Abstract
Background
Non-alcoholic steatohepatitis (NASH) is characterized by hepatic lipid accumulation combined with inflammation, which can ultimately progress into cirrhosis. Recently, we demonstrated that deletion of scavenger receptors (SR) CD36 and SR-A in haematopoietic cells reduced hepatic inflammation. In addition to uptake of modified lipoproteins, CD36 and SR-A are also involved in other functions that can activate the inflammatory response. Therefore, the actual trigger for SR activation during NASH is unclear. Here, we hypothesized that hepatic inflammation is triggered by recognition of oxidized LDL (oxLDL) by Kupffer cells (KCs).
Methods
To inhibit recognition of oxLDL by KCs, Ldlr−/− mice were immunized with heat-inactivated pneumococci, which were shown to induce the production of anti-oxLDL IgM antibodies, due to molecular mimicry with oxLDL. The mice received a high fat cholesterol (HFC) diet during the last 3 weeks to induce NASH.
Results
Immunization with pneumococci increased anti-oxLDL IgM levels and led to a reduction in hepatic inflammation, as shown by reduced macrophage, neutrophil and T-cell infiltration, and reduced gene expression of Tnf, Il-6, Il-1β, Mcp1 and fibrosis related genes. In immunized mice, KCs were smaller and showed less cholesterol crystals compared to non-immunized mice.
Conclusion
Antibodies to oxLDL play an important role in the pathogenesis of NASH. Therefore, the potential of PC-based vaccination strategies as a novel tool for the prevention and therapy of NASH should be tested in future.
Keywords: Liver, Inflammation, Kupffer cells, Cholesterol, Pneumococci
Non-alcoholic fatty liver disease (NAFLD) is a condition ranging from benign lipid accumulation in the liver (steatosis) to steatosis combined with inflammation. The latter is referred to as non-alcoholic steatohepatitis (NASH). NASH is considered as the hepatic component of metabolic syndrome. Estimates from the USA are that 5.7% to 17% of all adults have NASH, while 17% to 33% of Americans suffer from NAFLD (1, 2). As obesity and insulin resistance reach epidemic proportions in industrialized countries, the prevalence of both NAFLD and NASH is increasing. NAFLD is therefore a major health hazard (3). Steatosis alone is considered a relatively benign and reversible condition. However, the transition towards NASH represents a key step in the pathogenesis, as it sets the stage for the development of fibrosis, cirrhosis and liver cancer. While the mechanisms leading to steatosis are well described, little is known about the actual risk factors that drive hepatic inflammation during the progression towards NASH. Consequently, no therapeutic options are poor. Therefore, knowledge about the events that lead to hepatic inflammation is of great importance for the diagnosis and treatment of NASH.
Recently, we demonstrated that deletion of scavenger receptors (SR) CD36 and SR-A in haematopoietic cells reduced hepatic inflammation (4). In addition to uptake of modified lipids, scavenger receptors are involved in many other inflammatory pathways. These pathways include cellular adhesion, innate immune responses and phagocytosis of apoptotic cells (5). Based on the analogy between mechanisms for atherosclerosis and NASH, it is likely that the recognition of oxLDL by KCs, rather than other pathways, is the actual trigger for scavenger receptor-mediated inflammation. Therefore, we hypothesized that hepatic inflammation is triggered by the recognition of oxLDL by KCs.
It has recently been shown that the levels of IgM autoantibodies to modified LDL are inversely correlated with atherosclerosis (6–8). Oxidation-specific epitopes present in oxLDL are major targets of natural IgM antibodies (9). These antibodies arise spontaneously without prior infection or immune exposure and mainly consist of the IgM isotype (10). They are produced by innate-like B-1 cells, and provide a first line of defense against bacterial and viral pathogens (11),(12). In addition, natural IgM antibodies play an important role in providing house keeping functions by protecting from the accumulation of biological waste, such as oxLDL (10). Upon oxidation of LDL, reactive oxidation products from phospholipids retain the intact phosphorylcholine (PC) headgroup, which becomes available for immune recognition. These PC headgroups represent one of many so-called ‘oxidation-specific’ epitopes and are found on the outer side of the membrane of oxLDL (13). A panel of monoclonal autoantibodies directed to epitopes of oxLDL was cloned from the spleens of apoE−/− mice (14). In particular, one immunodominant clonotypic set of IgM autoantibodies was identified, EO6, which was shown to specifically bind to the PC moiety of oxidized PC-containing phospholipids, such as those present in oxLDL (13). EO6 antibodies were found to be identical to the natural T15 antibodies, which are germline encoded natural antibodies exclusively derived from B-1 cells. These T15 antibodies protect mice against Streptococcus pneumoniae infections, because PC is also present in the capsular polysaccharide of the cell wall of this bacterium. Based on this molecular mimicry, immunization of Ldlr−/− mice with heat-killed S. pneumonia resulted in higher serum titers of anti-oxLDL IgM antibodies and decreased atherosclerosis (15). These findings suggest that anti-oxLDL antibodies directed to the PC group present on oxLDL possibly inhibit the recognition of oxLDL by macrophage scavenger receptors, such as CD36.
