Abstract
Background
Liver injury has been reported in children treated with repeated doses of acetaminophen. The objective of this study is to identify and validate reports of liver injury or death in children younger than 6 years of age following repeated therapeutic doses of acetaminophen.
Methods
We reviewed United States (US) Poison Center data, peer-reviewed literature, US FDA Adverse event reports and US Manufacturer safety reports describing adverse effects following acetaminophen administration. Reports that described hepatic abnormalities (description of liver injury or abnormal laboratory testing) or death following administration to children younger than 6 years of age were included. The identified reports were double abstracted and then reviewed by an expert panel to determine if the hepatic injury was related to acetaminophen, and whether the dose of acetaminophen was therapeutic (≤75 mg/kg) or supra-therapeutic.
Results
Our search yielded 2531 reports of adverse events associated with acetaminophen use. From these cases, we identified 76 cases of hepatic injury and 26 deaths associated with repeated acetaminophen administration. There were 6 cases of hepatic abnormalities and no deaths associated with what our panel determined to be therapeutic doses. A large proportion of cases could not be fully evaluated due to incomplete case reporting.
Conclusions
While we identified numerous examples of liver injury and death following repeated doses of acetaminophen, all of the deaths and all but 6 cases of hepatic abnormalities involved doses greater than 75 mg/kg/day. This study suggests that the doses of less than 75 mg/kg/day of acetaminophen are safe for children younger than 6 years of age.
Keywords: acetaminophen, pediatrics, repeated dosing
INTRODUCTION
In 2000, there were approximately 20 million children under 5 years of age in the United States (US). Survey data indicate that approximately 26% of children younger than 24 months and approximately 10% of children between 2 and 5 years have received at least one dose of acetaminophen in the past week.1 Among these millions of exposures, there are several cases of liver injury, liver failure and a few deaths reported each year.2
The majority of these tragic cases are clearly attributable to overdose, but some case reports and case series have suggested that deaths may occur with therapeutic dosing. In 2000, Prescott reviewed the clinical details of 89 children who were reported to have liver damage after the use of acetaminophen for therapeutic purposes.3 He noted that in several of the cases, the alleged dose was less than 70 mg/kg/day (below the current recommended daily maximum of 75 mg/kg/day) but also noted that many of these cases had features suggesting that the dosing history was inaccurate or lacked sufficient detail for a valid assessment of dosing and causality.
The objective of this study was to evaluate cases of reported hepatic abnormalities that occurred following repeated doses of acetaminophen in children younger than 6 years of age. This study expands on prior work in several ways. First, we used multiple data sources to increase the number of cases and increase the generalizability of our findings. Second, the relationship of the hepatic abnormality to acetaminophen intake was validated by an expert panel. Finally, the expert panel also reviewed the clinical history and laboratory information to validate the reported dose of acetaminophen. The specific aims were to 1) determine the probability that the hepatic abnormalities described in the cases were due to acetaminophen, 2) determine if any of the cases occurred with therapeutic dosing, 3) estimate the lowest dose of acetaminophen that was likely to have caused hepatic abnormalities among the reported cases, and 4) determine the root cause for the cases where hepatic abnormalities were reported during repeated administration of acetaminophen.
METHODS
Study Design
This was a systematic review of cases reported in the published medical literature, the National Poison Data System (NPDS) of the American Association of Poison Control Centers (AAPCC), the United States (US) Food and Drug Administration Adverse Event Reporting System (AERS) and the McNeil Consumer Healthcare Manufacturer Safety Database. As de-indentified and published patient information was used, this study was deemed exempt research by our institutional review board.
Case definition
We defined cases as children who were younger than 6 years of age, who had repeated exposure to acetaminophen (≥ 2 doses over > 8 hours) and where a death or hepatic abnormality was mentioned in the report. For case identification a hepatic abnormality was defined as any mention of liver or hepatic injury, liver or hepatic failure, or documentation of an elevated serum transaminase, bilirubin, alkaline phosphatase or other suggestion in the report of an adverse event affecting the liver.
Case Identification
Literature Search
The literature search was conducted in MEDLINE (1966–2006) and EMBASE (1980–2006). We used the following strategy: Acetaminophen (MeSH keyword) OR [acetaminophen OR apap OR paracetamol OR 103-90-2].mp; CENTRAL (1968–2006) Acetaminophen [Search All Text] AND children [Search All Text]. OLDMEDLINE (1950–1965) Acetaminophen (MeSH keyword) OR [acetaminophen OR apap OR paracetamol OR 103-90-2].mp Limit: Humans.
