Abstract
Diallyl trisulfide (DATS) is a garlic organosulfide that is toxic to cancer cells, however, little is known about its effect in the initiation phase of carcinogenesis. We sought to determine whether DATS could inhibit the carcinogen, benzo(a)pyrene (BaP), from inducing precancerous activity, in vitro. MCF-10A cells were either pre-treated (PreTx) or concurrently treated (CoTx) with 1 μM BaP, and 6 or 60 μM DATS for up to 24 hours. The DATS 6 and 60 μM CoTx inhibited BaP-induced cell proliferation by an average of 71.1 and 120.8%, respectively, at 6 hours. The 60 μM DATS pretreatment decreased BaP-induced G2/M cell cycle transition by 127 %, and reduced the increase in cells in the S-phase by 42%; whereas 60 μM DATS CoTx induced a 177% increase in cells in G1. DATS effectively inhibited (P<0.001) BaP-induced peroxide formation by at least 54%, which may have prevented the formation of BaP-induced DNA strand breaks. In this study, we reveal mechanisms involved in DATS inhibition of BaP-induced carcinogenesis, including inhibition of cell proliferation, regulation of cell cycle, attenuation of ROS formation, and inhibition of DNA damage. At the doses evaluated, DATS appears to be an effective attenuator of BaP-induced breast carcinogenesis, in vitro.
Keywords: benzo(a)pyrene, diallyl trisulfide, precarcinogenic activity, breast cancer, MCF-10A, in vitro
1. Introduction
Garlic's health benefits were recorded by the early Kemetic and Nubian people and have also been incorporated into the medicinal practices of the early Egyptian, Greek, Indian, and Chinese cultures as a panacea plant (Block, 1985; Ali et al., 2000; Rivlin, 2001). Several epidemiological studies have indicated that diets with high garlic consumption decrease the risk of developing some cancers, particularly gastrointestinal, colon, prostate, and breast cancer (Mei et al., 1982; Steinmetz et al., 1994; Hsing et al., 2002; Challier et al., 1998). Modern research has indicated that many of garlic's medicinal properties, including its hypocholesterolemic, hypolipidemic, antioxidant, and antineoplastic activity can be primarily attributed to its organosulfur compounds, including diallyl sulfide, diallyl disulfide, diallyl trisulfide (DATS) (Augusti and Mathew, 1974; Sparnins et al., 1988; Corzo-Martinez et al., 2007).
DATS, also known as allitridium, is a garlic organosulfide compound (OSC) produced during the disruption of the garlic bulb, which releases alliinase converting alliin to allicin. Allicin is then converted to several polysulfides, 14.6% of which is DATS (Iciek et al., 2009; Brodnitz et al., 1971). DATS can represents up to 35-60% of garlic oil, depending on extraction method, and is thus a prominent organosulfide found in garlic (Tsao and Yin, 2001; Wu et al., 2002; Li et al., 2011).
A combination of approaches has demonstrated the efficacy of DATS in a variety of cancer types. An epidemiological study showed that when combined with selenium, DATS inhibited gastric cancer in males (Li et al., 2004). In vivo models have shown that DATS inhibits benzo(a)pyrene (BaP)-induced forestomach cancer in A/J mice when administered 48 to 96 hours prior to BaP exposure, and inhibited growth of PC-3, HepG2, and CT26 cancer tumor xenografts in nude mice (Sparnins et al., 1988; Xiao et al., 2006; Zhang et al., 2007; Wu et al., 2011). In vitro studies have shown that DATS inhibits carcinogenesis by inducing cell cycle arrest, reducing cell viability by inducing apoptosis through the generation of reactive oxygen species (ROS) in cancer cells (Antosiewicz et al., 2006; Herman-Antosiewicz et al., 2007, Herman-Antosiewicz and Singh 2005; Xiao et al., 2004 & 2005; Hosono et al., 2005). In normal cells, DATS has not been shown to elicit the same toxicity as seen in cancer cells (Kim et al., 2007; Powolny and Singh, 2008). In addition, the role of DATS in inhibiting carcinogenesis initiation in normal cells has not been explored. In this study, we evaluated two potentially physiological doses of DATS to determine their efficacy in the inhibition of early carcinogenic activity in a normal cell line. This study provides the first evidence that DATS can inhibit early carcinogenic activity in a normal human breast epithelial cell line treated with a known environmental and dietary carcinogen.
