 1,000 Medicare beneficiary-years, at a cost of $3.22 billion. In 2008, there were 227,298 cases of acute myocardial infarction in Medicare in 2008, an incidence of 7.79 per 1,000 Medicare beneficiary-years, at a cost to Medicare of $3.16 billion for the acute hospitalizations.
Figure 2.
Hospitalizations and Spending for Severe Sepsis and Acute Myocardial Infarction in Medicare. Spending is for the acute hospitalization only and is not inflation-adjusted.
The rising numbers of hospitalization with severe sepsis resulted primarily from a rise in the rate of organ dysfunction per hospitalization with infection. The absolute number of hospitalizations with infection increased from 108 per 1,000 Medicare beneficiary-years to 121 per 1,000 Medicare beneficiary-years (p<0.001). Rates of organ dysfunction among patients hospitalized with infection rose more rapidly, from 12.1% in 1996 to 28.8% in 2008 (p<0.001). Rates of severe organ dysfunction, defined as the presence of three or more organ dysfunctions, rose from 0.33% of hospitalizations with infection in 1996 to 1.64% in 2008, or from 2.7% of hospitalizations with severe sepsis to 5.7% of such hospitalizations (both p< 0.001).
Changes in Case Fatality Rate
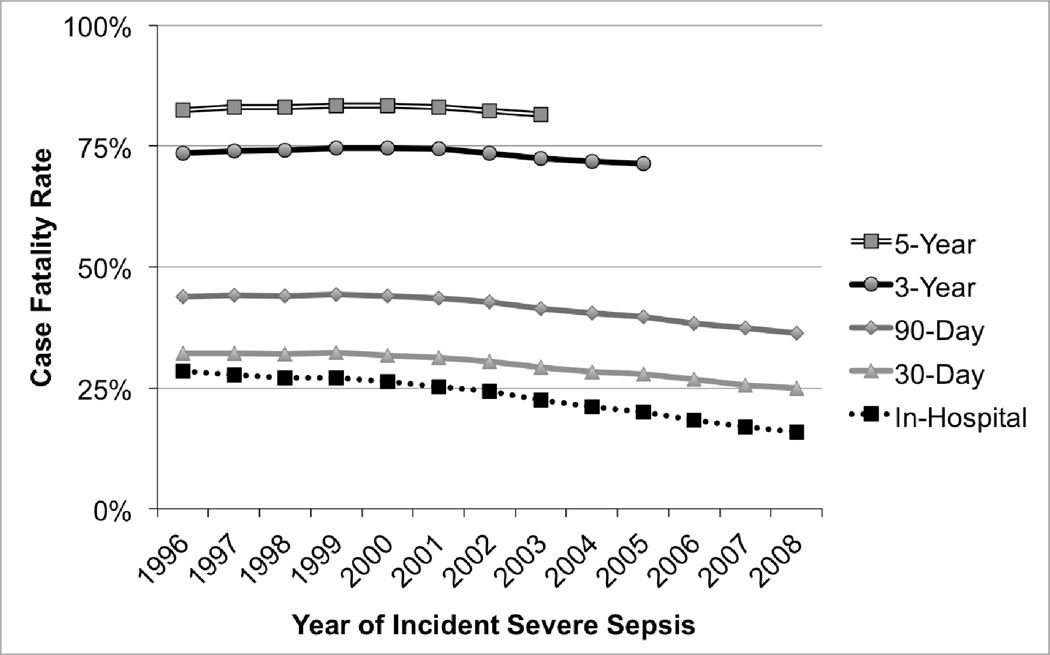
The potential contribution of changes in case fatality rate to the rising severe sepsis survivorship is shown in Figure 3. There were small improvements in long-term case fatality rate after severe sepsis during this period. Three-year case fatality for patients who developed severe sepsis between 1996 and 2005 fell from 73.5% to 71.3% (p<0.001). These relatively flat long-term case fatality rates contrast with short-term figures. The inpatient case fatality dropped from 28.5% in 1996 to 15.8% in 2008, with more modest declines seen in patient-centered short-term outcomes such as 30-day case fatality (32.2% to 24.9%) and 90-day case fatality (43.9% to 36.3%) (all p< 0.001).
Figure 3.
