CONSPECTUS
Short DNA or RNA oligonucleotides have tremendous potential as therapeutic agents. Because of their ability to engage in Watson-Crick base pairing they can interact with messenger mRNA or pre-mRNA targets with high selectivity and thus offer the possibility of precise manipulation of gene expression. This possibility has engendered extensive efforts to develop oligonucleotides as drugs, with many candidates already in clinical trials. However, a major impediment to the maturation of oligonucleotide-based therapeutics is the fact that these relatively large and usually highly charged molecules have great difficulty crossing cellular membranes and thus in penetrating to their sites of action in the cytosol or nucleus. In this Account we first summarize some basic aspects of the biology of antisense and siRNA oligonucleotides and then discuss chemical conjugation as an approach to improving the intracellular delivery and therapeutic potential of these agents. Our emphasis will be on the pharmacological ramifications of oligonucleotide conjugates rather than the details of conjugation chemistry. One important approach has been conjugation with ligands designed to bind to particular receptors and thus provide specificity to the interaction of cells with oligonucleotides. Another approach has been to couple antisense or siRNA with agents such as cell penetrating peptides that are designed to provoke escape of the conjugate from intracellular vesicular compartments. Both of these approaches have enjoyed some success. However, there remains much to be learned before oligonucleotide conjugates can find an important place in human therapeutics.
1. Basic Aspects of Oligonucleotide Biology
Classic Antisense Oligonucleotides
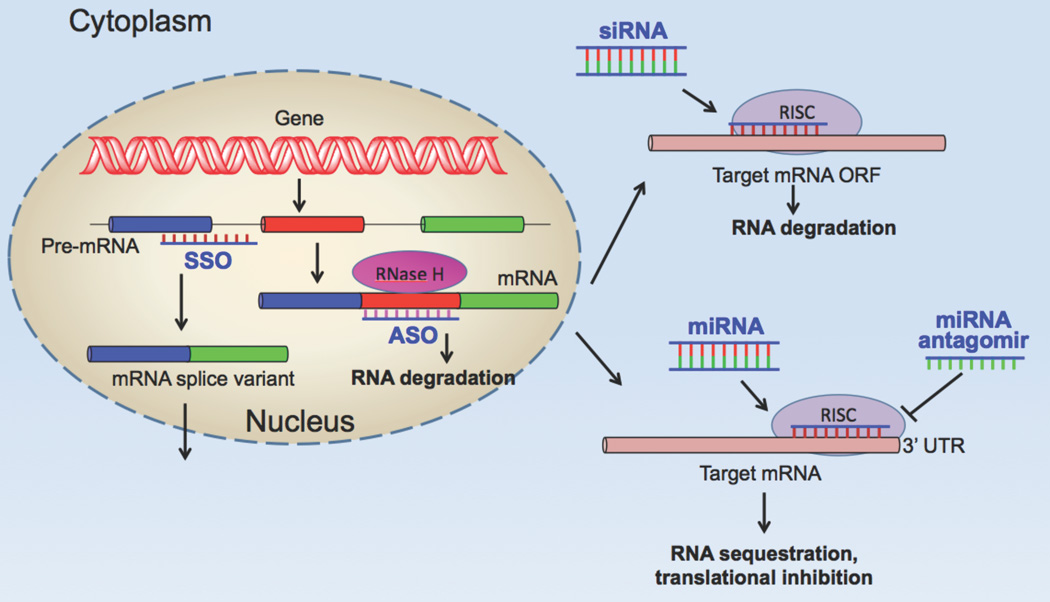
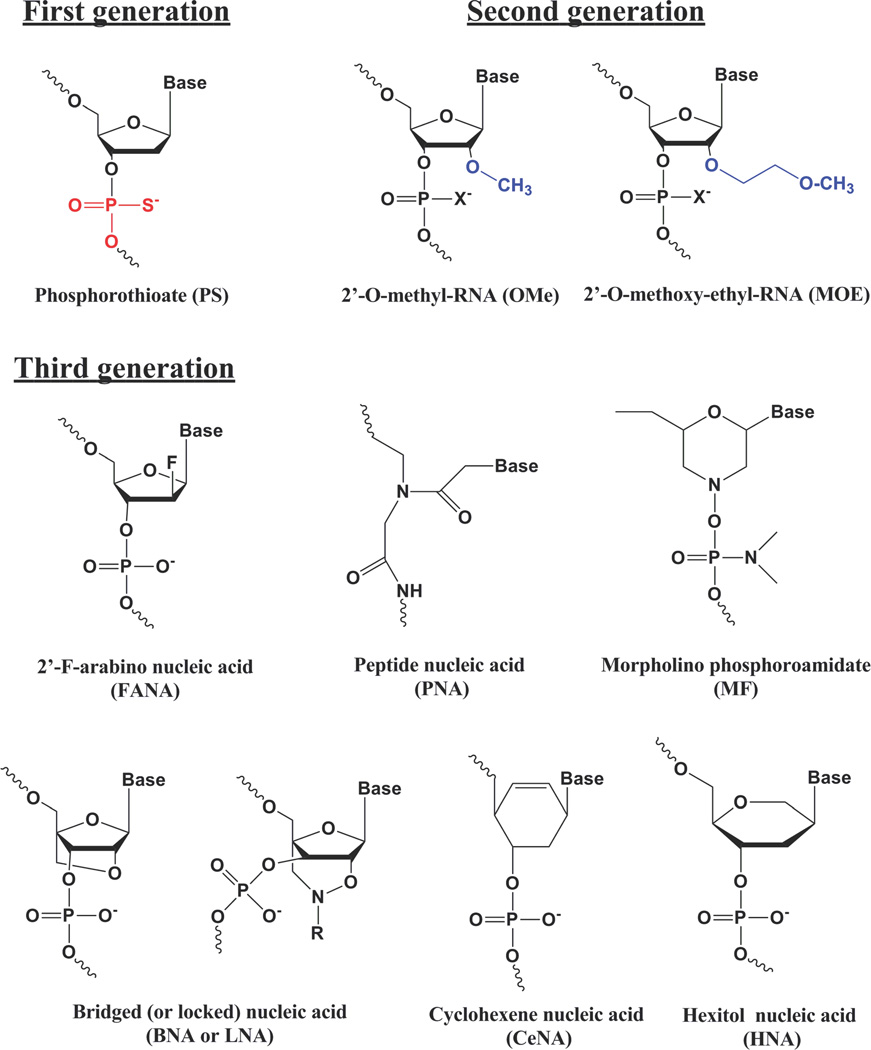
Relatively short (≥16 bases) oligonucleotides that are readily prepared by convenient solid phase synthesis can provide highly selective recognition among the multiple RNAs derived from the human genome. Upon introduction into cells, DNA antisense oligonucleotides can enter the nucleus and engage in specific base pairing with complementary sequences in pre-mRNA. The formation of a DNA/RNA hybrid results in the recruitment of the enzyme RNaseH that can cleave the RNA, which then triggers further degradation of the cleaved fragments by other enzymatic processes (Figure 1). Native DNA, with phosphodiester internucleotide linkages, proved to be too unstable for work in cell culture or in vivo. Thus a large number of chemical modifications were developed to enhance the biological effectiveness of antisense molecules (Figure 2). A common modification is the substitution of sulfur for oxygen in the phosphate backbone leading to phosphorothioate (PS) oligonucleotides. These are considerably more stable than native DNA in the biological milieu; however they have the potential disadvantage of being ‘sticky’ and binding non-specifically to proteins. Another important set of modifications involves substitution of aliphatic residues at the 2’ position of the nucleoside sugar thus creating 2’-O-Methyl and similar derivatives. This results in molecules that are more RNA-like and often bind with higher affinity to RNA. Another important modification is represented by locked nucleic acids (LNAs) where a 2’–4’ bridge results in oligonucleotides with very high binding affinity. Finally, families of uncharged oligonucleotides have been created by radical modification of the backbone. Replacement of the phosphodiester backbone with peptide linkages gives rise to peptide nucleic acids (PNAs), while inclusion of non-ionic phosphorodiamidate linkages gives rise to morpholino oligonucleotides. These backbone modified molecules, as well as the 2’ modifications, do not recruit RNAse H but do retain excellent base pairing specificity and the ability to influence other processes involving mRNA such as message splicing and protein translation1,2.
Figure 1. Oligonucleotide Mechanisms of Action.