2. Materials and Methods
2.1. Cell Line, Chemicals and Reagents
MCF-10A normal breast epithelial cells were purchased from American Type Culture Collection (ATCC, Rockville, Maryland). Phenol red-free DMEM/F-12 media, horse serum, penicillin/streptomycin, antibiotic/antimycotic, epidermal growth factor, human insulin (Novolin R), trypsin-EDTA (10X), Hanks Balanced Salt Solution (HBSS), and Phosphate Buffered Saline (PBS) were purchased from Invitrogen (Carlsbad, CA). Cholera toxin was obtained from Enzo Life Sciences (Plymouth Meeting, Pennsylvania). The CellTiter 96® AQueous One Solution Cell Proliferation Assay was obtained from Promega (Madison, Wisconsin). Diallyl trisulfide (DATS) was purchased from LKT Laboratories (St. Paul, Minnesota). Benzo(a)pyrene (BaP), PeroxiDetect™ Kit, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri).
2.2. Cell Culture
MCF-10A cells were cultured in DMEM/F12 media supplemented with cholera toxin (100 ng/ml), epidermal growth factor (20 ng/ml), horse serum (5%), human insulin (10 μg/ml), hydrocortisone (0.5 μg/ml), and penicillin-streptomycin. The cells were grown to 90-100% confluence by changing the media every 2-3 days, and sub culturing every 5-7 days, with maintenance in a 37°C, 5% CO2 humidified incubator. After DATS treatments, cells were examined during the first 24 hours for changes in cell viability, cell cycle, production of ROS, and DNA damage as biomarkers of early carcinogenic activity.
2.3. Cell Treatments and Harvesting
MCF-10A cells were categorized into two groups, DATS pretreatment (PreTx) and DATS concurrent treatment (CoTx). The PreTx group was treated with 6 or 60 μM of DATS for four hours, followed by 1μM of BaP. The CoTx group was treated with 1 μM BaP in combination with 6 or 60 μM of DATS. Cells were harvested at 3, 6, 12, or 24 hours. Both the DATS and BaP were dissolved in DMSO and for all experiments cells only (media), 0.1% DMSO vehicle, and 1 μM BaP only controls were also utilized. All treatments were prepared and conducted under low light conditions and incubated at 37°C, in a 5% CO2 humidified incubator. Cells were harvested by trypsinization and the cells suspended in PBS without Mg2+ or Ca2+ and frozen until further use.
2.4. MTS Assay for Cell Viability
Resuspended MCF-10A cells (2× 105/well) in supplemented serum-free media were plated into 88 wells (100 μl/well) of a flat bottom 96-well plate and allowed to attach to the bottom of the wells overnight. The media was then aspirated from each well by pipetting, followed by the application of 100 μl of supplemented serum-free media prepared with the treatments described above for n=8. Following incubation for 3, 6, 12 or 24 hours, cell viability was assessed according to the manufacturer's protocol of the CellTiter 96® Aqueous One Solution MTS Assay. Absorbance was measured using a PowerWave X-340 96-well Plate Reader (Bio-Tek Instruments, Inc., Winooski, Vermont). The results were normalized to the cell only media control which represented 100% cell viability.