As we only included the US Poison Center and Manufacturer Safety Reports, we limited the literature to reports of cases that occurred in the US.
National Poison Data System (NPDS) Search
We searched NPDS (2000–2006) to identify patients less than 6 years with a reported exposure to acetaminophen-containing product (based on NPDS coding) and an outcome coded as “moderate”, “major” or “death”. The NPDS data set captures standardized data fields for demographic characteristics, exposure, treatment and outcome information on all cases reported to US Poison Centers. However, regional poison centers maintain case notes that contain additional data (including how the history of exposure was obtained, complete clinical information and laboratory values). We were able to obtain full case notes from 14 major poison centers representing approximately one-third of all cases. In addition, we obtained all fatality abstracts from this time period from all US poison centers.
FDA Adverse Event Reporting System (AERS) Search
We queried the AERS (2004–2008) for cases with a child less than 6 years old and exposure to an acetaminophen-containing product. The full case record was obtained for each case from the FDA through the Freedom of Information Act.
Manufacturer Safety Reports Search
Manufacturer safety reports (1978–2008) were obtained from McNeil Consumer Healthcare. Safety reports included cases that occurred in the US, reported an adverse event involving a child less than 6 years of age and exposure to a single ingredient acetaminophen product.
Case Review and Processing
Abstraction
All cases were reviewed by two trained data abstractors using a standardized data collection tool. The abstractors recorded demographic information, information about the dose and duration of use for all products, the laboratory, pathology and autopsy findings (with times if available) and medical history. Discrepancies were resolved through discussion and by review with a senior investigator. The abstractors determined the reported dose of acetaminophen using a stepwise approach based on the available information. When sufficient information was provided, the abstractors determined the total daily dose in milligrams. In some cases the dose was reported as a range or maximum dose the range would be reported. If the child’s weight was not reported the abstractors assumed the 90th percentile weight for the age and sex in order to calculate a mg/kg/day dose, thereby erring towards a low mg/kg dosage of acetaminophen. Cases where there was not quantitative or qualitative information on the dose or where the clinical effect could not be determined were excluded from further analysis. A senior investigator reviewed all cases excluded for inadequate documentation of clinical effect and all deaths to verify that the case met exclusion criteria and that there was no dosing information available. Duplicate reports of a single case were identified and the documentation from all sources was provided to the panel. This methodology has been used in a previously reported project.4
Panel Review
The panel consisted of five physicians with substantial experience in the assessment and treatment of acetaminophen-poisoned children. The panel included four medical toxicologists (including two pediatricians) and a hepatologist. The review process consisted of several steps (Figure 1). As described in detail below, the panel members first assessed causality. Second, the panel categorized the dose as therapeutic (≤75 mg/kg/day) or supratherapeutic (>75 mg/kg/day). When possible, the panel also estimated a mg/kg/day dose. Finally, the panel was asked to identify potential root causes. After the cases were independently reviewed, the panel met and all cases were discussed to achieve a consensus categorization for causality, dose and root cause. The panel had access to all information available to the abstractors and could accept or reject any information provided in the abstracted format.
Figure 1.
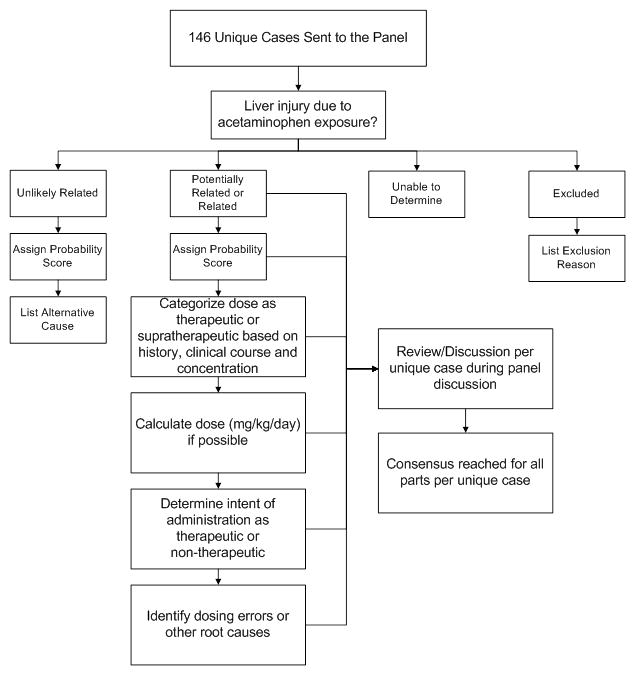
Panel review process for cases of hepatic abnormalities or death possibly related to acetaminophen.