Four mechanisms are illustrated. In the nucleus: (i) classical antisense (ASO) mediated mRNA degradation via ribonuclease H; (ii) alteration of exon choice using splice switching oligonucleotides (SSO). In the cytosol: (iii) siRNA mediated mRNA degradation via the Ago 2/RISC complex; (iv) miRNA modulation of mRNA function.
Figure 2. Common Chemical Modifications of Oligonucleotides.
Other than the morpholino and PNA backbone modifications that form uncharged molecules, the various chemistries shown can be applied to siRNA as well as to single stranded oligonucleotides.
siRNA and miRNA
A surge of recent discoveries have fundamentally altered our understanding of the role of RNA in the cell. Not only is mRNA the key link between the genome and proteome, but multiple other forms of RNA are now known to play critical roles in virtually all aspects of gene expression3. From the therapeutic perspective siRNA and miRNA have evoked the greatest interest. Short (~21 base pair) double stranded RNA segments can be loaded onto the multi-protein RISC complex4. For siRNA action the Argonaute 2 (Ago 2) protein is the critical element of the RISC complex; this protein degrades one of the two strands (sense strand) while retaining the other (the antisense or guide strand). The loaded RISC complex then surveys mRNAs in the cytosol and selects for perfect complementarity. The mRNA in the RNA/RNA duplex is then cut at a position10 bases from the 5’ end of the guide strand, and the two fragments of the mRNA are then further degraded by other enzymatic processes. See Figure 1. The actions of miRNAs are also mediated by the RISC complex but in this case there is usually not full complementarity between the guide strand and the target mRNA and the binding sites are often in the 3’ untranslated region of the mesage5. See Figure 1. This results in message sequestration into P-bodies6, interruption of translation, and often in degradation. Since only partial complementarity is needed this means that a single miRNA may interact with multiple mRNAs and thus be involved in the regulation of multiple genes. While in some cases miRNAs themselves have be used to modify expression of disease-related genes7, a more common approach is the use of ’antagomirs’, antisense oligonucleotides complementary to the miRNA that can bind and inactivate it8.
Splice Switching Oligonucleotides
An important approach involves the use of so-called ‘splice switching antisense oligonucleotides’ (SSOs). Although the human genome contains only about twenty thousand genes it can direct the production of hundreds of thousands of proteins via alternative splicing of pre-mRNA. Using oligonucleotides complementary to splice junctions it is possible to re-direct the nuclear splicing machinery to include or exclude particular exons (or segments of introns)9. See Figure 1. The SSO must be designed so that it does not recruit RNaseH or the result would be degradation rather than altered splicing. Fortunately this is easy to do using either oligonucleotides that are fully substituted at the 2’ position, or that have uncharged backbones. The SSO technology will hopefully be useful in the many human diseases that involve defects in splicing; additionally, through manipulation of the splicing of reporter genes, it has provided a very convenient assay for measuring the effectiveness of delivery of oligonucleotides to the nucleus10.
Off-Target Effects
In theory antisense, siRNA, and SSO oligonucleotides should be capable of modulating the expression of only the target gene and no other gene. However, in practice there are several sources of off-target effects of oligonucleotides. Simplest to understand are situations where there is partial complementarity between the target message and another message. Another source of off-target effects relates particularly to phosphorothioate oligonucleotides that tend to bind non-specifically to many proteins. In addition, any single-stranded oligonucleotide can fold to form a three-dimensional structure that can act as an aptamer11 and thus serve as a ligand for proteins. Finally, many types of oligonucleotides interact with Toll-Like Receptors (TLRs) or other components of the innate immune system12 to trigger the expression of cytokines and other genes involved in host defense mechanisms.