Case Fatality Rates After Severe Sepsis, by year in which severe sepsis developed. The case fatality rate is the fraction of hospitalizations after which the patient died by the specified time period.
Relative Importance of Alternative Mechanisms
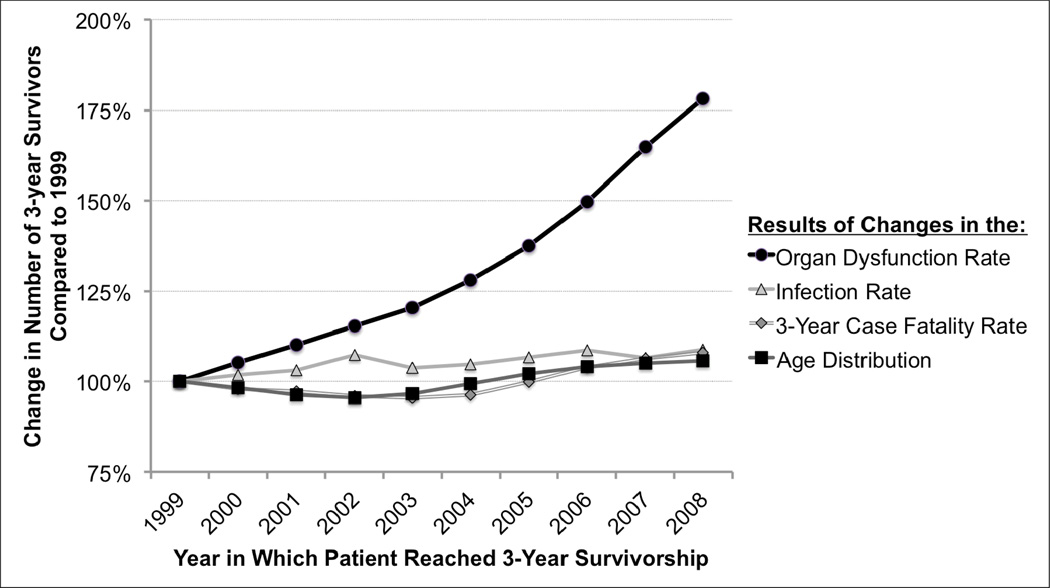
The majority of the increase in the number of survivors was driven by the increase in severe sepsis incidence – particularly driven by the increased incidence of organ dysfunctions among patients who were hospitalized with infections (Figure 4). Thus 3-year survivorship grew 78.2% due to the rising rate of organ dysfunctions among patients hospitalized with infection, whereas the changes in the age distribution, age-specific infection rate, and 3-year case fatality rate each contributed less than 10% to the growth in severe sepsis survivors.
Figure 4.
Contributions to the Rising Number of Survivors of Severe Sepsis. This Figure shows the relative number of 3-year survivors who would have been expected had only each given factor changed.
Interpretive Example: The aging of the population alone would have resulted in a 5.7% increase in the number of 3-year survivors between 1999 and 2008, had other rates remained the same. The Figure reveals that the most important contributor to the growth in the number of severe sepsis survivors over this decade was the increase in the rate of organ dysfunction per patient hospitalized with infection, not by better survival among patients with severe sepsis.
Sensitivity Analyses
We conducted several sensitivity analyses for our results—particularly to changes in coding practice—presented in Appendix 2, and found consistent results.
DISCUSSION
Sepsis survivorship is a common and growing feature of the health care of older Americans. This manuscript establishes for the first time the pervasiveness of a problem already proven to be important to individual patients and their families: sepsis survivors are known to be at substantial risk for poor quality of life, functional disability and cognitive impairment.3,4,25,31 Hundreds of thousands of patients with severe sepsis are surviving years after their illness and face these challenges. These Medicare data show consistently rising rates of incident severe sepsis, consistent with past work in the U.S. and more recent work in Europe.24,32 This study also demonstrates, for the first time, that the rise in survivorship is particularly being driven by a rising incidence of severe sepsis, rather than improvements in the case fatality rate after severe sepsis.