2.5. Flow Cytometry
The cell cycle phase distribution of DNA from MCF-10A cells treated for 24 hours, as described above, was assayed by flow cytometry. Cells were treated when cultures were sub-confluent (40-50%), and upon trypsin harvesting (as described above), the cells were approximately 90% confluent in T-75 cell culture flasks. For cell cycle analysis, cells were fixed with 5 ml of 100% ethanol (pre-cooled to -20°C) added drop-wise while vortexing and incubated at 4°C for at least 24 hours. The cells were then centrifuged at 2500 rpm for 5 minutes, and resuspended in residual ethanol, followed by 1 hour room temperature incubation with 1 ml of propidium iodide staining solution (0.5 mg/ml ribonuclease A, 1 mg/ml D-glucose and 100 μg/ml propidium iodide in PBS) in the dark. The proportion of cells in each cell cycle phase was determined by propidium iodide florescence using the FACSCalibur Flow Cytometer (Becton Dickson (BD) Biosciences, Franklin Lakes, NJ), evaluating 20,000 events per sample. Cell cycle distributions were calculated using ModFit LT 3.0 software (Verity Software House, Topsham, ME).
2.6. Aqueous Peroxide Detection
Resuspended MCF-10A cells (1× 105/well) in supplemented serum-free media were plated in each well of a 96-well flat bottom plate. Cell treatments were prepared in serum-free-media, with the addition of a 0.03% H2O2 positive control, and applied to the cells in triplicate wells for the times indicated. The media was then aspirated from each well by pipetting, followed by the application of 100 μl of supplemented serum-free media prepared with the treatments described above (Section 2.3). The extracellular media was analyzed according to the manufacturer's protocol for the PeroxiDetect Kit for the determination of aqueous peroxides (Sigma, 2005) and absorbance was determined spectrophotometrically at 560nm for nanomoles of hydrogen peroxide based on a standard curve (R2= 0.96) using a PowerWave X-340 96-well plate reader (Bio-Tek Instruments, Inc., Winooski, Vermont).
2.7. Single Cell Gel Electrophoresis (Comet Assay)
Cells were treated and harvested as described. The Comet assay was performed following the protocol of Aboyade-Cole et al. 2008, using Komet 5.5 software (Andor Technology, PLC Belfast, Northern Ireland), and Kinetic Imaging (Kinetic Imaging Ltd, Merseyside, United Kingdom).
2.8. Statistical Analysis
Unless otherwise stated, each assay was conducted for N=3. The data from all experiments were analyzed by one-way analysis of variance (ANOVA), followed by the Bonferroni's Multiple Comparison Test using GraphPad Prism 5.0 software. The results were displayed as the average values +/- SEM to determine significant differences (P<0.05) between the treatment groups and the DMSO vehicle (*) and BaP only (#) controls.
3. Results
3.1. Cell Viability
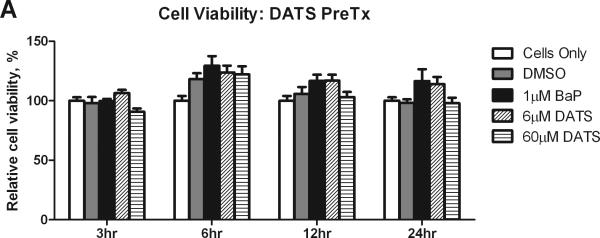
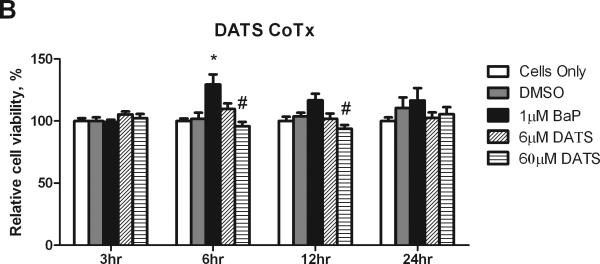
The MTS assay was used to evaluate changes in cell viability and proliferation after treatment with BaP and DATS. BaP was shown to significantly (P<0.05) increase MCF-10A cell proliferation at 6 hours, and sustained a slight increase in cell viability at 12 and 24 hours, relative to the DMSO control. The 6 μM CoTx concentration of DATS inhibited BaP-induced increases in cell proliferation at 6, 12, and 24 hours by an average of 71, 115, and 228%, respectively, relative to DMSO, but was not toxic to the cells, and thus returned the cell viability to that of the cells only control. The 60 μM DATS CoTx inhibited BaP induced cell proliferation at 6, 12, and 24 hours by an average of 121, 175, and 180%, respectively (Figure 1).
