Summary
Rotator cuff repairs are commonly performed to reduce pain and restore function. Tears are also treated successfully without surgical intervention; however, the effect that a torn tendon has on the glenohumeral cartilage remains unknown. Clinically, a correlation between massive rotator cuff tears and glenohumeral arthritis has often been observed. This may be due to a disruption in the balance of forces at the shoulder, resulting in migration of the humeral head and subsequently, abnormal loading of the glenoid. Our lab previously demonstrated changes in ambulation and intact tendon mechanical properties following supraspinatus and infraspinatus rotator cuff tendon tears in a rat model. Therefore, the purpose of this study was to investigate the effects of supraspinatus and infraspinatus rotator cuff tears on the glenoid cartilage. Nine rats underwent unilateral detachment of the supraspinatus and infraspinatus tendons and were sacrificed after four weeks. Cartilage thickness significantly decreased in the antero-inferior region of injured shoulders. In addition, equilibrium elastic modulus significantly decreased in the center, antero-superior, antero-inferior, and superior regions. These results suggest that altered loading after rotator cuff injury may lead to damage to the joint with significant pain and dysfunction. Clinically, understanding the mechanical processes involved with joint damage will allow physicians to better advise patients.
Keywords: glenoid cartilage, rotator cuff, animal model, glenohumeral arthritis
Introduction
Glenohumeral joint stability is provided by a balance between static and dynamic structures, including the glenoid articular cartilage and labrum, ligaments, joint capsule, osseous structures, and the rotator cuff. The close interactions between these structures allow for normal function (i.e., an intact rotator cuff allows for concentric rotation of the humeral head on the glenoid). In particular, the glenoid cartilage is characterized by maximum thickness localized to the periphery, deepening the glenoid cavity and allowing for increased stability when articulating with the humeral head.
Due to the interrelationships between shoulder joint structures, damage to one structure may affect others. Specifically, rotator cuff tendon injuries have been, in some cases, associated with the development of glenohumeral arthritis, termed cuff tear arthropathy1–3. Rotator cuff tendon tears are common musculoskeletal problems that can lead to altered joint mechanics and significant pain and dysfunction. A massive rotator cuff tear involving the infraspinatus and supraspinatus may disrupt the balance of forces at the shoulder4, resulting in superior and antero-superior migration of the humeral head and subsequently, abnormal loading of the glenoid. Management of rotator cuff tears typically focuses on pain reduction and functional improvements with a period of conservative treatment prior to consideration of surgery; however, the long term consequences of leaving the tendons torn is not well understood, and recurrent symptoms, such as joint arthritis, can only be managed by joint replacement.
Our lab previously demonstrated that the rat shoulder model mimics the bony architecture and soft tissue (tendon and ligament) anatomy of the human5. In this study, we aimed to determine if the glenoid cartilage in the rat is also similar to the human. Additionally in this rat model, our lab previously demonstrated decreased shoulder function6 and decreased intact tendon (biceps and subscapularis) mechanical properties7 following a supraspinatus and infraspinatus rotator cuff tear, but did not examine the effect of this injury on the glenoid cartilage (including the articular cartilage and labrum). To our knowledge, no animal models have investigated changes in the glenoid cartilage following rotator cuff tear. Therefore, our objectives were to characterize the rat glenoid cartilage and to investigate the effects of supraspinatus and infraspinatus rotator cuff tears on the glenoid cartilage. We hypothesized that: H1) in uninjured shoulders, the glenoid cartilage will be thinnest in the center and have no regional variations in modulus, as in human. In addition, H2) thickness and H3) modulus will both decrease in the superior and antero-superior regions, as a result of the altered loading due to the supraspinatus and infraspinatus rotator cuff tear.