Independent review
Panel members were provided with both the case abstractions and full case source documentation approximately six weeks prior to the meeting. For each case, each panel member independently determined the probability that the liver injury was caused by acetaminophen. Since liver injury that produced a peak serum alanine aminotransferase (ALT) below 100 IU/L was considered medically insignificant, these cases were excluded after panel review to verify that there was no other evidence of liver injury (e.g. elevated INR or encephalopathy). To determine causality, the reviewers were asked to consider the following: history of exposure to acetaminophen, the clinical findings (timing of injury, pattern of hepatic enzymes), presence of acetaminophen in serum (after consideration of timing) and other findings (such as shock liver or viral hepatitis) that might account for the hepatic abnormality. To minimize bias against low doses causing a hepatic abnormality, (i.e. panel members feeling that the dose was “too low” to cause injury), the panel was instructed not to consider the dose of acetaminophen when determining causality.
Each panelist rated the probability that the hepatic abnormality was due to acetaminophen using a categorical scale (unlikely related, potentially related, related and unable to determine). Members were asked to specifically note any possible alternative causes for cases categorized as unlikely rated. For cases judged to be potentially related or related, the panelists also provided a numeric rating of the probability that the hepatic abnormality was due to acetaminophen (0= not acetaminophen to 100= undoubtedly acetaminophen).
For all cases where the hepatic abnormality was categorized as potentially related or related to acetaminophen, the panel members reviewed the reported dose and the clinical and laboratory information to categorize the dose (the panel dose category) as therapeutic, supratherapeutic or unknown. When sufficient information was provided, the panel determined the reported daily dose in mg/kg and a dose of ≤75 mg/kg/day was used to define therapeutic. If less detailed information was provided (e.g. 0.5–1 tablets or every 4–6 hours) the panel assumed values that provided the lowest estimated daily dose. Finally, if no quantitative information was provided, the panel would consider qualitative history. For example, if the dosing was described as “therapeutic” in case notes or report, the panel would consider the reported dose as therapeutic.
The panel categorized the dose as therapeutic or supratherapeutic or unknown by using the reported quantitative dose, the qualitative description of the dose, the consistency of reported dosing within the case, and the clinical and laboratory information. For example, if the report stated a single dose administered 6 hours prior to the onset of acute liver failure, the history would be considered inconsistent with the clinical course and the panel had the option to invalidate the reported dose and determine the panel dose category using other information (such as serum concentrations). Similarly, serum acetaminophen concentrations were reviewed and compared to the dosing history. In the absence of liver failure, the reported dose was considered invalid if the serum concentration was higher than would be expected for the reported dose using a serum half-life of 4 hours (a conservative estimate). For example, if the reported dose was two 15 mg/kg doses 6 hours apart but the serum concentration 4 hours after the last dose was 100 mcg/ml, the panel would categorize the dose as supratherapeutic. If the patient was in liver failure, the panel would consider accumulation and prolonged half-life when determining the consistency of the report with the concentration.
After determining the relationship of liver injury to acetaminophen and determining the panel dose category, each member attempted to determine at least one root cause or contributing factor for the case. Members rated the intent of acetaminophen administration as therapeutic, non-therapeutic (e.g. off label, malicious or sedation) or unknown. The panel could select one or more causes from a list of common medication errors previously reported with acetaminophen products (e.g. use of an incorrect dosing device, administration of an infant concentrated formulation (100 mg/ml) using dosing recommendations for the children’s formulation (32 mg/ml), administration of multiple acetaminophen-containing products, and use of adult formulation) as well as identify other scenarios.
Panel meeting
After individual review of the cases, the panel met during a 2 day session to review each case as a group. Each case was presented by a panel member who provided his rating of the likelihood of relatedness to acetaminophen, other potential causes, the estimated dose and the root cause. The case was then discussed and a consensus was determined.
Analysis
We report descriptive statistics to characterize the subjects, dose and clinical effects. The doses are presented in two ways. First, the reported dose is the numeric or qualitative information on dose contained in the report. Second, the panel determined dose is the panel’s assessment of the dosing category after consideration of all available clinical information.