The aim of the current study was to determine whether oxLDL is causally involved in the pathogenesis of NASH. For this purpose, Ldlr−/− mice were used as a well-recognized model mimicking the human lipoprotein metabolism with high fidelity and is therefore also extremely useful to investigate the physiological triggers for hepatic inflammation, which can already develop upon short term treatment with high fat high cholesterol (HFC) diet (16). These mice were immunized with heat-inactivated pneumococci to investigate whether anti-oxLDL antibodies have a protective effect on NASH. Supporting our hypothesis, immunized Ldlr−/− mice showed reduced hepatic inflammation compared to non-immunized mice. These data clearly demonstrate the importance of antibodies to oxLDL in the pathogenesis of NASH. Therefore, the potential of PC-based vaccination strategies as novel tool for the prevention and therapy of NASH should be tested in future.
Experimental procedures
Preparation immunogen
For immunization, the heat-inactivated R36A strain of Streptococcus pneumoniae (Birmingham, Alabama) was used, still bearing the PC headgroup epitope similar to oxLDL. Colonies of the R36A strain were harvested at mid log phase after incubation at 37°C on blood agar plates and transferred to Todd-Hewitt plus 0.5% yeast broth. The mid log phase is characterized by an OD value of 0.425 to 0.45 at 600 nm. S. pneumoniae was heat-inactivated at 60°C for 30 minutes; afterwards no colonies of this suspension were detected on blood agar plates. For freezer stocks of strain R36A, small aliquots of S. pneumoniae at mid log density were harvested and suspended in Todd-Hewitt plus 80% sterile glycerol and stored at −80°C (17).
Mice, immunization and diet
Ldlr−/− mice on a C57BL/6 background were housed under standard conditions and had access to food and water ad libitum. Experiments were performed according to Dutch laws, approved by the Animal Experiment Committee of Maastricht University.
The immunization protocol started in 12 week old female mice, fed a normal chow diet. Mice were divided into 4 groups (n = 10 for each group) and received the equilivant of 108 CFU of the heat-killed pneumococcal immunogen emulsified in 200µl sterile 0.9% NaCl for the primary subcutaneous immunization, subsequently three intraperitoneal booster immunizations were administered every two weeks (15). The control group received a NaCl injection only. After immunization, the mice were given normal chow, the control group, or a HFC diet, the experimental group, for 3 weeks. Blood from the tail vein was collected after the dietary period and mice were then sacrificed by cervical dislocation. Liver tissue was isolated and snap-frozen in liquid nitrogen and stored at −80°C or fixed in 4% formaldehyde/PBS. The collection of blood and specimens, the biochemical determination of lipids in plasma and the liver, liver histology, ALT, RNA isolation, cDNA synthesis and qPCR and auto-antibody titers against IgG and IgM antibodies to CuOx-LDL and MDA-LDL were extensively described previously (4). Immune complex (IC) measurements were performed as described previously (15). Briefly, circulating ICs were determined by a capture assay in which a polycolonal antibody specific for muring apoB100 was coated on microtiter wells at 5µg/ml in PBS. Individual mouse sera (1:100) were added to the wells and incubated for 1 hour at room temperature. IgM bound to the captured apoB-containing particles was detected using an alkaline phosphatase conjugated goat anti-mouse IgM antibody by chemiluminescent ELISA. The amount of IgM bound to the captured LDL was then normalized for the amount of captured apoB, and expressed as a ratio of IgM counts (RLU/100ms) / apoB100 counts (RLU/100ms) or IgM/apoB.
Electron microscopy
A detailed overview about the (post)fixation, embedding, cutting and type of electron microscope was described previously (16). To stain the Kupffer cell lysosomes, acid phosphatase (ACPase) enzyme cytochemistry was performed. Small wedge biopsies of the liver were perfused by syringe injection with icecold 2% purified glutaraldehyde in 0.1M cacodylater buffer (pH 7.4) for 15 min. The wedge biopsies were cut in small pieces and kept in 0.1M cacodylate buffer + 7.5% sucrose at 4°C until further processing; the buffer solution was refreshed weekly. The samples were frozen for 1h at −30°C whereafter 50µm thick cryosections were made. These sections were incubated according to the cerium-based method of Robinson and Karnovsky for the localization of ACPase 32. After the incubation, the sections were washed two times in 0.1M cacodylate buffer supplemented with 5% sucrose, refixed in 3% glutaraldehyde in cacodylate buffer for 1h and rinsed overnight in veronal acetate buffer (pH 7.4, 4°C). The sections were then postfixed for 30 min in 2% osmium tetroxide in veronal buffer + 4% sucrose and then routinely processed for embedding in epon.
Statistical analysis
Data was statistically analyzed by performing two-tailed non-paired t-tests using GraphPad Prism, version 4.03 for Windows. Data were expressed as the mean ±SEM and considered significant at p < 0.05. *, ** and *** indicate p < 0.05, 0.01 and 0.001 resp.