Figure 1.
Cell viability and proliferation resulting from Pre- and CoTx with DATS and BaP. MCF-10A cells were pretreated with DATS for four hours followed by the addition of 1 μM BaP (A) or treated concomitantly with 1 μM BaP and DATS (B). To determine cell viability, the MTS solution was applied to the cells for 2-3 hours and analyzed at an absorbance of 490nm. The absorbance of each treatment group was normalized relative to the cells only control for 100% cell viability. The graphs represent the average relative cell viability for eight replicates for N=3, +/- the SEM (* and # indicate a P<0.05 significant difference from the DMSO control and the 1 μM BaP only control, respectively).
3.2. Cell Cycle Analysis
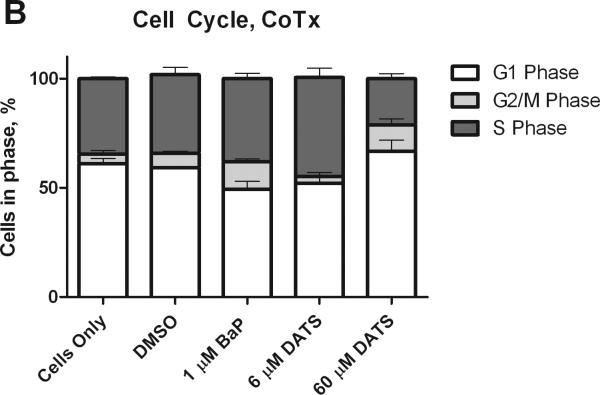
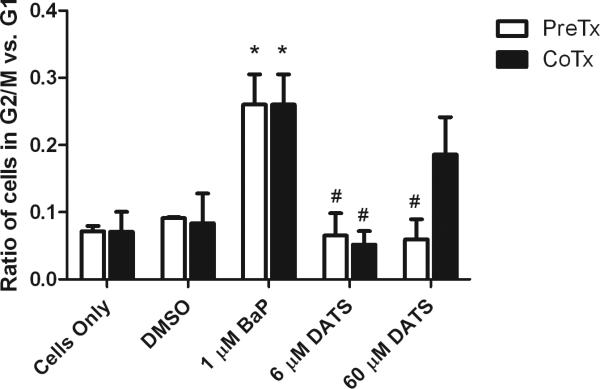
BaP has been shown to induce G2/M and S phase arrest in breast cancer cells (Sadikovic and Rodenhiser, 2006). MCF-10A cells were evaluated by flow cytometry for changes in cell cycle after treatment for 24 hours. BaP transitioned cells to the G2/M and S-phases, by 119 and 22%, respectively, relative to the DMSO control. DATS PreTx (6 and 60 μM) inhibited the BaP-induced G2/M phase shift by 127 and 123%, respectively (Figure 2). To numerically compare the concentration of cells arrested in the gap phases, the percentage of cells in G2/M was divided by the percentage of cells in G1 to produce a G2-M/G1 ratio. BaP produced a 1.8 and 2.0-fold increase in cells in the G2-M/G1 ratio, relative to the respective Pre- and CoTx DMSO controls. The 6 μM DATS concentration reduced the BaP-induced G2-M/G1 ratio by an average of 115 and 118%, respectively, as a Pre- and CoTx; whereas the 60 μM DATS concentration reduced the ratio by 120 and 40%, respectively (Figure 3).
Figure 2.