RESULTS
Our search identified a total of 2531 reports. The distribution of reports from each data source and their ultimate disposition is shown in Figure 2. Most cases were excluded due to a lack of qualifying hepatic abnormality. The majority of the cases were identified from AERS. The 14 poison centers that provided case information for this study managed approximately 1/3 of all acetaminophen exposures reported to NPDS that resulted in moderate or greater outcomes.
Figure 2.
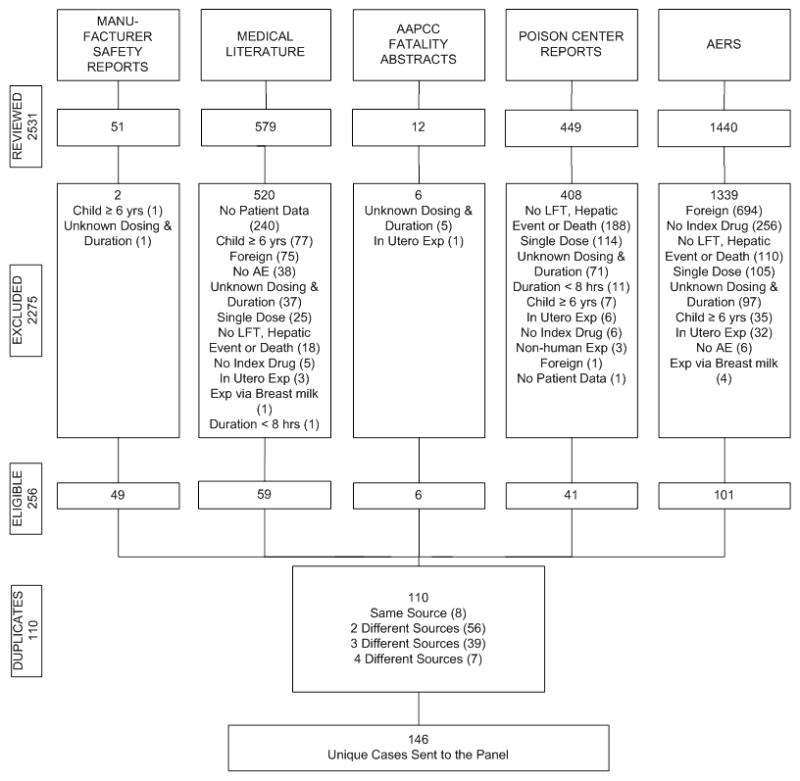
Case sources and disposition of cases of hepatic abnormalities or deaths related to acetaminophen exposure.
Ultimately, 146 unique cases (105 non-fatal hepatic abnormality and 41 deaths) met the inclusion criteria and 102 (76 non-fatal hepatic abnormality and 26 deaths) were rated to be at least potentially related to acetaminophen. Of the remaining 44 cases, 15 (3 non-fatal hepatic abnormality and 12 deaths) were judged as unlikely related with evidence for alternative cause of injury, 11 (3 non-fatal hepatic abnormality and 8 deaths) lacked sufficient information to determine if the hepatic abnormality was related to acetaminophen, and 18 were excluded based upon further review of inclusion criteria (Table 1).
Table 1.
Demographics and case characteristics of acetaminophen toxicity reports that were excluded from full assessment.