Results
Increased IgM antibody titers against modified LDL after immunization with heat-inactivated pneumococci
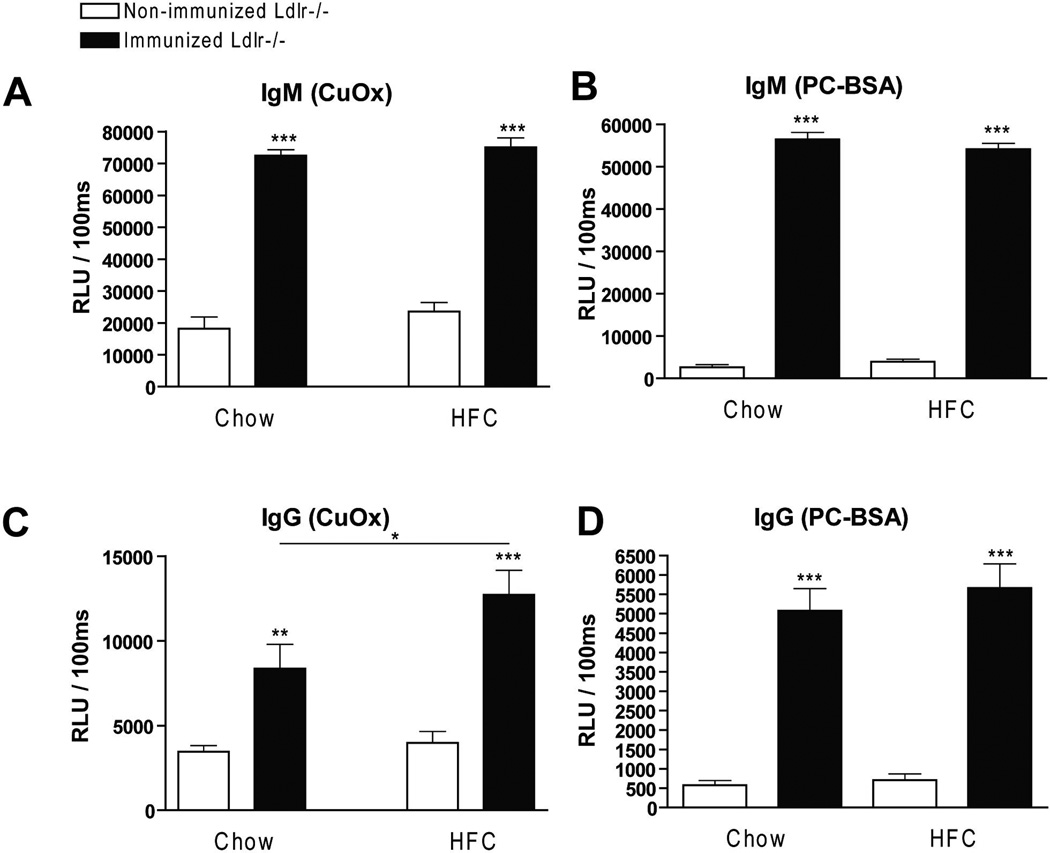
To determine whether IgM autoantibodies to oxLDL have a protective effect on liver inflammation, mice were immunized for 9 weeks with heat-inactivated pneumococci, known to induce high anti-oxLDL IgM titers dominated by T15-idiotypic IgM. To induce NASH, the mice received a HFC diet during the last 3 weeks. Total body weight and the ratio of liver weight to total body weight were not significantly different between the different groups (Supplemental Fig. 1). Immunization of Ldlr−/− mice with heat-inactivated pneumococci resulted in a strong increase in IgM titers to oxLDL (Fig. 1A+B). Only weak, although significant IgG responses were observed, consistent with previous reports that pneumococcal immunizations induce an IgM dominated thymus-independent type-2 response highly specific for PC (Fig. 1C+D). The levels of circulating IgM/apoB immune complexes did not differ between the groups, likely indicating efficient clearance of oxLDL (Supplemental Fig. 2)
Figure 1.
IgM autoantibodies in mice that received pneumococcal immunization. (A–D) IgM and IgG antibodies against oxLDL (CuOx and PC-BSA) were measured in plasma of pneumococci-immunized (n=10) and control (n=10) mice at a dilution of 1:200, respectively. Data is expressed as RLU = Relative light units / 100ms and were triplicate determinations. (*, ** and ***: p<0.05; 0.01; 0.001 respectively)
No difference in liver lipid levels between immunized and non-immunized Ldlr−/− mice after 3 weeks of HFC diet
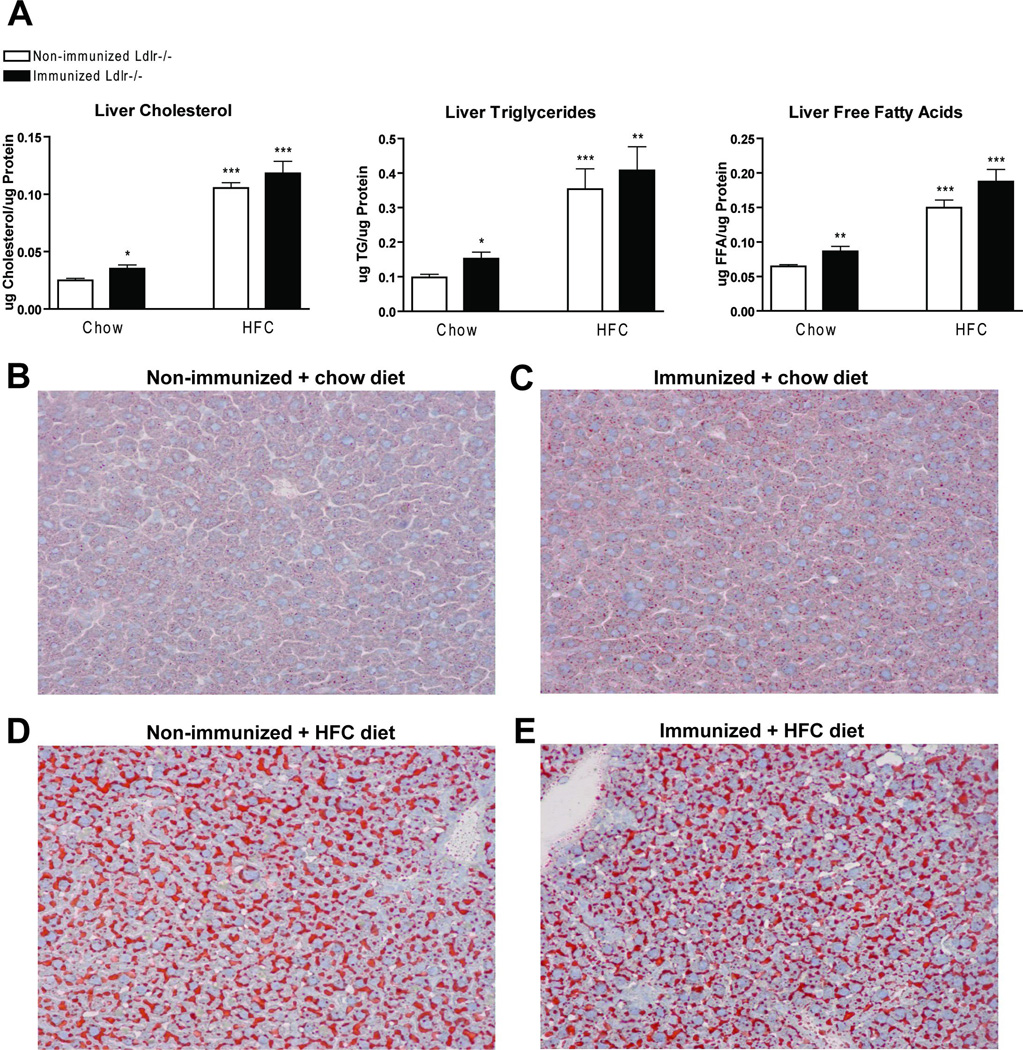