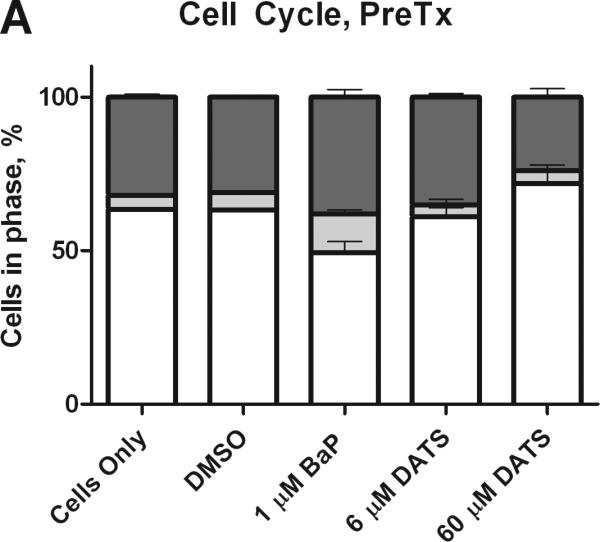
Cell cycle analysis of MCF-10A cells treated with BaP and DATS. MCF-10A cells were either pretreated with DATS for four hours, followed by treatment with 1 μM BaP for 24 hours (A) or co-treated with DATS and BaP for 24 hours (B). The cells were fixed in ethanol, stained with propidium iodide, and analyzed by flow cytometry. The values represent the average percent of cells in each phase, G1, G2/M, and S, +/- SEM.
Figure 3.
The ratio of cells in G2/M and G1 phases. The bars indicate the average percentage of cells in G2/M divided by the average percentage of cells in G1, +/- SEM for the DATS PreTx and DATS and BaP CoTx after 24 hours. The * indicates a P<0.05 significant difference from the DMSO control, and # indicates a P<0.05 significant difference from the 1 μM BaP only control.
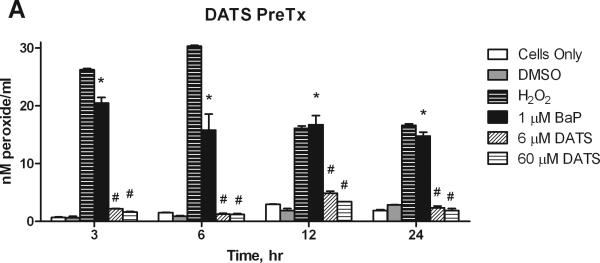
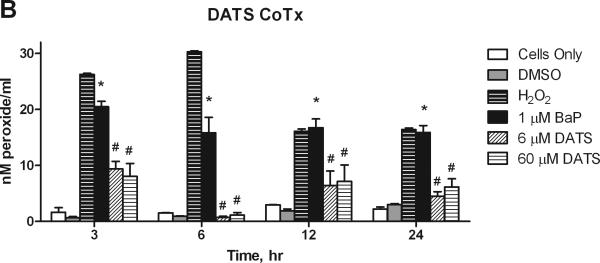
3.3. Hydrogen Peroxide Detection
We studied the effect of DATS on BaP-induced production of hydrogen peroxide (H2O2) in the media of treated MCF-10A cells. BaP induced a significant (P<0.001) and sustained increase in aqueous peroxide over 24 hours, though the levels of formation decreased over time, indicating endogenous repair. DATS PreTx and CoTx at 6 and 60 μM significantly (P<0.001) attenuated BaP-induced H2O2 at all time points (Figure 4). Pretreatment with DATS appeared to be more effective than DATS cotreatment for inhibiting BaP-induced ROS at 12 and 24 hours; however both treatments were significantly lower than the BaP alone and H2O2 controls. In the 12 and 24 hour CoTx groups, all DATS treatments resulted in a slight, but statistically insignificant increase in peroxide formation relative to the DMSO control.
Figure 4.
The suppression of BaP induced extracellular ROS formation by DATS. MCF-10A cells were exposed to a pretreatment of DATS followed by 1 μM BaP (A), or with a concomitant combination of 1 μM BaP and DATS (B), both for 3, 6, 12, or 24 hours. The results deem the average aqueous peroxides (as a model ROS), +/- SEM, as detected by the PeroxiDetect Kit in triplicate for N=3. The * indicates a P<0.05 significant difference from the DMSO control.