Methods
Nine adult male Sprague-Dawley rats (400 to 450 g) were used in this IACUC approved study. All animals underwent unilateral detachment of the supraspinatus and infraspinatus tendons in the left shoulder. Briefly, with the arm in external rotation, a 2 cm skin incision was made followed by blunt dissection down to the rotator cuff musculature. The rotator cuff was exposed, and the tendons were visualized at their insertion on the humerus. The supraspinatus tendon was then separated from the other rotator cuff tendons before sharp detachment at its insertion on the greater tuberosity using a scalpel blade. The infraspinatus tendon was then detached in the same manner. Any remaining fibrocartilage at the insertion was left intact, and detached tendons were allowed to freely retract. The overlying muscle and skin were closed, and the animals were allowed unrestricted cage activity. All rats were sacrificed 4 wks after detachment and frozen.
Animals were thawed, and the left and right scapulae were dissected out. The biceps tendon was sharply detached at its insertion at the superior rim of the glenoid using a scalpel blade. The scapula was then embedded in a holding fixture using PMMA. Specimens were then secured in a custom fixture and immersed in PBS containing a protease inhibitor cocktail (5 mM Benz-HCl, 1mM PMSF, 1 M NEM) at room temperature.
Thickness Measurement
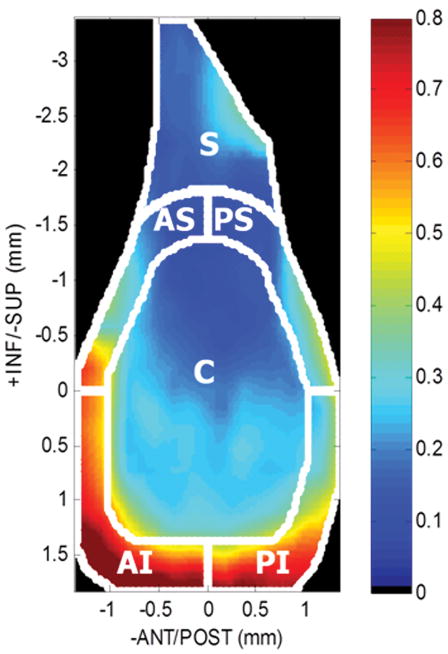
Specimens were scanned at 0.25 mm increments using a 55 MHz ultrasound probe (Visualsonics, Inc) in plane with the scapula. Using methods consistent with previous studies8,9, captured 2D B-mode images of each scan were manually segmented (three times and averaged) by selecting the cartilage and bony surfaces of the glenoid (Fig. 1). The 3D positions of these surfaces were reconstructed with a custom program (MATLAB) and used to determine cartilage thickness maps by subtracting the cartilage and bony surfaces (Fig. 2A). Each thickness map was divided into six regions (center (C), postero-superior (PS), postero-inferior (PI), antero-superior (AS), antero-inferior (AI), and superior (S)), and an average thickness was computed for each region for both injured (LEFT) and control (RIGHT) shoulders. Following ultrasound scanning, specimens were refrozen until mechanical testing.
Figure 1.
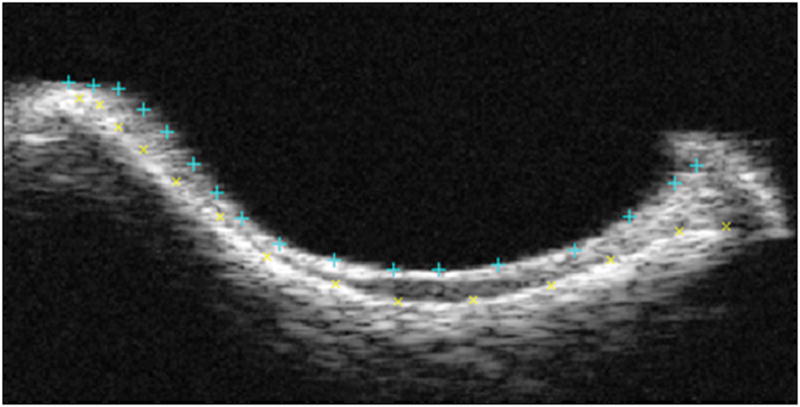
Coronal view of a representative B-mode image slice of the central portion of the glenoid. The top and bottom surfaces, cartilage and bone, respectively, were each selected to segment the articulating surface.
Figure 2.