| Unable to Determine (n=11) | Unlikely Related (n=15) | Excluded (n=18) | |
|---|---|---|---|
|
| |||
| Age | |||
| < 2 years | 8 (73%) | 12 (80%) | 10 (56%) |
| 2 to < 4 years | 1 (9%) | 1 (7%) | 4 (22%) |
| 4 to < 6 years | 2 (18%) | 2 (13%) | 4 (22%) |
|
| |||
| Gender | |||
| Male | 3 (27%) | 8 (53%) | 11 (61%) |
| Female | 5 (46%) | 7 (47%) | 7 (39%) |
| Not Reported | 3 (27%) | 0 (0%) | 0 (0%) |
|
| |||
| Death | |||
| Fatal | 3 (27%) | 12 (80%) | 0 (0%) |
| Non-Fatal | 8 (73%) | 3 (20%) | 18 (100%) |
|
| |||
| Drug Administered by | |||
| Parent | 5 (46%) | 6 (40%) | 8 (44%) |
| Other | 3 (27%) | 6 (40%) | 5 (28%) |
| Self | 0 (0%) | 1 (7%) | 4 (22%) |
| Healthcare Facility | 1 (9%) | 1 (7%) | 0(0%) |
| Not Reported | 2 (18%) | 1 (7%) | 1 (6%) |
|
| |||
| Exposure Site | |||
| Home | 2 (18%) | 3 (20%) | 12 (67%) |
| Healthcare Facility | 1 (9%) | 1 (7%) | 0 (0%) |
| Other Residence | 0 (0%) | 2 (13%) | 0 (0%) |
| Not Reported | 8 (73%) | 9 (60%) | 6 (33%) |
|
| |||
| Reason for Exclusion | |||
| No Hepatic Event | N/A | N/A | 16 (89%) |
| Acute Overdose | 2 (11%) | ||
|
| |||
| Alternative Causes | |||
| Underlying Illness/Condition | 9 (60%) | ||
| Exposure to Non-APAP Product | N/A | 3 (20%) | N/A |
| Inconsistent Clinical Course | 2 (13%) | ||
| Asphyxia Due to Overlay | 1 (7%) | ||
Of the 76 related non-fatal cases with hepatic abnormality, 48 (63%) were younger than 2 years and 28 (37%) were male (Table 2). The median (interquartile range) of probability scores assigned by the panel for events being caused by acetaminophen were 85 (70 to 90). The reported dose was therapeutic (≤75 mg/kg/day) in 9 cases (12%), supratherapeutic (>75 mg/kg/day) in 43 cases (57%) and unknown in 24 cases (32%). The clinical information on the 9 cases that developed hepatic abnormalities following reported therapeutic doses are detailed in Table 3. For these cases, the median (range) panel probability that the injury was caused by acetaminophen was 60 (30 to 95). After panel review, the reported dose was felt to be inconsistent with the therapeutic dosing in 3 of the cases. For one case (Case 6) the panel felt that the serum acetaminophen concentration of 77 mg/L suggested a higher dose so the dose categorization for the case was determined to be supratherapeutic. For another case (Case 7) the dose would have only been therapeutic if the child’s weight exceeded the 90th percentile for age, and for another case (Case 10) the child was reported as severely malnourished so it is likely that his weight was below the 90th percentile used to calculate the reported dose, making the actual mg/kg dose higher than our estimate.
Table 2.
Demographic and Case Characteristics for All at Least Potentially Related Cases by Fatal Outcome
| Non-Fatal (n=76) | Fatal (n=26) | Total (n=102) | |
|---|---|---|---|
|
| |||
| Age | |||
| < 2 years | 48 (63%) | 14 (54%)* | 62 (61%)* |
| 2 to < 4 years | 15 (20%) | 8 (31%) | 23 (23%) |
| 4 to < 6 years | 13 (17%) | 4 (15%) | 17 (17%) |
|
| |||
| Gender | |||
| Male | 28 (37%) | 9 (35%) | 37 (36%) |
| Female | 36 (47%) | 10 (39%) | 46 (45%) |
| Not Reported | 12 (16%) | 7 (27%) | 19 (19%) |
|
| |||
| Drug Administered by | |||
| Parent | 33 (43%) | 7 (27%) | 40 (39%) |
| Other | 28 (37%) | 9 (35%) | 37 (36%) |
| Not Reported | 7 (9%) | 9 (35%) | 16 (16%) |
| Health Care Facility** | 3 (4%) | 0 (0%) | 3 (3%) |
| Relative | 2 (3%) | 1 (4%) | 3 (3%) |
| Multiple Caregivers | 2 (3%) | 0 (0%) | 2 (2%) |
| Caregiver | 1 (1%) | 0 (0%) | 1 (1%) |
|
| |||
| Exposure Site | |||
| Not Reported | 45 (59%) | 23 (89%) | 68 (67%) |
| Home | 27 (36%) | 3 (12%) | 30 (29%) |
| Multiple Locations*** | 3 (4%) | 0 (0%) | 3 (3%) |
| Health Care Facility | 1 (1%) | 0 (0%) | 1 (1%) |
|
| |||
| Measuring Device Reported | 4 (5%) | 3 (12%) | 7 (7%) |
Due to rounding of percentages totals may not equal 100%.
Includes 1 case of Health Care Facility and Not Reported and 1 case of Health Care Facility and Other.
Includes 2 cases of Home and Health Care Facility and 1 case Health Care Facility and Home.
Table 3.