These findings constitute a set of challenges to physicians and policy-makers. These data suggest a substantial population burden of older patients suffering with important disability after severe sepsis—over 100,000 with moderate-to-severe cognitive impairment, and nearly 500,000 with functional disability. These numbers of disabled severe sepsis survivors are of the same order of magnitude as other recognized public health problems such as breast cancer survivorship.10 Moreover, there is evidence that some—perhaps much—of this disability is attributable to severe sepsis, rather than severe sepsis being simply a marker for disability. (From individual-level longitudinal data on severe sepsis survivors, a hospitalization for severe sepsis was associated with 3.34-fold increase in the odds of moderate-to-severe cognitive impairment. For those with no disability before severe sepsis, such a hospitalization was associated with the development of 1.57 new limitations in ADLs and IADLs, and for those with mild-to-moderate limitations beforehand, sepsis was associated with the development of 1.50 new limitation.5,19) Such population burden of disability after severe sepsis argues for an important role of sepsis prevention and disability mitigation in preserving the independence of older Americans. Moreover, the downstream costs of severe sepsis may be quite substantial—both in terms of direct health care costs from disability, but also in terms of caregiver burden, lost productivity, and informal care needs. Investments by Medicare in programs such as early mobility33 or delirium prevention34 may offer long-term returns—in both improved patient functioning and in reduced health care costs – but this needs to be studied.
From a clinical perspective, the importance of increasing rates of organ dysfunction to rising severe sepsis incidence (and hence total numbers of surviving patients) argues for particular focus on the early inpatient detection and management of severe sepsis. It has been hypothesized that there may be a critical window during which effective treatment of infection (the rates of which are rising only slightly) may prevent the inflammatory and coagulopathic cascade of severe sepsis that leads to organ dysfunction.35 If this hypothesis is correct, preventing the conversion of infection to severe sepsis may have marked influence on the population burden of sepsis survivorship. Interventions such as early antibiotics36 and pneumococcal vaccination may be effective in this arena – and their cost effectiveness analysis should consider their potential to avert the high societal cost of disability after severe sepsis. The rising rates of organ dysfunction may be hypothesized to result from several causes, including growing use of immunosuppressive medications37, higher thresholds for hospital admission in an era of increasing shift towards outpatient treatment38, and increased diagnosis of organ injury with more sensitive and ubiquitous testing39; establishing the relative contributions of these different mechanisms may be an important topic for future research.
There is little evidence of significant declines in case fatality rates over longer patient-centered time horizons. Cohorts that experienced declines in short-term mortality did not have corresponding declines in long-term mortality, even though sepsis appears to exert a mortality effect for at least 5 years after diagnosis14,15. This unchanged long-term mortality occurred despite the publication of several landmark trials during this period, including early-goal directed therapy for severe sepsis40 and the introduction of low tidal volume ventilation for acute lung injury41, and an international campaign focused on sepsis-related quality of care.42,43 A crucial question is whether these therapies are ineffective at altering the long-term natural history of severe sepsis, whether the attributable long-term mortality of severe sepsis is lower than previously believed,14,15 or whether the implementation of effective therapies has been too incomplete to impact population health.44
Our results have several limitations. We focused on severe sepsis within the fee-for-service Medicare population. While this is a population in which previous work demonstrated clear functional and cognitive disability among survivors,5 it does not include patients with severe sepsis not yet eligible for Medicare. As such, the total population burden of severe sepsis survivorship is likely greater. Second, we used a widely accepted operationalization of severe sepsis for national epidemiologic work; while this definition has been clinically validated, this is not the same as prospective assessment of patients, were such a national assessment feasible. Third, previous estimates of the associations of severe sepsis with disability showed that there was less attributable disability after severe sepsis in patients who were already severely disabled5; if there are relatively more severely disabled patients in the US national population, our estimated burden of severe sepsis survivorship may be an overestimate. Finally, we used administrative data, and so our results could be influenced by secular trends in coding practice for specific organ dysfunctions; Appendix 2 presents several sensitivity results. An overall increased attention to secondary diagnoses might account for some of the overall trend, although we speculate that this would more likely represent a failure to code organ dysfunction in the past than a fraudulent coding of organ dysfunction in the current period.
Severe sepsis has emerged as a dominant cause of serious illness in Medicare among older Americans, and survivorship after severe sepsis is common. There are nearly 500,000 disabled survivors of severe sepsis, yet few proven therapies or specific support programs exist to help or support their caregivers. Given severe sepsis survivors’ enduring cognitive, physical and quality of life decrements, these patients and their families may benefit from efforts to improve and integrate their care—from the prevention of severe sepsis through its acute treatment and long-term follow-up.