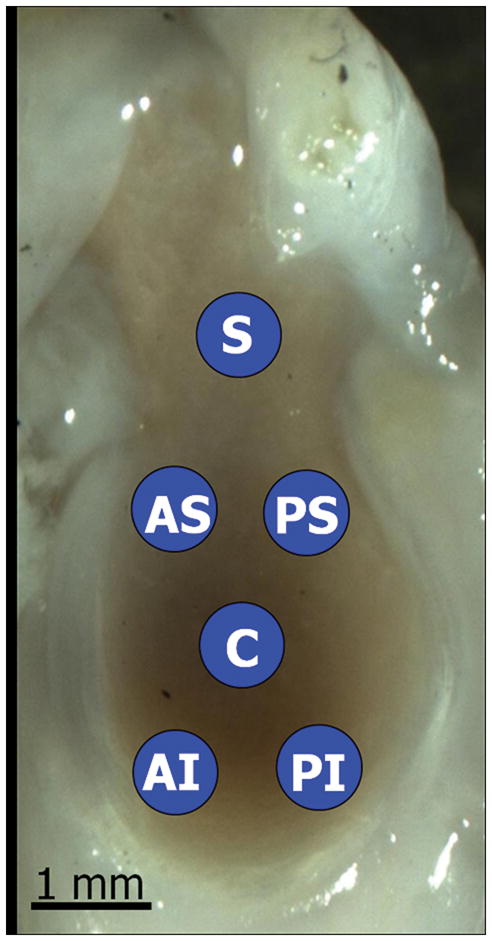
Figure 2A: Sagittal view of a representative cartilage thickness map determined by ultrasound and divided into six regions (center (C), antero-superior (AS), antero-inferior (AI), postero-superior (PS), postero-inferior (PI), and superior (S)).
Figure 2B: Saggital view of a representative image of the rat glenoid, highlighting the six locations for mechanical indentation testing. Cartilage thickness was calculated at each location using ultrasound.
Mechanical Testing
Utilizing a 0.5 mm diameter, non-porous spherical indentor tip, cartilage indentation testing was performed: preload (0.005 N), 8 step-wise stress relaxation tests (8 μm ramp at 2 μm/s followed by a 300 s hold). The scapula was repositioned for each localized region using angular, rotational, and linear stages such that the indenter tip was perpendicular to the cartilage surface. Cartilage thickness for indentation testing was determined by identifying the indentation location on each cartilage thickness map (determined by ultrasound, Fig. 2B). Equilibrium elastic modulus was calculated, as described previously10, at 20% indentation and assuming Poisson’s ratio (v=0.30).
Statistics
For statistical analysis, normality was assessed, and, due to the non-parametric distribution of the cartilage thickness data, non-parametric statistics were performed for our first and second hypotheses. Specifically, to determine if the regional glenoid cartilage thickness and mechanics in the rat mimics the human (with the center region thinnest compared to the periphery and no differences in regional mechanics), the uninjured shoulder of each rat was evaluated using a Kruskal Wallis test with Fisher’s post hoc (significance set at p<0.01 to account for multiple comparisons) comparing the center region with the peripheral regions and one-way ANOVA with Fisher’s post hoc (significance set at p<0.002 to account for multiple comparisons) comparing all regions (for thickness and mechanics, respectively). To determine if rotator cuff tears lead to decreased cartilage thickness and decreased cartilage mechanics, a Wilcoxon signed ranked test and paired t-test (for thickness and mechanics, respectively) were performed separately comparing each region for the paired injured and uninjured control shoulders.
Results
For uninjured shoulders, consistent with our first hypothesis for cartilage thickness, the glenoid cartilage was thinnest in the center compared to the inferior peripheral regions (postero-inferior and antero-inferior). However, no significant differences were seen between the center and superior peripheral regions (superior, antero-superior, and postero-superior, Table 1, LEFT). Contrary to our first hypothesis for cartilage mechanics, regional differences in equilibrium elastic modulus were observed in the uninjured rat shoulder with modulus being larger in the superior region compared to both posterior regions (superiorly and inferiorly) (Table 1, RIGHT).