To investigate liver lipid levels in hyperlipidemic mice with or without immunization, biochemical assessment of liver cholesterol, triglycerides (TG) and free fatty acids (FFA) was carried out (Fig. 2A). After 3 weeks on HFC diet, a clear increase in all liver lipid levels was observed compared to mice on a chow diet. Liver lipid levels did not differ between immunized and non-immunized Ldlr−/− mice on the HFC diet. Mice on the chow diet showed a small increase in liver lipid levels after immunization when compared to non-immunized Ldlr−/− mice. Oil red O and HE staining confirmed the biochemical liver lipid measurements (Fig. 2B–E and Supplemental Fig. 3).
Figure 2.
Liver lipid levels. (A) Liver cholesterol, triglycerides (TG) and free fatty acids (FFA) after chow and 3 weeks of HFC diet. (B–E) Oil red O staining after 3 weeks of HFC diet in (B+D) non-immunized (C+E) and immunized Ldlr−/− mice after (B+C) chow and (D+E) 3 weeks of feeding on the HFC diet, respectively. * Significantly different from non-immunized mice on chow diet. (*, ** and ***: p<0.05; 0.01; 0.001 respectively)
Decreased plasma cholesterol in immunized Ldlr−/− mice on the HFC diet compared to control mice
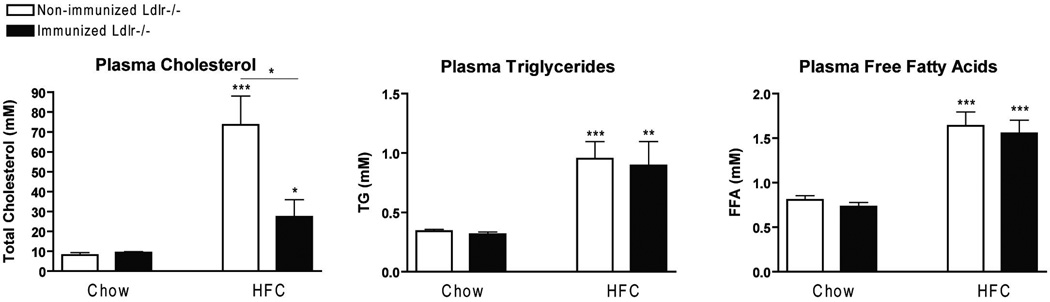
The effect of immunization on plasma lipids was assessed by measuring the levels of plasma cholesterol, TG and FFA. After feeding on the HFC diet, a significant increase was observed for all plasma lipids compared to mice on a chow diet. Interestingly, plasma cholesterol was reduced in immunized Ldlr−/− mice compared to non-immunized mice on the HFC diet. Plasma TG and FFA did not differ between the groups following the HFC diet. On chow diet, plasma lipid levels did not differ between the groups (Fig. 3).
Figure 3.