Demographic and clinical characteristics of cases with death or hepatic abnormalities and reported acetaminophen dose below 76 mg/kg.
| Case# | Source | Age | Sex | Panel score* | Reported daily dose (mg/kg/d) | Duration | Peak Transaminase | Comments |
|---|---|---|---|---|---|---|---|---|
| Fatal Case | ||||||||
| 41 | Man | 5 m | M | 95 | 9.4 | 2 doses | 10960 | Serum APAP 36 mg/L >24 hr post last dose |
| Non-Fatal Cases | ||||||||
| 53 | Lit | 3 m | NR | 50 | 23 | 2d | >2000 | 3 day diarrhea/viral illness |
| 56 | Lit | 5m | RM | 50 | 42 | 2d | >2000 | 3 weeks of fever, viral illness |
| 110 | FDA | 2 y | M | 30 | 50–75 | 14 d | 230 IU/L | Given 4–6 doses/day of APAP product for up to 2 weeks for treatment of mastoiditis. |
| 112 | Lit | 2 y | M | 90 | 60 mg | 1 d | 36,755 | Hydrocephalus, admitted for UTI received APAP in hospital, long chain 3 hyoxyacyl CoA dehydrogenase deficient |
| 123 | PC | 3 | F | 85 | 80–160 | 2 d | 17000 | Hist prior liver failure, given either 4 × 80 or 4×160 mg tabs daily |
| 137 | PC | 4 y | M | 60 | 68# | 2.5 d | 799 | Fever, vomiting. Given adult formulation. |
| 138 | Lit | 4.5y | NR | 75 | 60 | 3 d | 5060 | |
| 139 | Lit | 4.5 | NR | 60 | 36 | 4 d | >2000 | 1 week fever, URI |
| 143 | PC | 5 y | m | 40 | 45.5# | 1 d | 8,000 IU/L | Nemalin Rod Neuropathy, ventilator dependent. 7 day illness- Received 2 doses (1g) of infant drop. Admitted for pneumonia with hepatic injury 2 days after APAP |
Panel score is a consensus score where the panel rated the probability that the liver injury or death was related to acetaminophen. 0= not acetaminophen 100= without a doubt acetaminophen.
Dose estimated using 90th percentile body weight for age and sex.
There were 26 deaths that were at least potentially related to acetaminophen; 14 (54%) were less than 2 years old and 9 (35%) were male (Table 2). The median (interquartile range) for deaths being caused by acetaminophen were 90 (85 to 95). The reported dosing was therapeutic (< 75 mg/kg/day) in 1 (4%) case, supratherapeutic (> 75 mg/kg/day) in 15 (58%) cases and could not be determined in 10 (38%) cases. For the cases where an exact dose could not be determined, the panel categorized the dose as supratherapeutic in 6 and unknown in 4. The lowest reported dose associated with death was 9.4 mg/kg/day. However, the panel considered the dosing history to be inconsistent with the clinical information (serum acetaminophen concentration of 36 mcg/ml 24 hours after two reported 4.2 mg/kg doses) and this case was categorized as supratherapeutic by the panel. A description of this case is shown in Table 3 (Case 1).
The panel indentified a root cause for 83% of fatal and non-fatal cases (Table 4). Common reasons for inadvertent overdose included administration of the wrong product (n=27) administration of a concentrated infant product (100mg/ml) using the children’s product (32 mg/ml) dose and schedule (n=21), use of adult product (n=14), too frequent dosing (n=8) and administration of more than one acetaminophen-containing product (n=7).
Table 4.
Contributing factor mentions/exposure conditions for all cases rated as at least potentially related to acetaminophen.