Table 1.
Average glenoid regional thickness (median (IQR=Q1–Q3), *p<0.01 significantly different from center region) and equilibrium modulus (mean±SD), *p<0.002 significantly different from superior region) for uninjured controls
| UNINJURED RAT GLENOID PROPERTIES | ||
|---|---|---|
| Glenoid Region | Regional Thickness (mm) | Equilibrium Modulus (MPa) |
| C | 0.21(IQR=0.19–0.23) | 1.54±0.29 |
| AS | 0.23(IQR=0.22–0.24) | 1.22±0.22 |
| AI | 0.54(IQR=0.49–0.59)* | 1.55±0.34 |
| PS | 0.18(IQR=0.17–0.19) | 0.89±0.26* |
| PI | 0.54(IQR=0.51–0.54)* | 0.77±0.32* |
| S | 0.17(IQR=0.17–0.20) | 1.97±0.93 |
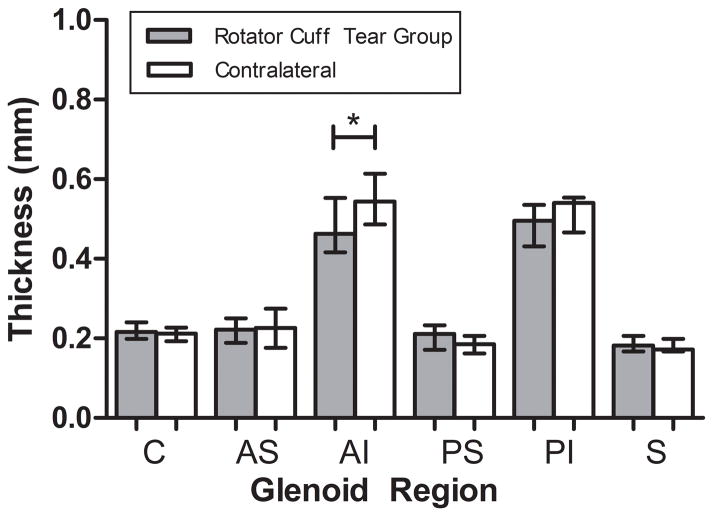
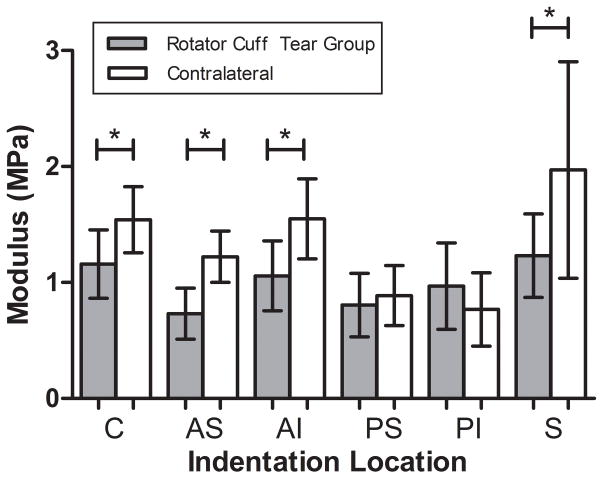
Following rotator cuff tears, decreased cartilage thickness was not observed in the superior and antero-superior regions (Fig. 3). Interestingly, decreased cartilage thickness was observed in the antero-inferior region. Consistent with our third hypothesis and degeneration observed in the human condition, decreased cartilage modulus was observed in the superior and antero-superior regions (Fig. 4). Additionally, decreased modulus was also observed in the center and antero-inferior regions. No other regional changes were identified.
Figure 3.
Rotator cuff tears resulted in cartilage thinning in the antero-inferior (AI) region of the glenoid (median±IQR) (*p<0.05).
Figure 4.
Rotator cuff tears resulted in decreased modulus in the center (C), antero-superior (AS), antero-inferior (AI), and superior (S) regions of the glenoid (mean±SD) (*p<0.05).