Pharmacokinetics and Biodistribution of Oligonucleotides
There is a vast literature on this topic including excellent recent reviews13,14; however the essence can be described rather briefly. Systemically injected oligonucleotides rapidly (minutes) leave the blood compartment and are redistributed to various tissues15,16. Since the molecular mass of most oligonucleotides is well below the cut-off for renal filtration, there is a second phase of clearance (minutes-hours) when the oligonucleotides exit from tissues stores and are excreted in the urine. An exception to this is the case of phosphorothioates, since they tend to bind to plasma and tissue proteins thus substantially prolonging their lifetime in the body14. While systemically administered oligonucleotides can enter most tissues to some degree (other than the CNS), both antisense and siRNAs tend to be most highly accumulated in tissues that are rich in reticuloendothelial cells including the liver and spleen, or in kidney proximal tubule cells13,17. This natural biodistribution has influenced the choice of therapeutic targets for oligonucleotide pharmacology.
Cellular Uptake and Subcellular Trafficking of Oligonucleotides
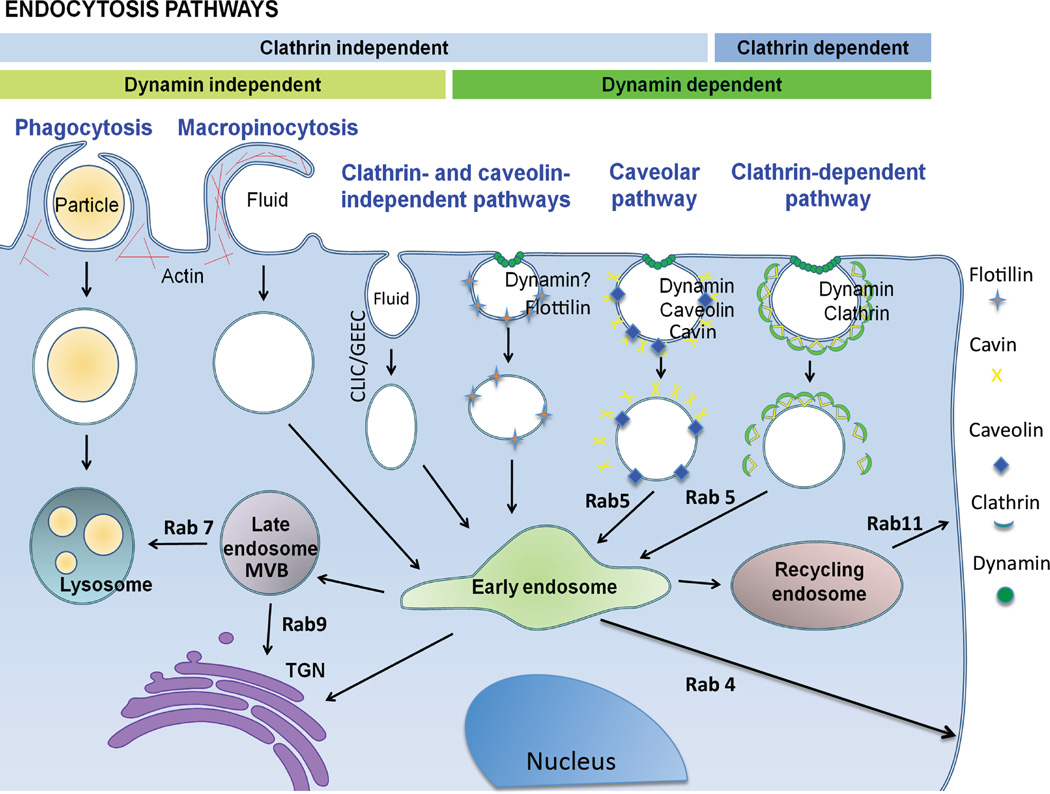
Being large, polar molecules oligonucleotides do not permeate across biological membranes. In general oligonucleotides enter cells by some form of endocytosis and then traffic to diverse subcellular compartments. Recently the complexity of oligonucleotide uptake and intracellular trafficking and its role in determining the functional effectiveness of these molecules has become better appreciated10,18–21. As depicted in Figure 3 there are multiple mechanisms of endocytosis leading to distinct intracellular membrane-bound compartments including early and late endosomes, lysosomes, the Golgi apparatus and the endoplasmic reticulum22. Much of the oligonucleotide that accumulates in cells becomes non-productively trapped in endomembrane compartments. However, because of the dynamic nature of intracellular trafficking processes, a small amount of oligonucleotide can ‘leak’ to the cytosol and diffuse into the nucleus23. It is this minor component of the total cellular pool that is pharmacologically active. In general phosphorothioate-based antisense molecules are taken up more effectively than either siRNA or uncharged single-strand oligonucleotides; however even the phosphorothioates are subject to endosome trapping.
Figure 3. Pathways of Endocytosis and Intracellular Trafficking.
Several of the illustrated endocytotic pathways have been associated with the uptake of various types of oligonucleotide conjugates or of ‘free’ oligonucleotides. Various intracellular membranous organelles are also illustrated as are some of the proteins associated with trafficking pathways. The Rab proteins are GTPases that help to guide intracellular traffic. (reproduced with permission23).