ACKNOWLEDGMENTS
This work was supported by a grant from the U.S. National Institutes of Health, K08, HL091249. We appreciate the expert programming of Letitia Shapiro, A.M. at the University of Michigan.
Sponsor’s Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Appendix 1
Example of Demographic Decomposition
In order to understand the relative contributions to the changing rates of severe sepsis survivorship, the following approach is used. First, consider the women aged 65 to 69. We calculate the following values for 1996 from the Medicare data:
POP: The number of patients in Medicare in this group
HOSP: Their age- and gender-specific rate of hospitalization with infection
DYSFUNC: The aggregate rate of organ dysfunction per hospitalization
SURVIVE: The aggregate 3-year survival rate for patients with severe sepsis
The total number of 3-year survivors in this age group of women is mathematically equivalent to POP * HOSP * DYSFUNC * SURVIVE. Adding subscripts to indicate the reference year, the gender, and the age group, then the
Number of 3-Year Survivors96,W,65–69 =
= POP96,W,65–69 * HOSP 96,W,65–69* DYSFUNC96 * SURVIVE96
The total Number of 3-Year Survivors is the sum over both genders and all age groups of these age- and gender-specific Numbers of 3-Year Survivors.
To calculate the effect of population-aging between 1996 and 2005, we consider what would have happened if POP96,W,65–69 were replaced in the above equations by POP2005,W,65–69. We hold HOSP 96,W,65–69, DYSFUNC96, and SURVIVE96 constant, but recalculate the sum. The ratio of this simulated number for 2005 to the actual number 3-year survivors in 1996 was 1.057. Thus the change in the age-distribution accounted for a 5.7% increase in the number of 3-year survivors over that period, as presented in Figure 4.
A similar logic could be used changing only the year-specific rates for each of the other 3 terms. This was done to produce Figure 4.
Appendix 2
Sensitivity Analyses to Alternative Severe Sepsis Definitions
In 2002, a new ICD-9-CM code for “severe sepsis” was introduced. Excluding cases of severe sepsis with this explicit code had little effect on these results, as most such hospitalizations also had codes for a specific infection and at least one acute organ dysfunction. We further replicated our analyses with alternative definitions of severe sepsis that excluded each organ dysfunction, to ensure that unspecified changes in coding practice for other conditions did not drive our results. (That is, we considered a definition of severe sepsis as an infection plus an organ dysfunction other than, for example, hepatic injury.) Severe sepsis survivorship rose significantly in all such sensitivity tests, although less so if acute kidney injury is excluded as a severe-sepsis-defining organ dysfunction.
This Table is organized as follows. Each column of the Table presents the results of the analyses as they occurred under alternative definitions of severe sepsis. The first column, for reference, provides the results presented in the body of the manuscript. Key findings are in the italicized rows – they show, for example, a consistent percentage increase in the number of 3-year survivors across the definitions. The rows entitled “Contributions to 3-Year Survivorship Change” parallel the analysis summarized in Figure 4.
Finally, note that results for 5-year survival, as is commonly used in oncology, are presented here as well, and show a consistent pattern.