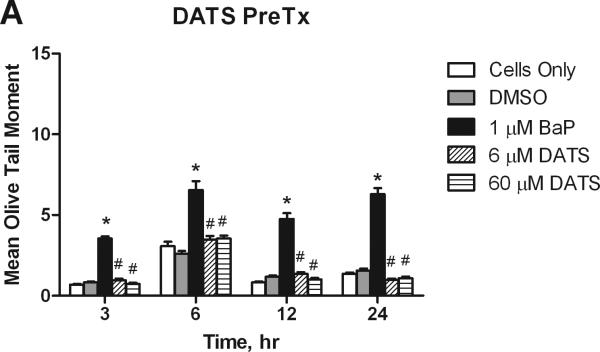
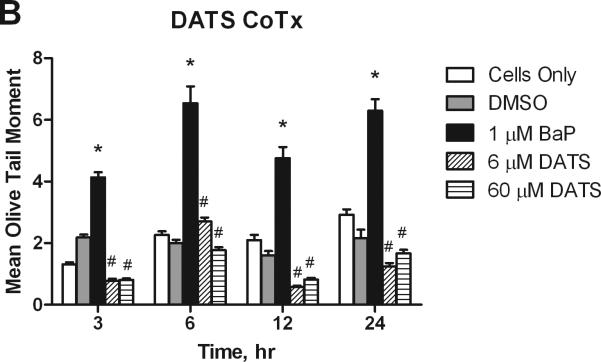
3.4. DNA Damage
The Alkaline Comet Assay quantifies single strand DNA breaks and alkali labile sites. DNA strand breaks produce a comet-like tail when cells are electrophoresed, which can be quantified to determine the extent of DNA damage. The olive tail moment is a (OTM) parameter that compares the amount of DNA in the nucleus, or head of the comet, to the amount in the tail; the larger the OTM, the greater the extent of damage. Under alkaline conditions, the OTM specifically reveals single strand DNA damage and alkaline liable sites. BaP induced significant DNA damage in the MCF-10A cells. At all concentrations, DATS pre- and concurrent treatment significantly (P<0.05 at 3 hours, and P<0.001 at 6-24 hours) attenuated BaP-induced damage at the time pointes studied. DATS CoTx at 6 hours demonstrated the least effective attenuation of BaP-induced DNA strand breaks, though it reduced strand breaks by 72.8%. Concurrent treatment DATS also protected the DNA of the cells, reducing DNA damage to levels below that of the DMSO and cells only controls at 12 and 24 hours (Figure 5).
Figure 5.
DATS inhibition of BaP induced DNA strand breaks. Utilizing the Comet assay, MCF-10A cells treated with a PreTx of DATS followed by BaP (A), or DATS and BaP concurrently (B) were analyzed for DNA single strand breaks and alkali liable sites. The results present the mean olive tail moment (OTM), as an indicator of DNA damage, +/- SEM for 150 cells for N=2. The * indicates a P<0.05 significant difference from the DMSO control.
4. Discussion
BaP is a polycyclic aromatic hydrocarbon produced during the incomplete combustion of organic material (Edwards, 1983). BaP has been shown to induce the carcinogenic transformation of normal cells, in vitro (Siriwardhana and Wang, 2008). As a procarcinogen, BaP's metabolites and intermediates elicit the cellular damage primarily responsible for its carcinogenic potential, including formation of DNA adducts and strand breaks, oxidative damage from ROS, mutations, chromosomal aberrations, and tumorigenesis (Morris and Seifter, 1992; Caruso et al., 2001; Hanelt et al., 1997). Siriwardhana and Wang (2008) concluded that in MCF-10A cells, a single exposure to BaP resulted in cellular acquisition of cancerous properties and spontaneous mutations.
Garlic OSCs have demonstrated chemopreventive potential that has not been fully elucidated. The proposed mechanisms for their inhibition of mutagenesis include the suppression of procarcinogen biotransformation, to prevent DNA adduct formation; enhancement of phase II detoxifying enzymes; scavenging of free-radicals; induction of apoptosis in cancerous cells; cell cycle arrest; inhibition of tumor proliferation; and post-translational modification of proteins by formation of mixed disulfides, hydropersulfides, and trisulfides (Shukla and Kalra 2007; Iciek et al., 2009).