Discussion
This is the first study to characterize the rat glenoid cartilage and to investigate the effects of supraspinatus and infraspinatus rotator cuff tears on the glenoid cartilage in an animal model. Using high frequency ultrasound, we found that the glenoid cartilage was thinnest in the center compared to the inferior peripheral regions (as in the human), but not different than the superior peripheral regions. Additionally, contrary to our first hypothesis, regional differences in equilibrium elastic modulus were observed. For injured shoulders, the glenoid cartilage thickness significantly decreased in the antero-inferior region compared to the contralateral control. We also observed decreased mechanical properties in the anterior (superiorly and inferiorly), center, and superior regions. Despite additional regional changes (we hypothesized changes would occur only in the superior and antero-superior region), these results support our hypothesis that glenoid cartilage thickness and mechanics would both decrease as a result of the massive cuff tear, and are consistent with cartilage degeneration observed in human shoulders.
The articulation of the glenoid of the scapula and the humerus is often referred to as a golf ball on a tee, allowing for large range of motion during normal shoulder function. Several techniques have been utilized to quantitatively evaluate cartilage volume and thickness in the shoulder, including stereophotogrammetry11, magnetic resonance imaging12, and ultrasound12. These studies showed, that in the human, the maximum cartilage thickness is localized to the periphery, deepening the glenoid cavity and allowing for increased glenohumeral stability. Our results showed that cartilage thickness in the uninjured rat glenoid was maximal in the periphery, particularly in the inferior region, consistent with that in the human.12 Additionally, regional variations in mechanical properties were observed in uninjured shoulders, suggesting that loading in the rat glenoid may be differently distributed during normal function compared to the human, which has similar mechanical properties across regions13,14.
Clinically, altered mechanical loading due to joint instability has been implicated as the primary initiating mechanism of osteoarthritis.15 As a result, surgically induced destabilization of joints is a widely used animal model of the disease.16 Animal models provide a useful tool to track the progression of osteoarthritis from its earliest stages and exhibit changes similar to the human condition.17 Previous animal models in the dog, rabbit, guinea pig, and rat include induction methods that mimic human injuries including ACL transection, meniscectomy, and other combination injuries.16,18,19 Specifically, Setton et al. showed a 77% decrease in compressive stiffness 6 wks following ACL transection that was sustained at 12 wks20. Consistent with our results in the rat shoulder, these animal models demonstrated decreased cartilage compressive modulus and decreased tissue thickness in the knee.17
Using quantitative ambulatory measures, previous studies in this animal model demonstrated that detachment of the supraspinatus and infraspinatus leads to decreased joint kinetics, as indicated by significantly larger lateral forces, decreased braking force, and decreased vertical ground reaction force in the injured limb. In addition, decreased step width, indicative of “tripoding” and decreased shoulder function, was also observed.21 When combining the current study with previous findings, this model has shown that massive two-tendon rotator cuff tears decreases both glenoid cartilage properties and joint function. These data support studies that suggest altered loading due to joint instability may be a causative factor for cartilage damage.15
Clinically, physicians often advise patients with rotator cuff tears on conservative versus operative treatment without clearly understanding the potentially damaging effects on adjacent intact tissues. Results from our study suggest that a patient with a massive rotator cuff tear involving the supraspinatus and infraspinatus may develop cartilage damage if the tendons are left torn.
This study has several limitations. Although the rat shoulder has similar anatomy (in bony architecture and soft tissue insertions) to the human and has been widely used as a model of rotator cuff injury, the use of a quadruped animal does not exactly replicate the human condition. In addition, while we previously showed that rat ambulation is altered in this rotator cuff model, in the absence of glenohumeral kinematic data, it is unknown whether the damage to the cartilage is a direct result of altered loading at the shoulder joint. However, cartilage changes did occur without direct injury to the cartilage lending support to the explanation of altered joint loading. Additionally, we only evaluated one time point, therefore, the mechanisms that lead to joint damage cannot be fully elucidated. A limitation must also be acknowledged in our mechanical testing indentation protocol, which prescribed 8 step indentations in each region, corresponding to a tissue depth of greater than 20% cartilage thickness in some regions. However, modulus values were always calculated using Hayes equation at load-displacement values of 20% cartilage thickness. Lastly, due to the potential compensatory loading effects on the uninjured shoulder, the use of the contralateral limb as a control should also be acknowledged. Despite these limitations, our results clearly demonstrate the concept of structural and mechanical consequences following rotator cuff injury. Future studies will examine additional time points to examine the progression of cartilage changes as well as tissue biological properties to gain insight into the relationships between, and mechanisms governing, cuff tears and subsequent joint damage.