| Sensitivity Analyses Excluding Codes for… | ||||
|---|---|---|---|---|
| Full Definition |
Severe Sepsis and Septic Shock |
Cardiovascular | Neurologic | |
| New 3-Year Survivors, 1999 (Got Sepsis in 1996) | 102,767 | 102,767 | 73,051 | 87,398 |
| New 3-Year Survivors, 2008 (Got Sepsis in 2005) | 225,251 | 207,410 | 164,540 | 203,084 |
| % Change, 2008 vs. 1999 | 219% | 202% | 225% | 232% |
| New 5-Year Survivors, 2001 (Got Sepsis in 1996) | 67,799 | 67,799 | 46,966 | 58,033 |
| New 5-Year Survivors, 2008 (Got Sepsis in 2003) | 121,029 | 119,756 | 85,743 | 108,803 |
| % Change, 2008 vs. 2001 | 179% | 177% | 183% | 187% |
| Incident Cases 1996 | 387,330 | 387,330 | 289,614 | 341,586 |
| Incident Cases 2003 | 656,194 | 645,379 | 488,511 | 594,821 |
| Incident Cases 2005 | 791,809 | 689,826 | 590,572 | 722,414 |
| Incident Cases 2008 | 1,015,432 | 881,334 | 751,334 | 853,751 |
| % Change, 2008 vs. 1996 | 262% | 228% | 259% | 250% |
| Case Fatality Rates | ||||
| 5-year case fatality rate, incident cases from 1996 | 82.5% | 82.5% | 83.8% | 83.0% |
| 5-year case fatality rate, incident cases from 2003 | 81.6% | 81.4% | 82.5% | 81.7% |
| 3-year case fatality rate, incident cases from 1996 | 73.5% | 73.5% | 74.8% | 74.4% |
| 3-year case fatality rate, incident cases from 2005 | 71.3% | 69.7% | 71.9% | 71.7% |
| In-Hospital case fatality rate, incident cases from 1996 | 28.5% | 28.5% | 27.6% | 30.0% |
| In-Hospital case fatality rate, incident cases from 2008 | 15.8% | 12.4% | 13.0% | 16.0% |
| Contributions to 3-Year Survivorship Change | ||||
| Population Aging | 5.7% | 5.7% | 5.7% | 5.7% |
| Hospitalizations with Infection per Person | 8.6% | 8.6% | 8.6% | 8.6% |
| Organ dysfunction per Hospitalization with Infection | 78.2% | 55.2% | 77.7% | 84.3% |
| Long-term Survival | 8.0% | 14.2% | 11.3% | 10.7% |
| Contributions to 5-Year Survivorship Change | ||||
| Population Aging | 4.2% | 4.2% | 4.2% | 4.2% |
| Hospitalizations with Infection per Person | 8.6% | 8.6% | 8.6% | 8.6% |
| Organ dysfunction per Hospitalization with Infection | 49.7% | 47.3% | 49.1% | 53.9% |
| Long-term Survival | 5.4% | 6.1% | 8.2% | 7.7% |
| Sensitivity Analyses Excluding Codes for… | ||||
| Hematologic | Hepatic | Renal | Respiratory | |
| New 3-Year Survivors, 1999 (Got Sepsis in 1996) | 83,898 | 102,332 | 82,327 | 73,260 |
| New 3-Year Survivors, 2008 (Got Sepsis in 2005) | 188,885 | 223,797 | 124,560 | 189,416 |
| % Change, 2008 vs. 1999 | 225% | 219% | 151% | 259% |
| New 5-Year Survivors, 2001 (Got Sepsis in 1996) | 54,794 | 67470 | 55,122 | 48,832 |
| New 5-Year Survivors, 2008 (Got Sepsis in 2003) | 98,814 | 120262 | 76,004 | 97,170 |
| % Change, 2008 vs. 2001 | 180% | 178% | 138% | 199% |
| Incident Cases 1996 | 320,965 | 384,128 | 284,144 | 240,175 |
| Incident Cases 2003 | 544,488 | 650,126 | 376,227 | 467,504 |
| Incident Cases 2005 | 668,707 | 783,373 | 413,788 | 597,456 |
| Incident Cases 2008 | 921,573 | 1,002,340 | 425,198 | 824,543 |
| % Change, 2008 vs. 1996 | 287% | 261% | 150% | 343% |
| Case Fatality Rates | ||||
| 5-year case fatality rate, incident cases from 1996 | 82.9% | 82.4% | 80.6% | 79.7% |
| 5-year case fatality rate, incident cases from 2003 | 81.9% | 81.5% | 79.8% | 79.2% |
| 3-year case fatality rate, incident cases from 1996 | 73.9% | 73.4% | 71.0% | 69.5% |
| 3-year case fatality rate, incident cases from 2005 | 71.6% | 71.2% | 69.7% | 68.1% |
| In-Hospital case fatality rate, incident cases from 1996 | 29.7% | 28.2% | 25.8% | 20.2% |
| In-Hospital case fatality rate, incident cases from 2008 | 16.1% | 15.4% | 15.7% | 10.5% |
| Contributions to 3-Year Survivorship Change | ||||
| Population Aging | 5.7% | 5.7% | 5.7% | 5.7% |
| Hospitalizations with Infection per Person | 8.6% | 8.6% | 8.6% | 8.6% |
| Organ dysfunction per Hospitalization with Infection | 81.6% | 77.8% | 26.9% | 116.8% |
| Long-term Survival | 8.9% | 8.0% | 4.7% | 4.7% |
| Contributions to 5-Year Survivorship Change | ||||
| Population Aging | 4.2% | 4.2% | 4.2% | 4.2% |
| Hospitalizations with Infection per Person | 8.6% | 8.6% | 8.6% | 8.6% |
| Organ dysfunction per Hospitalization with Infection | 49.9% | 49.6% | 17.0% | 72.0% |
| Long-term Survival | 6.3% | 5.4% | 4.1% | 2.2% |
Footnotes
Conflict of Interest
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Study concept and design: all, acquisition of subjects and/or data: TJI, analysis of data: TJI, interpretation of data: all, and preparation of manuscript: all.