| Root Causes | Non-Fatal (n=76) | Fatal (n=26) | Total (n=102) |
|---|---|---|---|
| Wrong Dose Administered | 33 (25%) | 15 (35%) | 48 (27%) |
| Wrong Product | 22 (17%) | 5 (12%) | 27 (15%) |
| Confused Drop for Suspension | 18 (14%) | 3 (7%) | 21 (12%) |
| Unknown | 12 (9%) | 5 (12%) | 17 (10%) |
| Adult Preparation | 9 (7%) | 5 (12%) | 14 (8%) |
| Doses Administered Too Close Together | 7 (5%) | 1 (2%) | 8 (5%) |
| Multiple Products | 4 (3%) | 3 (7%) | 7 (4%) |
| Adverse Event at Correct Dose | 6 (5%) | 0 (0%) | 6 (3%) |
| Suppository | 5 (4%) | 1 (2%) | 6 (3%) |
| Underlying Illness or Condition | 4 (3%) | 0 (0%) | 4 (2%) |
| Wrong Unit/Device | 2 (2%) | 1 (2%) | 3 (2%) |
| Alternating Products | 3 (2%) | 0 (0%) | 3 (2%) |
| More Than 1 Caregiver | 2 (2%) | 0 (0%) | 2 (1%) |
| Non-Acetaminophen Ingredient | 0 (0%) | 2 (5%) | 2 (1%) |
| Child Abuse | 0 (0%) | 1 (2%) | 1 (< 1%) |
| Iatrogenic Error | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| Combination Product | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| Crushed Tablets | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| Incorrect Dosage Form | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| English as Second Language | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| Wrong Concentration | 0 (0%) | 1 (2%) | 1 (< 1%) |
| Wrong Dose Schedule | 1 (< 1%) | 0 (0%) | 1 (< 1%) |
| TOTAL Number of Frequency Mentions** | 133 (100%) | 43 (100%) | 176 (100%) |
More than one contributing factor mention/exposure condition may have been identified for each case.
Percentages are calculated out of the total number of mentions for each column; non-fatal, fatal and total.
DISCUSSION
After an extensive review of the published literature, NPDS, manufacturer and AERS reports, the panel identified numerous cases of children younger than 6 years of age who developed hepatic abnormalities, some culminating in death, when acetaminophen was administered with therapeutic intent. The panel identified a small number of cases where hepatic abnormalities in children were described following reported therapeutic doses of acetaminophen. However, the panel found no cases where a death occurred due to acetaminophen toxicity from repeated administration of therapeutic doses of acetaminophen. The panel identified a small number of cases where hepatic abnormalities in children were described following reported therapeutic doses of acetaminophen.
Several of the reports alleging significant liver injury at therapeutic doses were of low quality and the relationship of the injury to acetaminophen could not be fully assessed. The low quality of published reports of drug induced liver injury has been previously highlighted.5 Recently, formal guidelines have been published outlining the elements needed for reporting cases of drug induced liver injury,6 and overall compliance with these guidelines is low7. While it is understandable that voluntary reporting (especially by consumers) may be incomplete, it seems unreasonable to include these cases when making regulatory decisions regarding recommended dosing.
In addition to insufficient information for causality assessment, in several cases the panel felt the reports lacked sufficient detail to allow accurate assessment of the dosing or contained objective evidence that suggested that the dose was actually supratherapeutic. This raises the possibility of publication bias where authors may underestimate dosing. A report that describes liver injury at a therapeutic dose is more likely to be published, while liver injury following overdose is well established. Clearly there is a need for meticulous dose assessment in cases where liver injury is alleged at therapeutic doses.
Finally, several of the cases where the reported dose was therapeutic involved administration of adult products to children- a clear misuse of the product by a caregiver.
We did not identify any characteristics that were associated with an increased risk of injury at therapeutic dosing. The age range was 3 months to 5 years old. While fever and vomiting may have been contributing factors and were reported in the majority of cases, it is impossible to assess the role of these common conditions as possible risk factors without a control group for comparison. Two of the hepatic abnormality cases (Cases 5 and 10) had more unusual conditions (in one malnutrition and long chain 3 hydroxyacyl CoA dehydrogenase deficiency and in the other malnutrition and Nemalin Rod neuropathy) that may have increased their susceptibility to acetaminophen toxicity. (Table 3) There are several recent reports of liver injury in patients with complex medical conditions who were administered therapeutic doses of acetaminophen in a healthcare setting.8–10 These cases included chronically malnourished patients who were hospitalized with acute illness.
The root cause analysis suggests several areas where interventions may effectively reduce the number of cases of acetaminophen induced hepatic abnormalities. Several of the causes involve incorrect dosing by a caregiver. Methods are needed to assure that caregivers can understand the correct dose. Interventions such as unit dosing, improved labeling and providing dosing instructions for children younger than 2 years on the product label may decrease these errors. The recent removal of infant concentrated formulations of cough and cold medications, some of which contained acetaminophen, may prevent dosing errors due to confusion between the children’s and infant formulation for these products. Although, there are still two concentrations of the single-agent acetaminophen liquid products available over-the-counter, industry is transitioning to a single concentration, eliminating the higher concentrated infant formulation and only offering the current children’s concentration of 160 mg/5 ml = 32 mg/ml beginning in 2011.11
There are limitations to our study. The external validity of our results is limited by our selection criteria. While our search was comprehensive and included several data sources, we recognize the likelihood of missed cases. Although our search was limited to reports from the US, we included a large number of cases and have no reason to believe that doses indentified in cases outside the US would systematically differ from domestic cases. Therefore we feel our results can reasonably be generalized to other countries. However, as labels and cultural approaches to using medications may differ, it is likely that the root cause for overdoses may differ in other countries. While we were able to review detailed reports of deaths reported to poison centers, we were only able to review liver injury cases from a limited number of centers. However, these centers managed a significant proportion of the US cases (approximately 1/3) with moderate or greater outcomes during the study period. We therefore believe that our results are generalizable to all poison center cases.