Plasma lipid levels. Plasma cholesterol, triglycerides and free fatty acids after chow and 3 weeks of HFC diet in non-immunized and immunized Ldlr−/− mice. * Significantly different from non-immunized mice on chow diet. (*, ** and ***: p<0.05; 0.01; 0.001 respectively)
Decreased hepatic inflammation in Ldlr−/− mice immunized with heat-inactivated pneumococci
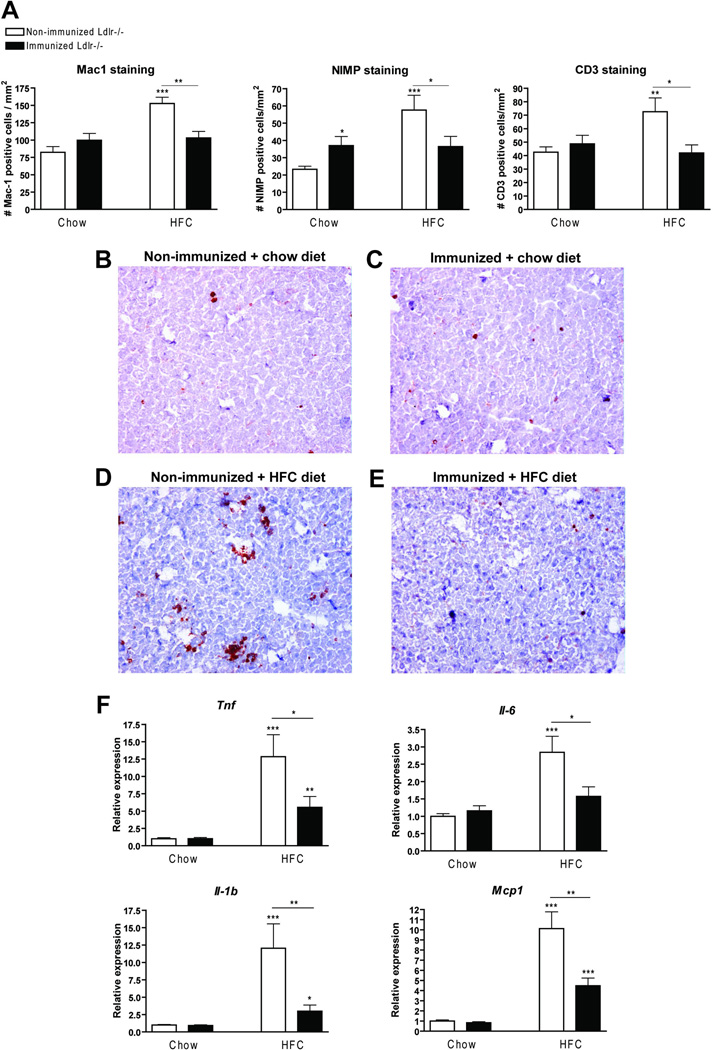
To determine whether immunization of Ldlr−/− mice with heat-inactivated pneumococci affects hepatic inflammation, liver sections were stained for the inflammatory cell markers Mac-1 (infiltrated macrophages and neutrophils), NIMP (neutrophils) and CD3 (T cells). As shown in figure 4A, the number of infiltrated macrophages, neutrophils and T cells was lower in immunized Ldlr−/− mice compared to non-immunized mice after feeding the HFC diet. These data on cell infiltration are confirmed by HE staining (Supplemental Fig. 3). Moreover, the normal chow diet induced a significant increase in the number of neutrophils in immunized chow-fed mice compared to non-immunized mice. Representative histological pictures of the Mac-1 staining for all four experimental groups are shown in Figure 4B–E. Further confirming the reduced hepatic inflammation in immunized Ldlr−/− mice on the HFC diet, gene expression analysis showed a significant decrease in the inflammatory markers tumour necrosis factor (Tnf), interleukin-1beta (Il-1b), interleukin 6 (Il-6) and monocyte chemoattractant protein-1 (Mcp1) in livers of immunized Ldlr−/− mice on the HFC diet compared to non-immunized mice (Fig. 4F). However, hepatic inflammation in Ldlr−/− mice on the HFC diet after immunization was still higher than chow-fed immunized mice according to the inflammatory markers Tnf, Il-1b and Mcp1. The presence of elevated transaminases in plasma like alanine aminotransferase (ALT) did not differ between the different groups (Supplemental Fig. 4).
Figure 4.
Parameters of hepatic inflammation. (A) Liver sections were stained for infiltrated macrophages and neutrophils (Mac-1), neutrophils (NIMP) and T cells (CD3), respectively, and counted. (B–E) Representative pictures of Mac-1 staining (200×) after feeding on the chow (B+C) and HFC diet (D+E) in non-immunized (B+D) and immunized (C+E) Ldlr−/− mice, respectively. (F) Gene expression analysis for tumour necrosis factor (Tnf), interleukin 6 (Il6) and 1β(Il1β) and monocyte chemoattractant protein 1 (Mcp1). * Significantly different from non-immunized mice on chow diet. *, ** and *** indicate p < 0.05, 0.01 and 0.001 respectively.