Clinical Evaluation of Oligonucleotides
There has been an enormous effort to mature oligonucleotide pharmacology to the point of human application with numerous ongoing clinical trials of both antisense and siRNA13,24. In most cases oligonucleotides in current studies in humans are given as ‘free’ molecules, not associated with any sort of carrier or delivery system. However, a few trials involved use of lipid-based delivery systems. The oligonucleotides being tested clinically mainly incorporate relatively simple chemical modifications such as use of 2’-O-Methoxyethyl residues in antisense or F or 2’-O-Methyl modifications of siRNA. Many of the intended targets are cancer-related; however, there are also examples of trials that focus on local administration such as inhalation25, or on targets in the liver26, an organ that tends to accumulate oligonucleotides. A very exciting development is the testing of both morpholino27 and 2’-O-Methyl28 SSOs to correct (at the RNA level) the genetic defect in Duchenne muscular dystrophy, an otherwise incurable disease.
Key Challenges for Oligonucleotide Pharmacology
Despite some promising clinical developments with ‘free’ antisense or siRNA molecules, there remain concerns about the inability of these compounds to permeate membranes, their unproductive accumulation in endosomes, and their poor pharmacokinetic and biodistribution profiles16,29. Additionally, it would be highly desirable to be able to ‘target’ oligonucleotides to specific cells or tissues and thus enhance their selective therapeutic actions. Much work has been devoted to addressing these challenges with the major thrusts being the development of lipid based or polymer based nanocarrier systems as summarized in recent reviews30,31. In this Account, however, we will focus solely on molecularly defined chemical conjugates as an alternative strategy for overcoming the challenges of oligonucleotide pharmacology.
2. Chemical Conjugation of Oligonucleotides
Much of the work on chemical modification of oligonucleotides has been directed at increasing stability in the biological milieu, improving potency, and reducing off-target effects1,2. More directly related to this Account is the chemistry underlying conjugation of oligonucleotides with molecules designed to improve intracellular delivery or in vivo pharmacokinetics and biodistribution32. An excellent recent review delineates many of the issues involved in the conjugation of oligonucleotides with various partners33. For example, the merits of solid phase versus solution phase conjugation are discussed. The former is highly efficient and facilitates purification; however, it is limited by the availability of appropriate synthons and by the need for both partners of the conjugate to be stable under the conditions of synthesis. In contrast, solution phase conjugation allows synthesis of each component under the most appropriate conditions, but the conjugation reaction may be inefficient and substantial purification problems can occur. This review also discusses specific aspects of the conjugation of oligonucleotides with peptides, carbohydrates and lipophilic molecules. Another useful review focuses entirely on solid phase synthesis of oligonucleotide conjugates34. Recent reports have brought new conjugation strategies to bear; this includes use of “click chemistry”35,36 and conjugation via phosphoramidation reactions37.
Peptides
Perhaps the most extensive studies on peptide-oligonucleotide conjugation have been done in the context of PNA and morpholino SSOs38,39. While it is possible to produce peptide/PNA chimeras by solid phase synthesis, in most cases peptide-oligonucleotide conjugates are formed by solution phase conjugation of the peptide to the oligonucleotide followed by purification. A variety of linkages have been used including amide, thioether, thiol-maleimide, ester, and disulfide. Conjugates of peptides with non-charged oligonucleotides are purified by reversed phase HPLC while conjugates with charged oligonucleotides are usually purified by ion exchange or by large scale PAGE. In our hands we have found that analysis by MALDI-TOF mass spectrometry is suitable for many peptide-oligonucleotide conjugates but that some of the larger conjugates require use of electrospray ionization mass analysis. An important question is whether it is essential to use a bioreversible linkage such as a disulfide in order to attain biological activity. This does not seem to be the case however, and experience in our laboratory40,41 as well as by others42 has shown that both bioreversible and non-reversible linkages can work well.
Lipids
The addition of lipophilic moieties to antisense and especially siRNA has proven to be a powerful approach to modification of the pharmacokinetic and pharmacodynamic characteristics of oligonucleotides. In particular conjugation with cholesterol promotes the interaction of the oligonucleotide with albumin and serum lipoproteins and subsequently promotes tissue uptake primarily via the hepatic lipoprotein receptors43. Tocopherol (Vitamin E) conjugates have also been used in a similar context44. In general simple lipophilic groups can be incorporated via solid phase synthesis using the appropriate phosphoramidites, some of which are commercially available. Chemical aspects of lipid conjugation to oligonucleotides as well as the physical properties of such conjugates have been extensively discussed in a recent review45.
Carbohydrates
Conjugation of oligonucleotides with carbohydrate moieties can provide a powerful approach to targeting the lectin-like proteins that exist on many cell types. While linking a monosaccharide to an oligonucleotide is relatively simple and can be approached through the preparation of carbohydrate containing phosphoramidites, synthesis of oligonucleotides bearing the more complex oligosaccharide structures needed for optimal lectin recognition is far more challenging34. Recently ‘click’ chemistry has been applied to the preparation of several types of oligonucleotide glycoconjugates including rather complicated branched oligosaccharides46,47.
Nucleic Acids
The formation of conjugates or chimeras of antisense or siRNA oligonucleotide with other nucleic acids moieties has some very interesting ramifications. One approach has been to prepare chimeras of siRNA with aptamers that target specific cell surface receptors. Typically a polynucleotide comprising the ‘passenger’ (sense) strand of the siRNA linked to the aptamer is synthesized by in vitro transcription, including the incorporation of 2’-F pyrimidines; thereafter the ‘guide’ (antisense) stand is complexed by hybridization48,49.