The internal validity of our results is limited by the quality of the reports we reviewed. We had to exclude a large number of cases where the dose was not documented. It is possible that some of these cases may have had therapeutic dosing that was not recorded and this may have resulted in misclassification bias and underestimated the number of cases with therapeutic dosing. We attempted to minimize the exclusion by accepting qualitative reports. Still, the extent of this misclassification cannot be determined. We also excluded some cases where the outcome was not known. However, it seems unlikely that a case with a fatal outcome would be reported as unknown, so the extent of this misclassification is unlikely to be significant.
In conclusion, there are very few cases of children who develop hepatic abnormalities when given repeated doses of acetaminophen. A recent systematic review reported no cases of hepatic injury among 32,414 children who received therapeutic doses of acetaminophen.12 In the current study, the vast majority of cases had reported doses that exceeded the maximum recommended daily dose of 75 mg/kg. Additionally, our expert panel felt that many hepatic abnormalities attributed to therapeutic doses of acetaminophen were due to other causes or that the dosing history was inaccurate and likely exceeded the maximum recommended daily dose.
Footnotes
Conflict of interests: This project was supported by an investigator initiated grant from McNeil Consumer Healthcare to Denver Health. Panel members not employed by Denver Health (GRB, EK, RFC, RSK) received stipends for their participation. KH, AB, JLG, SLM, and RCD are employees of Denver Health and received only their salaries for work performed on this project. Dr. Heard was supported by K08DA020573 from the National Institute on Drug Abuse.
References
- 1.Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Medication use among children <12 years of age in the United States: results from the Slone Survey. Pediatrics. 2009 Aug;124(2):446–454. doi: 10.1542/peds.2008-2869. [DOI] [PubMed] [Google Scholar]
- 2.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila) 2010 Dec;48(10):979–1178. doi: 10.3109/15563650.2010.543906. [DOI] [PubMed] [Google Scholar]
- 3.Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction? Am J Ther. 2000 Mar;7(2):99–114. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]
- 4.Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009 Apr;53(4):411–417. doi: 10.1016/j.annemergmed.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Kelly WN. The quality of published adverse drug event reports. Ann Pharmacother. 2003 Dec;37(12):1774–1778. doi: 10.1345/aph.1D202. [DOI] [PubMed] [Google Scholar]
- 6.Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010 Aug;52(2):730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal VK, McHutchison JG, Hoofnagle JH. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010 May;8(5):463–470. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceelie I, James LP, Gijsen V, et al. Acute liver failure after recommended doses of acetaminophen in patients with myopathies. Crit Care Med. 2011 Jan 14; doi: 10.1097/CCM.0b013e318206cc8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krahenbuhl S, Brauchli Y, Kummer O, et al. Acute liver failure in two patients with regular alcohol consumption ingesting paracetamol at therapeutic dosage. Digestion. 2007;75(4):232–237. doi: 10.1159/000111032. [DOI] [PubMed] [Google Scholar]
- 10.Pearce B, Grant IS. Acute liver failure following therapeutic paracetamol administration in patients with muscular dystrophies. Anaesthesia. 2008 Jan;63(1):89–91. doi: 10.1111/j.1365-2044.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 11.Healthcare MC. [Accessed May 11, 2011];Transition to single concentration- Pediatric liquind acetaminophen products. 2011 http://www.tylenolprofessional.com/letter_pediatric_liquid_acetaminophen_products.html.
- 12.Lavonas EJ, Reynolds KM, Dart RC. Therapeutic acetaminophen is not associated with liver injury in children: a systematic review. Pediatrics. 2010 Dec;126(6):e1430–1444. doi: 10.1542/peds.2009-3352. [DOI] [PubMed] [Google Scholar]