REFERENCES
- 1.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Angus D, Linde-Zwirble W, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson S, Ruokonen E, Varpula T, et al. for the Finnsepsis Study Group. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 4.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis: A systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Doig CJ, Ghali WA, et al. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32:981–985. doi: 10.1097/01.ccm.0000120053.98734.2c. [DOI] [PubMed] [Google Scholar]
- 8.Langa KM, Chernew ME, Kabeto MU, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milbrandt EB, Eldadah B, Nayfield S, et al. Toward an integrated research agenda for critical illness in aging. Am J Respir Crit Care Med. 2010;182:995–1003. doi: 10.1164/rccm.200904-0630CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt M, Greenfield S, Stovall E, editors. From cancer patient to cancer survivor: Lost in translation. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 11.Cheung WY, Neville BA, Cameron DB, et al. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27:2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullan F. Seasons of survival: reflections of a physician with cancer. N Engl J Med. 1985;313:270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- 14.Quartin AA, Schein RMH, Kett DH, et al. Magnitude and duration of the effect of sepsis on survival. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 15.Wunsch H, Guerra C, Barnato AE, et al. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 16.Hatten J. Medicare's Common Denominator: The Covered Population. Health Care Financing Review. 1980 Fall;:53–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 18.Barnato AE, Alexander SL, Linde-Zwirble WT, et al. Racial variation in the incidence, care, and outcomes of severe sepsis. Am J Respir Crit Care Med. 2007;177:279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153:204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 20.Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303:2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour CW, Iwashyna TJ, Cooke CR, et al. Marital status and the epidemiology and outcomes of sepsis. Chest. 2010;137:1289–1296. doi: 10.1378/chest.09-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 23.Angus DC. The Lingering Consequences of Sepsis: A Hidden Public Health Disaster. JAMA. 2010;304 doi: 10.1001/jama.2010.1546. 1833-184. [DOI] [PubMed] [Google Scholar]
- 24.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Kahn JM, Hayward RA, et al. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at non-revascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3:468–475. doi: 10.1161/CIRCOUTCOMES.110.957993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 27.Epstein AJ, Rathore SS, Krumholz HM, et al. Volume-based referral for cardiovascular procedures in the United States: A cross-sectional regression analysis. BMC Health Serv Res. 2005;5:42. doi: 10.1186/1472-6963-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog AR, Wallace RB. Measure of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52B:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 29.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the ederly using telephone screening of cognitive status. Neuropsychol Behavioral Neurol. 1996;6:103–110. [Google Scholar]
- 30.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 31.Lazosky A, Young GB, Zirul S, et al. Quality of life after septic illness. J Crit Care. 2010;25:406–412. doi: 10.1016/j.jcrc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelms SB, Huss FR, Granath G, et al. Assessment of incidence of severe sepsis in Sweden using different ways of abstracting International Classification of Diseases codes: difficulties with methods and interpretation of results. Crit Care Med. 2010;38:1442–1449. doi: 10.1097/CCM.0b013e3181de4406. [DOI] [PubMed] [Google Scholar]
- 33.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 37.Khan MM. Immunopharmacology. New York: Springer US; 2009. Immunosuppressive Agents; pp. 1–19. [Google Scholar]
- 38.Bernstein AB, Hing E, Moss AJ, et al. Health care in America: Trends in utilization. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- 39.Song Y, Skinner J, Bynum J, et al. Regional variations in diagnostic practices. N Engl J Med. 2010;363:45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 41.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 42.Slade E, Tamber PS, Vincent JL. The surviving sepsis campaign: Raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 44.Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]