3. Receptor Targeted Conjugates
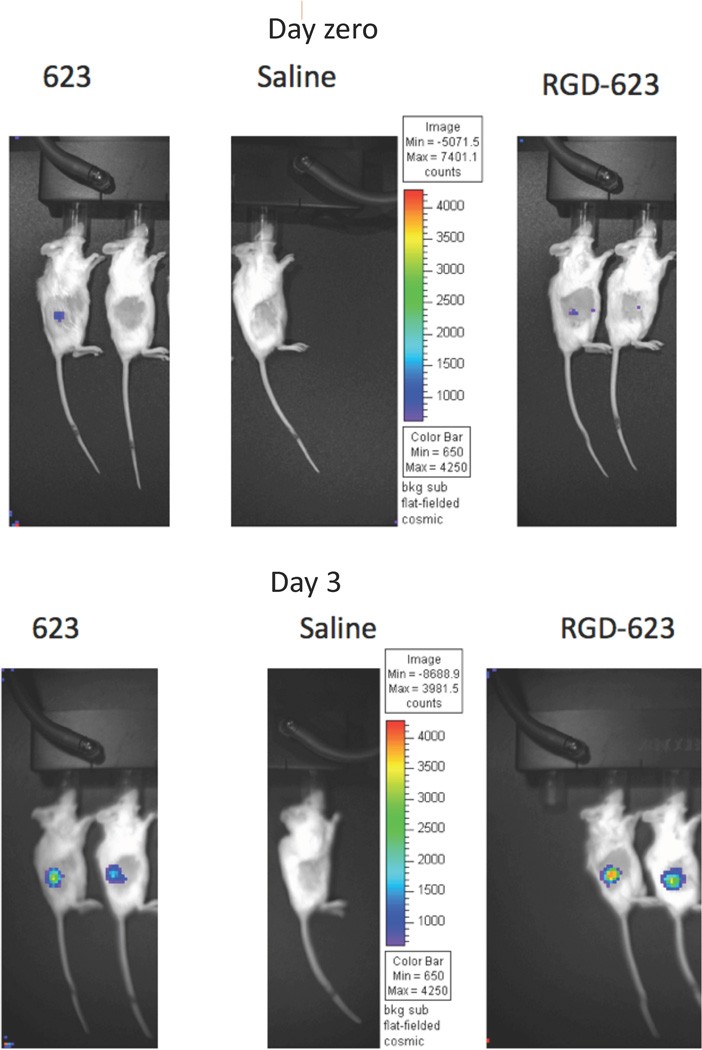
Lately there has been a substantial increase in work on receptor targeting of oligonucleotides via conjugation50,19. For example, our laboratory has described Arg-Gly-Asp (RGD) peptide conjugates of splice switching antisense oligonucleotides (SSOs) that can be effectively delivered to melanoma cells in culture via the αvβ3 integrin40. The RGD-SSO conjugates have also been tested to a limited degree in xenograft models of melanoma and have shown some activity (Figure 4). We have also studied conjugates of SSOs to bombesin, a peptide that binds with high affinity to BB2, a G Protein-Coupled Receptor that is over-expressed in some tumors including prostate cancer10 and have demonstrated substantial receptor-dependent effects in this system. We have also used anisamide, a high affinity small molecule ligand for the sigma receptor, to target oligonucleotides selectively to tumor cells. The anisamide–SSO conjugates were prepared by a novel solid phase method that involves creation of phosphoramidites of the ligand51. We have recently extended the RGD targeting approach to siRNA. Thus bi-, tri-, and tetra-valent RGD conjugates of a potent luciferase siRNA were synthesized52. As compared to un-conjugated siRNA, each of the RGD-conjugates displayed a remarkably increased and highly receptor-selective uptake into M21 melanoma cells that express the αvβ3 integrin. Interestingly however, only the tri- and tetra-valent conjugates displayed significant ‘knockdown’ effects. The issue of valency is a complex one in the context of conjugates between oligonucleotides and high affinity ligands. Initially we had assumed that increasing valency would lead to increased avidity and thus increased cell uptake and biological effect. However, in our experience this simple pattern does not necessarily prevail. For example, increasing valency does not necessarily lead to increased cell uptake. We have found this both with RGD-siRNA conjugates52 and with bombesin-SSO conjugates (unpublished). However, in the case of the RGD-siRNA conjugates there was an increased biological effect of the higher valency conjugates even though uptake levels were similar. We hypothesize that this behavior may be based on different intracellular trafficking of the various conjugates. Recently evidence has been accumulating that the route of intracellular trafficking can profoundly influence the pharmacological activity of oligonucleotides18,21.
Figure 4. In Vivo Effects of a Targeted Oligonucleotide Conjugate.
A RGD-SSO conjugate was used in these experiments. Human A375 melanoma cells were stably transfected with a luciferase reporter cassette that responds to an appropriate SSO by increasing production of properly spliced luciferase message and functional luciferase protein. The cells were then used as xenografts in SCID mice. After the tumors were established the animals were treated with 10 mg/kg of RGD-SSO or unconjugated SSO or with saline. Three days later luciferin was injected and photon emission due to luciferase was measured on an optical imaging system. Induction of luciferase was approximately 3-fold stronger in the animals treated with RGS-SSO as compared to free SSO while treatment with saline had no effect.
As mentioned above, there has been considerable recent interest in using nucleic acid conjugates or chimeras for the targeting of siRNA. One example involves the delivery of siRNA by targeting TLRs53. An un-methylated CpG DNA oligonucleotide known to bind to TLR9 was chemically conjugated to a siRNA. This resulted in enhanced ‘knockdown’ of endogenous and reporter genes in immune system cells known to express TLR9. In in vivo studies, a CpG siRNA targeting the immunosuppressive regulator Stat3 resulted in enhanced antitumor immune responses. A very promising approach to selective delivery of oligonucleotides involves use of nucleic acid aptamers11. A pioneering report described the biological effects of chimeric molecules comprised of an aptamer with high affinity for the Prostate Specific Membrane Antigen (PMSA) surface glycoprotein linked to siRNAs that affected key survival genes including Plk1 and Bcl254. In cell culture, the aptamer-siRNA chimeras were taken up efficiently by cells that expressed PSMA and they effectively reduced the target mRNAs. When administered by direct intra-tumoral injection the chimeras produced a significant tumor growth inhibition. A chemically optimized version of a PSMA aptamer-Plk1siRNA chimera displayed antitumor activity against PMSA-expressing tumors when given by systemic administration48. Other important examples of aptamer-siRNA chimeras are also beginning to emerge. Thus an aptamer siRNA chimera that bound to the viral gp120 protein was able to substantially reduce viral loads in HIV infected humanized mice49. Similarly an aptamer targeting the T-cell CD4 receptor linked to antiviral siRNAs was able to protect against vaginal HIV transmission in humanized mice55. In another study, PSMA-siRNA chimeras inhibited tumor growth in vivo using siRNAs directed against Upf2 and Smg1, two genes involved in nonsense mediate mRNA decay and thus in immune regulation of tumors56.