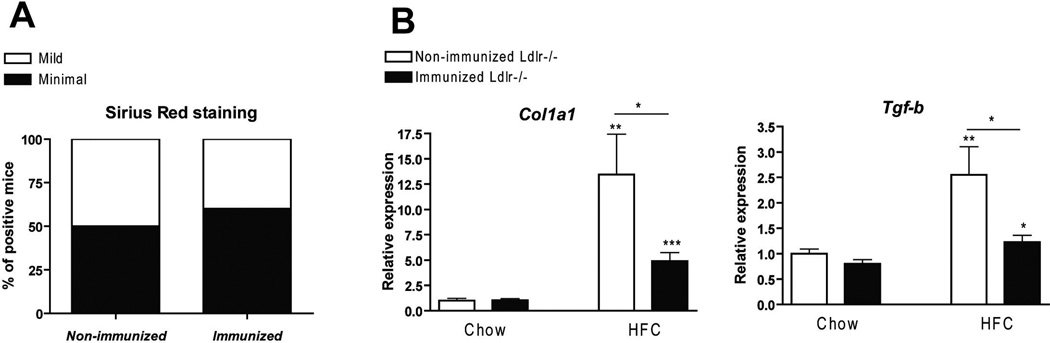
After 3 weeks of HFC diet, immunization prevented expression of fibrosis-related genes in Ldlr−/− mice
Fibrosis is considered to be an advanced stage of NASH. Collagen staining (Sirius Red) was performed to determine the degree of fibrosis. No differences were observed between the experimental groups after 3 weeks of HFC diet (Fig. 5A), which is probably related to the short duration of the HFC diet. However, gene expression analysis for collagen type 1A1 (Col1A1) and transforming growth factor beta (Tgf-β) demonstrated that the mRNA levels of these fibrogenic genes were lower in immunized mice compared to non-immunized mice on the HFC diet (Fig. 5B).
Figure 5.
Parameters of hepatic fibrosis. (A) Quantification of sirius red (collagen) after 3 weeks of HFC diet. Livers were quantified as minimal, mild or moderate positive for collagen around and in between the blood vessels of the liver. (B) Gene expression analysis of the fibrosis markers, collagen (Col1a1) and transforming growth factor beta (Tgf-β). * Significantly different from non-immunized mice on chow diet. *, ** and *** indicate p < 0.05, 0.01 and 0.001 respectively.
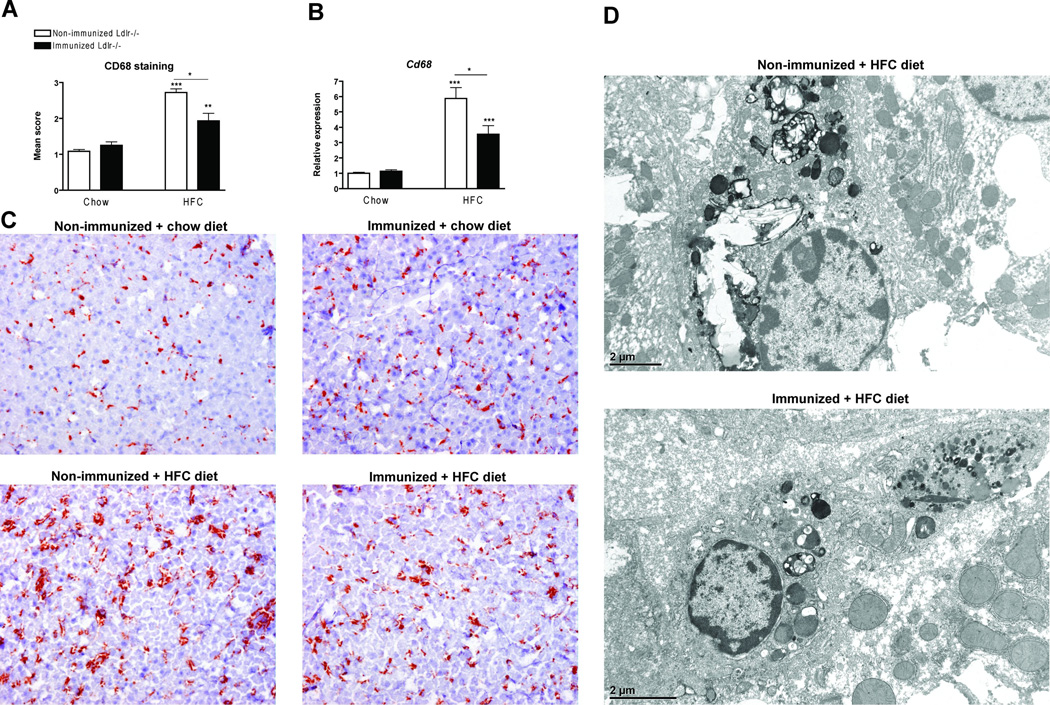
Decreased foamy appearance of Kupffer cells in immunized Ldlr−/− mice on the HFC diet
Immunohistochemistry for CD68 was performed to characterize the KCs. Scoring of the CD68 positive sections revealed a reduction in size of foamy KCs in immunized Ldlr−/− mice compared to non-immunized mice on the HFC diet (Fig. 6A+C). Gene expression of Cd68 was reduced in the immunized Ldlr−/− mice compared to non-immunized mice on the HFC diet (Fig. 6B). Electron microscopy of KCs confirmed the differences in size of the KCs between immunized and non-immunized Ldlr−/− mice, and showed that the immunized mice upon HFC diet had less lysosomal cholesterol accumulation and cholesterol crystals compared to non-immunized mice (Fig. 6D).
Figure 6.
Foamy Kupffer cells. (A) Liver sections were stained for CD68 (Kupffer cells) and scored for the level of foamy appearance: 1 (mild foamy appearance) to 3 (severe foamy appearance). Mean scores were calculated from six microscopic views. (B) Gene expression analysis of the Kupffer cell activation marker, CD68. (C) Representative images of liver sections stained for CD68 for the Ldlr−/− mice on a chow diet without and with immunization and for the Ldlr−/− mice on the HFC diet without and with immunization respectively at a 200× magnification. (D) Electron microscopy of foamy Kupffer cells. Acid phosphatase staining indicating the lysosomes of the Kupffer cells in non-immunized and immunized Ldlr−/− mice on the HFC diet.* Significantly different from non-immunized mice on chow diet. * and *** indicate p < 0.05 and 0.001 respectively.