In conclusion, detachment of the supraspinatus and infraspinatus tendons has been shown to decrease shoulder function6, decrease biceps and subscapularis tendon mechanical properties7, and result in thinning and softening of the glenoid articular cartilage, as observed in this study. This animal model can be utilized to understand the cartilage injury process and ultimately to develop and evaluate treatment modalities to prevent cartilage damage.
Acknowledgments
The authors acknowledge Leann Dourte and Daniel Cortes for their assistance. This study was supported by NIH/NIAMS.
References
- 1.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 2.Petersson CJ. Degeneration of the gleno-humeral joint. An anatomical study. Acta Orthop Scand. 1983;54:277–283. doi: 10.3109/17453678308996570. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HC, Luo ZP, Stone JJ, et al. Correlation between rotator cuff tear and glenohumeral degeneration. Acta Orthop Scand. 2003;74:89–94. doi: 10.1080/00016470310013725. [DOI] [PubMed] [Google Scholar]
- 4.Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clin Orthop Relat Res. 2001:107–118. doi: 10.1097/00003086-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 6.Perry SM, Getz CL, Soslowsky LJ. Alterations in function after rotator cuff tears in an animal model. J Shoulder Elbow Surg. 2009;18:296–304. doi: 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry SM, Getz CL, Soslowsky LJ. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J Shoulder Elbow Surg. 2009;18:52–57. doi: 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koff MF, Chong le R, Virtue P, et al. Validation of cartilage thickness calculations using indentation analysis. J Biomech Eng. 2010;132:041007. doi: 10.1115/1.4000989. [DOI] [PubMed] [Google Scholar]
- 9.Noble JA. Ultrasound image segmentation and tissue characterization. Proc Inst Mech Eng H. 2010;224:307–316. doi: 10.1243/09544119JEIM604. [DOI] [PubMed] [Google Scholar]
- 10.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 11.Soslowsky LJ, Flatow EL, Bigliani LU, Mow VC. Articular geometry of the glenohumeral joint. Clin Orthop Relat Res. 1992:181–190. [PubMed] [Google Scholar]
- 12.Graichen H, Jakob J, von Eisenhart-Rothe R, et al. Validation of cartilage volume and thickness measurements in the human shoulder with quantitative magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11:475–482. doi: 10.1016/s1063-4584(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 13.Mow VC, Bigliani LU, Flatow EL, Ratcliffe A, Soslowsky LJ. Material properties of the inferior glenohumeral ligament and glenohumeral joint cartilage. In: Matsen FA, FFH, Hawkins RJ, editors. The Shoulder: A Balance of Stability and Mobility. Rosemont: American Academy of Orthopaedic Surgeons; 1993. pp. 29–67. [Google Scholar]
- 14.Huang CY, Stankiewicz A, Ateshian GA, Mow VC. Anisotropy, inhomogeneity, and tension-compression nonlinearity of human glenohumeral cartilage in finite deformation. J Biomech. 2005;38:799–809. doi: 10.1016/j.jbiomech.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 16.Little CB, Smith MM. Animal Models of Osteoarthritis. Curr Rheum Reviews. 2008;4:1–8. [Google Scholar]
- 17.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 18.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–376. [PubMed] [Google Scholar]
- 19.Pritzker KPH. Animal-Models for Osteoarthritis - Processes, Problems and Prospects. Annals of the Rheumatic Diseases. 1994;53:406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473–482. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 21.Hsu JE, Reuther KE, Sarver JJ, et al. Restoration of anterior-posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J Orthop Res. 29:1028–1033. doi: 10.1002/jor.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]