The various studies discussed above, using receptor-targeted conjugates of antisense or siRNA strongly suggest that monomeric ligand-oligonucleotide conjugates can produce significant biological effects both in cell culture and in vivo without the use of any transfection agents. In many cases strong pharmacological effects can be attained at nanomolar levels of targeted SSO or siRNA. Despite these promising early studies there are several issues that must be addressed to allow further progress. A major impediment is the dearth of knowledge about the uptake and trafficking mechanisms involved in receptor mediated oligonucleotide delivery. Additionally many synthetic challenges remain. While it is relatively easy to make monovalent peptide-oligonucleotide conjugates, more complex conjugations are much more demanding. Thus, conjugates incorporating large nucleic acid aptamers, or multi-valent peptide ligands, can be challenging to synthesize and hard to purify.
4. Conjugates with Cell Penetrating Peptides
Cell Penetrating Peptides (CPPs) are peptides, usually cationic, that purportedly possess the ability to cross membranes and also to convey attached ‘cargos’ such as other peptides, proteins, or nucleic acids across the membrane as well. The TAT and Antennepedia peptides are early examples of CPPs, but a large variety of new CPPs have been described more recently57,58. Initially it was believed CPPs or CPP-cargo conjugates could directly translocate across the plasma membrane. However, this view has been radically revised and most studies suggest that cationic CPPs initially bind to negatively charged proteoglycans at the cell surface, are subsequently internalized into endosomes, and may then escape from endosomes to enter the cytosol. An important consideration is that the nature of the attached cargo plays a major role in CPP uptake mechanisms and in the effectiveness of cytosolic delivery; as might be anticipated, smaller cargos are more effectively delivered to the cytosol while larger moieties are primarily retained within endosomes59,60.
Although there have been numerous studies of both chemical conjugates and noncovalent complexes of antisense and siRNA with CPPs, the utilization of CPPs for intracellular delivery of oligonucleotides has had a somewhat checkered history38,50. In general, the conjugation of CPPs with charged oligonucleotides has not led to effective compounds. One issue is the tendency of the positively charged CPPs and the anionic oligonucleotides to aggregate and form insoluble complexes. However, conjugation with uncharged oligonucleotides such as morpholinos or PNAs has been more promising. Thus studies with conjugates of novel CPPs to PNA61 or morpholino62 SSOs have shown excellent splice-correction activity in cell culture, and promising therapeutic performance in mouse models of Duchenne muscular dystrophy63,64. An interesting approach involves synthesizing CPP-oligonucleotide conjugates that also contain a lipid moiety65; this seems to lead to enhanced endosomal escape and thus greater efficacy of the conjugated PNA SSO. A parallel approach has been to create nanoparticle complexes of anionic oligonucleotides and cationic CPPs; this strategy has been recently reviewed in detail66, but is really outside of the scope of the current article and will not be further discussed. A potential limitation with CPP-SSO conjugates is that, as with unconjugated SSOs, they usually require micromolar concentrations (or the in vivo equivalent) to attain significant biological effects. This may indicate that CPPs are only partially effective in releasing endosomal stores of oligonucleotides. As mentioned previously, SSOs are being evaluated clinically27,28; hopefully the further development of novel, non-toxic CPP-SSO conjugates may provide enhanced therapeutic effects.
5. Conclusions
While chemical modification has been an important part of the entire history of oligonucleotide research, the sub-topic of oligonucleotide conjugation has become particularly active recently. New strategies such as ‘click chemistry’ and various solid-state approaches have made conjugation with complex partners more accessible. The field is developing experience with the conjugation of a variety of moieties including peptides, lipids, carbohydrates and other forms of nucleic acids, and the biological ramifications of various types of conjugates are becoming better understood. A somewhat surprising generalization is that the linker between oligonucleotide and its partner moiety can be quite varied with relatively little impact on the biological potency of the conjugate (although exceptions will no doubt occur). Coupling to ligands that bind selectively to cellular receptors seems a very promising approach for both single stranded oligonucleotides and siRNA. Impressive successes in cell culture and in vivo have been attained with nucleic acid aptamers as ligands, while peptide-oligonucleotide conjugates also have demonstrated potency and specificity. Reports on the biological properties of lipid and carbohydrate conjugates have not been as abundant in the published literature, but there is certainly a good deal of activity concerning these approaches. Cell penetrating peptides have found an important niche in the delivery of uncharged oligonucleotides, primarily of the SSO type that have such great potential in the treatment of genetic diseases. Further developments in the chemistry of CPPs should augment their potential as delivery agents. As interesting concept is whether some of the beneficial properties of receptor-targeted ligands and of CPPs could be combined in the same oligonucleotide conjugate thus improving both cellular specificity and intracellular delivery.
Table 1.