DATS has been shown to inhibit DNA adduct formation (Yu et al., 2003), increase phase II biotransformation enzymatic expression (Fukao et al., 2004; Iciek et al., 2009; Chen et al., 2004), induce apoptosis in cancer cells (Xiao and Singh, 2006; Song and Lee, 2003; Wang et al., 2010a; Hosono et al., 2005; Wu et al., 2004), and induce cell cycle arrest in damaged cells (Wang et al., 2010a; Xiao et al., 2005; Hosono et al., 2005; Wang et al., 2010b; Xiao and Singh, 2003; Seki et al., 2008). In this study, DATS CoTx inhibited the BaP-induced increase in cellular proliferation. DATS at 6 μM inhibited BaP-induced G2/M and S-phase transitions. DATS Preand CoTx attenuated BaP-induced peroxide formation and DNA strand breaks at all time points evaluated. DATS was shown to be an effective attenuator of early carcinogenic activity and genotoxicity.
Studies vary as to BaP's impact on cell proliferation in normal and cancerous breast cell lines. In normal primary breast tissue and several neoplastic breast cell lines (MCF-7, HCC 1806, T47-D, and MDA MB 231), BaP was not shown to significantly alter cell viability following a single exposure after 24 hours (Keshava et al., 2005a&b; Sigounas et al., 2010; Tampio et al., 2009; Sadikovic and Rodenhiser, 2006). Our results were similar, as BaP did not significantly increase cell proliferation at 24 hours, but increases were significant at 6 hours, a time point that was not evaluated in other studies. Siriwardhana and Wang (2008), demonstrated that MCF-10A cells treated for 10-20 rounds of 0.1 μM BaP for 48 hours under growth-factor starvation resulted in cell proliferation (Siriwardhana and Wang, 2008). Tannheimer et al. (1997 and 1998) concluded that in the absence of growth factors, BaP treated MCF-10A cells induced growth factor signaling pathways, and prolonged exposure resulted in BaP-induced cell proliferation. BaP and it metabolites activate epidermal growth factor receptor (EGFR) genes that can promote cell proliferation and tumorgenesis in normal and neoplastic breast tissue (Burdick et al., 2003; Burchiel et al., 2007; Pliskova et al., 2005). In this study, the mechanisms underlining the temporary spike in BaP-induced cell proliferation have not been elucidated and require further evaluation. However, we hypothesize that BaP stimulates the EGFR pathway following the short term (~5 minute) serum starvation resulting from growth media removal and replacement with treatment media during the cell treatment process. As a result, normal cell growth patterns usually interrupted by this short term environmental change may be by-passed in the BaP treatment group through stimulation of the EGFR pathway. Benzo(a)pyrene quinones (BPQ) are BaP intermediates and metabolites formed during the biotransformation of BaP. According to Burdick et al. (2003), BPQ induce a biphasic response in normal breast cells, causing cell proliferation at lower concentrations and growth inhibition at higher concentrations. We predict that the low levels of BPQs formed after short term exposure to BaP increase cell proliferation through activity of the EGFR pathway, however, as their concentration increases, their stimulation of growth is attenuated, suppressing extensive cell proliferation (Burdick et al., 2003).