Discussion
Until now, the actual risk factors that drive hepatic inflammation during the progression to NASH are unknown. To determine whether oxLDL is causally involved in the pathogenesis of NASH, serum anti-oxLDL IgM antibody levels were increased by immunizing Ldlr−/− mice with heat-inactivated pneumococci, which dramatically decreased hepatic inflammation. These data point towards oxLDL as a trigger for hepatic inflammation. Furthermore, our data suggest that PC-based vaccination strategies could be the basis for a vaccination protocol towards the therapy of NASH. However, the long term consequences of immunization are unknown at the moment and should be tested in the future.
Scavenger-receptor-mediated uptake of oxidized lipoproteins by macrophages sets off a cascade of pro-inflammatory events leading to the initiation of the inflammatory response. OxLDL is phagocytosed by macrophages via binding of the oxPC molecules present in oxLDL to macrophage scavenger receptors, and results in foam cells (18, 19). Furthermore, CD36 has been implicated in inflammatory signaling induced by oxLDL (20). Previously, we have shown that both CD36 and SR-A play an important role in diet-induced NASH (4). Since scavenger receptors have a wide spectrum of functions (21–23), it is not clear whether the recognition of modified lipoproteins is the actual trigger for hepatic inflammation during NASH. Our data demonstrate for the first time that inflammation is reduced in the livers of pneumococci-immunized mice. These results are in line with earlier findings demonstrating decreased atherosclerotic lesion formation after pneumococcal immunization (15). Similarly, apoE −/−mice immunized with PC, one of the epitopes of anti-oxLDL autoantibodies present in oxLDL but also in the CPS of S. pneumoniae, demonstrated an increase in anti-oxLDL autoantibodies together with a reduction in atherosclerotic lesions (24). A number of in vitro studies suggest that the induced IgM antibodies against oxLDL prevented binding and uptake of oxLDL by macrophages and/or neutralized its pro-inflammatory signaling (11, 15, 25, 26). Indeed, the inflammatory process associated with atherosclerotic plaque formation is linked to the cytotoxicity and macrophage chemo-attractivity of oxLDL. Moreover, oxLDL is thought to be an atherogenic factor because its uptake by macrophages results in foam cells formation, the hall mark cells of atherosclerotic lesions (18, 19, 27). Our data provide evidence for similar mechanisms between atherosclerosis and NASH. Thus, the reduced inflammation in mice in which the scavenger receptors on haematopoietic cells had been deleted is likely to be related to the reduced recognition of oxLDL by KCs.
Interestingly, plasma cholesterol levels were significantly reduced in our immunized Ldlr−/− mice. Previously, it was shown that anti-oxLDL antibodies directed to the PC group present on oxLDL inhibit the recognition of oxLDL by macrophage scavenger receptors (28). We speculate that the formed immune complexes containing both LDL and oxLDL particles may be cleared faster by alternative pathways, as this is the case for IgM mediating apoptotic cell clearance. However, measurements of IgM/apoB immune complexes indicated that there were no differences between our groups of Ldlr−/− mice, also suggesting that the induced IgM mediate their protective effect by directly neutralizing the pro-inflammatory effects of oxLDL. These findings are in line with Binder et al, who demonstrated that under these conditions, no differences in immune complexes were observed (15). It is possible that the protective effect of these antibodies in vivo is further enhanced via a reduction in plasma cholesterol levels, since plasma cholesterol levels are an important trigger for hepatic inflammation (16).
NASH patients are often associated with high levels of lipid peroxidation products such as those present in oxLDL. Therefore, it has been suggested that the elevated levels of lipid peroxidation might make an important contribution to the pathogenesis of NASH (29, 30). In literature, it is demonstrated that the presence of immune responses towards lipid peroxidation products can be a predictor of progression of NAFLD (31). In addition, it was demonstrated that oxidized phosphatidylcholines (oxPC) were found predominantly in steatotic hepatocytes and macrophages/KCs and were more abundant in NAFLD/NASH livers than in normal control livers (32). Moreover, we have previously shown that NASH patients display increased hepatic myeloperoxidase activity which is also associated with lipid peroxidation (33). The role of oxidative stress as a key factor contributing to hepatic injury in patients with NASH (34, 35), has been underlined by a study with vitamin E therapy of non-diabetic NASH patients (36).
As fibrosis is one of the later consequences of NASH, we investigated the effect of immunization with heat-inactivated pneumococci on hepatic fibrosis. Gene expression of fibrosis-related genes was decreased, yet not confirmed by Sirius Red staining. This is probably due to the short time period of 3 weeks on the HFC diet, as Ldlr−/− mice only develop fibrosis after 3 months on a mild atherogenic diet (4). However, we report for the first time that uptake of oxLDL is associated with fibrogenesis in vivo. In line with these observations, a study by Kang et al demonstrated that oxLDL can activate hepatic stellate cells in vitro (37, 38). These findings indicate a crucial role for oxLDL in the fibrogenic process.