Selected Examples of Biologically Active Oligonucleotide Conjugates
| Oligonucleotide | Conjugation Partner |
Linkage | Target | Cell Uptake Via |
Reference |
|---|---|---|---|---|---|
| 2’-O-Me PS SSO | Bombesin peptide | Maleimide terminated peptide linked to 5’ SH oligonucleotide | Abnormal exon in luciferase reporter | BB2 receptor | 10 |
| 2’-F modified siRNA | None:chimera with RNA aptamer made by in vitro transcription | NA | Plk-1 mRNA | PSMA | 48 |
| siRNA | CpG DNA oligonucleotide | Carbon linker between the CpG and antisense siRNA strand | Stat 3 mRNA | TLR-9 | 53 |
| Morpholino SSO | Novel CPP with 6-aminohexanoic acids and arginines | Amide linker | Exon and intron 23 of the mouse dystrophin gene | CPP mediated non-specific uptake | 64 |
| siRNA | Tocopherol | 5’ phosphate linkage using a tocopherol phosphoramidite | ApoB mRNA | Lipoprotein Receptors? | 44 |
Acknowledgement
This work was supported by NIH grant RO1 CA151964 to RLJ. The authors wish to thank Ben Parise for expert assistance with graphics.
References
- 1.Corey DR. RNA learns from antisense. Nat Chem Biol. 2007;3:8–11. doi: 10.1038/nchembio0107-8. [DOI] [PubMed] [Google Scholar]
- 2.Kurreck J. RNA interference: from basic research to therapeutic applications. Angewandte Chemie. 2009;48:1378–1398. doi: 10.1002/anie.200802092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–420. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331:550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nature reviews. Molecular cell biology. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 7.Rossbach M. Small non-coding RNAs as novel therapeutics. Curr Mol Med. 2010;10:361–368. doi: 10.2174/156652410791317048. [DOI] [PubMed] [Google Scholar]
- 8.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 9.Sazani P, Kole R. Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest. 2003;112:481–486. doi: 10.1172/JCI19547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ming X, Alam MR, Fisher M, Yan Y, Chen X, Juliano RL. Intracellular delivery of an antisense oligonucleotide via endocytosis of a G protein-coupled receptor. Nucleic Acids Res. 2010;38:6567–6576. doi: 10.1093/nar/gkq534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiel KW, Giangrande PH. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides. 2009;19:209–222. doi: 10.1089/oli.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake K. Nucleic acid-sensing Toll-like receptors: beyond ligand search. Adv Drug Deliv Rev. 2008;60:782–785. doi: 10.1016/j.addr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 14.Geary RS. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2009;5:381–391. doi: 10.1517/17425250902877680. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Nakatani M, Narukawa K, Obika S. Antisense drug discovery and development. Future Med Chem. 2011;3:339–365. doi: 10.4155/fmc.11.2. [DOI] [PubMed] [Google Scholar]
- 16.Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–695. doi: 10.1021/mp900093r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam MR, Ming X, Dixit V, Fisher M, Chen X, Juliano RL. The biological effect of an antisense oligonucleotide depends on its route of endocytosis and trafficking. Oligonucleotides. 2010;20:103–109. doi: 10.1089/oli.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ming X. Cellular delivery of siRNA and antisense oligonucleotides via receptor-mediated endocytosis. Expert Opin Drug Deliv. 2011;8:435–449. doi: 10.1517/17425247.2011.561313. [DOI] [PubMed] [Google Scholar]
- 20.Ming X, Sato K, Juliano RL. Unconventional internalization mechanisms underlying functional delivery of antisense oligonucleotides via cationic lipoplexes and polyplexes. J Control Release. 2011;153:83–92. doi: 10.1016/j.jconrel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koller E, Vincent TM, Chappell A, De S, Manoharan M, Bennett CF. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 2011;39:4795–4807. doi: 10.1093/nar/gkr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annual review of biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 23.Juliano RL, Ming X, Nakagawa O. Cellular Uptake and Intracellular Trafficking of Antisense and siRNA Oligonucleotides. Bioconjug Chem. 2011 doi: 10.1021/bc200377d. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J. A status report on RNAi therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- 26.Bell DA, Hooper AJ, Burnett JR. Mipomersen, an antisense apolipoprotein B synthesis inhibitor. Expert Opin Investig Drugs. 2011;20:265–272. doi: 10.1517/13543784.2011.547471. [DOI] [PubMed] [Google Scholar]
- 27.Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, Abbs S, Garralda ME, Bourke J, Wells DJ, Dickson G, Wood MJ, Wilton SD, Straub V, Kole R, Shrewsbury SB, Sewry C, Morgan JE, Bushby K, Muntoni F. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond SM, Wood MJ. PRO-051, an antisense oligonucleotide for the potential treatment of Duchenne muscular dystrophy. Curr Opin Mol Ther. 2010;12:478–486. [PubMed] [Google Scholar]
- 29.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura A, Nagasaki Y. Smart siRNA delivery systems based on polymeric nanoassemblies and nanoparticles. Nanomedicine (Lond) 2010;5:1089–1102. doi: 10.2217/nnm.10.76. [DOI] [PubMed] [Google Scholar]
- 32.Marlin F, Simon P, Saison-Behmoaras T, Giovannangeli C. Delivery of oligonucleotides and analogues: the oligonucleotide conjugate-based approach. Chembiochem. 2010;11:1493–1500. doi: 10.1002/cbic.201000138. [DOI] [PubMed] [Google Scholar]
- 33.Singh Y, Murat P, Defrancq E. Recent developments in oligonucleotide conjugation. Chem Soc Rev. 2010;39:2054–2070. doi: 10.1039/b911431a. [DOI] [PubMed] [Google Scholar]
- 34.Lonnberg H. Solid-phase synthesis of oligonucleotide conjugates useful for delivery and targeting of potential nucleic acid therapeutics. Bioconjug Chem. 2009;20:1065–1094. doi: 10.1021/bc800406a. [DOI] [PubMed] [Google Scholar]
- 35.Meyer A, Spinelli N, Dumy P, Vasseur JJ, Morvan F, Defrancq E. Oligonucleotide sequential bis-conjugation via click-oxime and click-Huisgen procedures. J Org Chem. 2010;75:3927–3930. doi: 10.1021/jo100599m. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T, Peng CG, Matsuda S, Addepalli H, Jayaprakash KN, Alam MR, Mills K, Maier MA, Charisse K, Sekine M, Manoharan M, Rajeev KG. Versatile site-specific conjugation of small molecules to siRNA using click chemistry. J Org Chem. 2011;76:1198–1211. doi: 10.1021/jo101761g. [DOI] [PubMed] [Google Scholar]
- 37.Wang TP, Chiou YJ, Chen Y, Wang EC, Hwang LC, Chen BH, Chen YH, Ko CH. Versatile phosphoramidation reactions for nucleic acid conjugations with peptides, proteins, chromophores, and biotin derivatives. Bioconjug Chem. 2010;21:1642–1655. doi: 10.1021/bc1001505. [DOI] [PubMed] [Google Scholar]
- 38.Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, Iversen PL, Arzumanov AA, Gait MJ. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv Drug Deliv Rev. 2008;60:517–529. doi: 10.1016/j.addr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said Hassane F, Saleh AF, Abes R, Gait MJ, Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell Mol Life Sci. 2010;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam MR, Dixit V, Kang H, Li ZB, Chen X, Trejo J, Fisher M, Juliano RL. Intracellular delivery of an anionic antisense oligonucleotide via receptor-mediated endocytosis. Nucleic Acids Res. 2008;36:2764–2776. doi: 10.1093/nar/gkn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Astriab-Fisher A, Sergueev D, Fisher M, Shaw BR, Juliano RL. Conjugates of antisense oligonucleotides with the Tat and antennapedia cell-penetrating peptides: effects on cellular uptake, binding to target sequences, and biologic actions. Pharm Res. 2002;19:744–754. doi: 10.1023/a:1016136328329. [DOI] [PubMed] [Google Scholar]
- 42.Turner JJ, Arzumanov AA, Gait MJ. Synthesis, cellular uptake and HIV-1 Tat-dependent trans-activation inhibition activity of oligonucleotide analogues disulphide-conjugated to cell-penetrating peptides. Nucleic Acids Res. 2005;33:27–42. doi: 10.1093/nar/gki142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 44.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16:734–740. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 45.Patwa A, Gissot A, Bestel I, Barthelemy P. Hybrid lipid oligonucleotide conjugates: synthesis, self-assemblies and biomedical applications. Chem Soc Rev. 2011 doi: 10.1039/c1cs15038c. [DOI] [PubMed] [Google Scholar]
- 46.Kiviniemi A, Virta P, Drenichev MS, Mikhailov SN, Lonnberg H. Solid-Supported 2'-O-Glycoconjugation of Oligonucleotides by Azidation and Click Reactions. Bioconjug Chem. 2011;22:1249–1255. doi: 10.1021/bc200097g. [DOI] [PubMed] [Google Scholar]
- 47.Pourceau G, Meyer A, Vasseur JJ, Morvan F. Synthesis of a Glycomimetic Oligonucleotide Conjugate by 1,3-Dipolar Cycloaddition. Methods Mol Biol. 2011;751:167–193. doi: 10.1007/978-1-61779-151-2_11. [DOI] [PubMed] [Google Scholar]
- 48.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, 2nd, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27:839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+. T cell decline in humanized mice. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001581. 66ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa O, Ming X, Huang L, Juliano RL. Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. J Am Chem Soc. 2010;132:8848–8849. doi: 10.1021/ja102635c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam MR, Ming X, Fisher M, Lackey JG, Rajeev KG, Manoharan M, Juliano RL. Multivalent Cyclic RGD Conjugates for Targeted Delivery of Small Interfering RNA. Bioconjug Chem. 2011 doi: 10.1021/bc200235q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 55.Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, Seung E, Deruaz M, Dudek T, Einarsson JI, Yang L, Allen TM, Luster AD, Tager AM, Dykxhoorn DM, Lieberman J. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 2011;121:2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindgren M, Langel U. Classes and prediction of cell-penetrating peptides. Methods Mol Biol. 2011;683:3–19. doi: 10.1007/978-1-60761-919-2_1. [DOI] [PubMed] [Google Scholar]
- 58.Juliano RL, Alam R, Dixit V, Kang HM. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:324–335. doi: 10.1002/wnan.4. [DOI] [PubMed] [Google Scholar]
- 59.El-Andaloussi S, Jarver P, Johansson HJ, Langel U. Cargo-dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: a comparative study. Biochem J. 2007;407:285–292. doi: 10.1042/BJ20070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J. 2006;20:1775–1784. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- 61.Ivanova GD, Arzumanov A, Abes R, Yin H, Wood MJ, Lebleu B, Gait MJ. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abes R, Moulton HM, Clair P, Yang ST, Abes S, Melikov K, Prevot P, Youngblood DS, Iversen PL, Chernomordik LV, Lebleu B. Delivery of steric block morpholino oligomers by (R-X-R)4 peptides: structure-activity studies. Nucleic Acids Res. 2008;36:6343–6354. doi: 10.1093/nar/gkn541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin H, Saleh AF, Betts C, Camelliti P, Seow Y, Ashraf S, Arzumanov A, Hammond S, Merritt T, Gait MJ, Wood MJ. Pip5 Transduction Peptides Direct High Efficiency Oligonucleotide-mediated Dystrophin Exon Skipping in Heart and Phenotypic Correction in mdx Mice. Mol Ther. 2011 doi: 10.1038/mt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goyenvalle A, Babbs A, Powell D, Kole R, Fletcher S, Wilton SD, Davies KE. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koppelhus U, Shiraishi T, Zachar V, Pankratova S, Nielsen PE. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjug Chem. 2008;19:1526–1534. doi: 10.1021/bc800068h. [DOI] [PubMed] [Google Scholar]
- 66.Nakase I, Akita H, Kogure K, Graslund A, Langel U, Harashima H, Futaki S. Efficient Intracellular Delivery of Nucleic Acid Pharmaceuticals Using Cell-Penetrating Peptides. Acc Chem Res. 2011 doi: 10.1021/ar200256e. [DOI] [PubMed] [Google Scholar]