As expected, immunized mice on the HFC diet showed decreased foamy KCs compared to non-immunized mice. This reduction in size is probably due to decreased plasma cholesterol levels, as the size of the foamy KCs is not always correlated with the inflammatory state of the liver (4, 16). Overloading of macrophages with oxLDL was shown to lead to the formation of cholesterol monohydrate crystals (39). In line with these findings, we showed that after immunization with heat-inactivated pneumococci, KCs were less foamy, had less lysosomal cholesterol accumulation and therefore also less cholesterol crystals. These data indicate that the increased cholesterol accumulation inside KCs, together with the crystallization, is linked to hepatic inflammation.
To date, no therapy for NASH is available. Our novel data in mice clearly suggest that future research should focus on oxLDL as a trigger for NASH. Therefore, the potential of PC-based vaccination strategies to be used as a novel tool for the prevention and therapy of NASH should be tested in future.
Supplementary Material
Weight. (A) Total body weight and (B) ratio of liver weight to total body weight in in non-immunized and immunized Ldlr−/− mice on the HFC diet.
Circulating immune complexes in plasma. The ratio of IgM/apoB immune complexes in non-immunized and immunized Ldlr−/− mice on chow and HFC diet.
General liver histology. Represantative pictures (200× magnification) of Hematoxylin Eosin (HE) staining after chow and 3 weeks of HFC diet in non-immunized and immunized Ldlr−/− mice.
Plasma ALT levels. Aminotransferase (ALT) levels in plasma of non-immunized and immunized Ldlr−/− mice after chow and HFC feeding.
Acknowledgements
FINANCIAL SUPPORT:
Veni: 916.76.070 (2006/00496/MW); Maag Lever Darm Stichting (MLDS) (WO 08-16 + WO 09-46); DEB NIH R01 AI21548
We are grateful to Mag. Maria Ozsvar Kozma (Medical University of Vienna), Hans Duimel and Marie-Hélène Lenders (Maastricht University, the Netherlands) for their excellent technical help.
ABBREVIATIONS
- acLDL
acetylated LDL
- EM
electron microscopy
- FFA
free fatty acid
- HFC
high-fat, high-cholesterol
- IC
immune complexe
- Ig
immunoglobulin
- KC
Kupffer cell
- LDL
low density lipoprotein
- LDLR
low density lipoprotein receptor
- MDA
malondialdehyde
- MPO
myeloperoxidase
- MSR
macrophage scavenger receptor
- NASH
non-alcoholic steatohepatitis
- ORO
oil red o staining
- oxLDL
oxidized LDL
- PC
antigenic epitope of an exposed phosphorylcholine end group; oxLDL contains PC epitopes
- QPCR
quantitative PCR
- RLU
relative light unit
- SR
scavenger receptors
- TG
triglycerides
- WT
wild type
References
- 1.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124:248–250. doi: 10.1053/gast.2003.50032. [DOI] [PubMed] [Google Scholar]
- 3.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, Binder CJ, Lutjohann D, et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 138:2477–2486. 2486, e2471–e2473. doi: 10.1053/j.gastro.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 7.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 12.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 13.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 16.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lutjohann D, Kerksiek A, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 17.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itabe H, Suzuki K, Tsukamoto Y, Komatsu R, Ueda M, Mori M, Higashi Y, et al. Lysosomal accumulation of oxidized phosphatidylcholine-apolipoprotein B complex in macrophages: intracellular fate of oxidized low density lipoprotein. Biochim Biophys Acta. 2000;1487:233–245. doi: 10.1016/s1388-1981(00)00098-6. [DOI] [PubMed] [Google Scholar]
- 19.Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 20.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J Immunol. 2004;173:6427–6432. doi: 10.4049/jimmunol.173.10.6427. [DOI] [PubMed] [Google Scholar]
- 24.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, et al. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 25.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 26.Chang MK, Bergmark C, Laurila A, Horkko S, Han KH, Friedman P, Dennis EA, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayo S, Ohsawa M, Ehara S, Naruko T, Ikura Y, Hai E, Yoshimi N, et al. Oxidized low-density lipoprotein levels circulating in plasma and deposited in the tissues: comparison between Helicobacter pylori-associated gastritis and acute myocardial infarction. Am Heart J. 2004;148:818–825. doi: 10.1016/j.ahj.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50(Suppl):S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 30.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634–1636. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 31.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, et al. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 33.Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, Malle E, et al. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 35.McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang Q, Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1. Lab Invest. 2009;89:1275–1290. doi: 10.1038/labinvest.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 39.Tangirala RK, Jerome WG, Jones NL, Small DM, Johnson WJ, Glick JM, Mahlberg FH, et al. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J Lipid Res. 1994;35:93–104. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Weight. (A) Total body weight and (B) ratio of liver weight to total body weight in in non-immunized and immunized Ldlr−/− mice on the HFC diet.
Circulating immune complexes in plasma. The ratio of IgM/apoB immune complexes in non-immunized and immunized Ldlr−/− mice on chow and HFC diet.
General liver histology. Represantative pictures (200× magnification) of Hematoxylin Eosin (HE) staining after chow and 3 weeks of HFC diet in non-immunized and immunized Ldlr−/− mice.
Plasma ALT levels. Aminotransferase (ALT) levels in plasma of non-immunized and immunized Ldlr−/− mice after chow and HFC feeding.