The antiproliferative effect on cancer cells by OSCs such as DATS is likely associated with the stimulation of apoptosis (Shukla and Kalra, 2007; Nishino et al., 1989). DATS has been shown to induce apoptosis in human lung fibroblast, lung cancer, and gastric cancer cells (Sakamoto et al., 1997; Li and Lu 2002). DATS also suppressed insulin-like growth factor receptor 1 protein level and phosphorylation, while impacting the BAD proapoptotic protein in prostate cancer cells to suppress tumorgenesis (Xiao and Singh, 2006). Normal cells have been shown to be more resistant to OSC-induced apoptosis than neoplastic cells (Karmakar et al., 2007; Kim et al., 2007; Sakamoto et al., 1997; Xiao and Singh, 2003), though high doses of DATS have been shown to be toxic in mouse models (Li et al., 2011). Malki and colleagues found that MCF-12A normal breast epithelial cells exposed to 100 μM of DATS for 24 hours induced apoptosis and resulted in a 40% decrease in cell viability; which was observed to a greater extent (60%) in MCF-7 breast cancer cells (Malki et al. 2009). In our study, apoptosis was not observed in the flow cytometry histograms (not shown), nor was cell viability significantly decreased by the two concentrations of DATS evaluated, and thus apoptosis was not evaluated. We also did not analyze DATS alone on MCF-10A cells, but only in combination with BaP, which may account for the differences in our results with those of Malki and colleagues (Malki et al., 2009). The 60 μM dose of DATS slightly reduced MCF-10A cell viability, though it was not deemed to be statistically significant relative to the cells only control.
DATS is a potent inducer of cell cycle arrest in cancer cells (Seki et al., 2008; Wu et al. 2004). Cell cycle arrest is an endogenous response to stress through activation of signal transduction checkpoints (Hartwell and Weinert, 1989). G1/S phase checkpoints prevent the replication of damaged DNA, while G2/M checkpoints inhibit the segregation of damaged chromosomes during mitosis (Iciek et al., 2009). Most studies have shown that DATS induces G2/M arrest (Wang et al., 2010a&b; Xiao et al., 2005; Xiao and Singh, 2003); however, in colon and gastric cancer cells, DATS induced G1/S arrest, revealing a cell cycle-dependent induction of apoptosis when cells were transitioned from G2/M to G1, possibly the result of tubulin polymerization (Hosono et al., 2005; Li and Lu, 2002).
In this study, DATS PreTx at 6 μM was the most effective attenuator of BaP induced G2/M and S-phase shifts, maintaining the percentage of cells in each phase to the concentrations observed in the controls; whereas 60 μM DATS Pre and CoTx shifted cells to G1 and G2/M Phases, respectively. The differences in cell cycle in the Pre- and CoTx groups may be attributed to the protective mechanisms employed by DATS as a PreTx against BaP induced shift to G2/M and S phases. As a pretreatment, DATS is preventing the replication of DNA damage and allowing for DNA repair. As a co-treatment, DATS is inducing G2/M arrest to allow for the repair of damaged chromosomes. Further evaluation of cell cycle and apoptosis beyond 24 hours may demonstrate more significant changes in BaP-induced cell cycle changes, as BaP induced S-and G2/M phase arrest in MCF-7 and T47-D breast cancer cells, respectively, after 96 hours of exposure (Sadikovic and Rodenhiser, 2006). Our results differ from what was previously reported about BaP's effects on transition to G0/G1 phase in MCF-10A cells (Siriwardhana and Wang, 2008). In their study, the cells underwent growth factor starvation for the duration of treatment, and in our study, the cells were not subjected to growth starvation, which may account for the differences in our results.
ROS are free radicals that can initiate the cellular injury responsible for aging and disease (Weisburger, 2001; Valko et al., 2006). During the biotransformation of BaP, reactive intermediates and metabolites can induce DNA damage through the generation of ROS initiating oxidative damage to nucleic acids and proteins (Leadon et al., 1988). BaP can induce ROS, which overwhelm the total antioxidant capacity of primary breast tissue, and are correlated to clustered DNA damage, chromosomal aberrations, and inducing the carcinogenic transformation of normal breast cells (Sigounas et al., 2010; Caruso et al., 2001; Siriwardhana and Wang, 2008).
Here, we demonstrated that DATS inhibited BaP-induced ROS in normal cells, thus potentially inhibiting cellular and DNA injury that can lead to cancer. DATS PreTx was more effective in ROS inhibition, further demonstrating a protective effect on the cells. BaP has previously been shown to induce DNA single and double strand breaks in primary breast epithelial cells (Sigounas et al., 2010); thus DATS inhibition of ROS may have also inhibited the observed BaP-induced DNA strand breaks. The mechanisms for the inhibition of BaP-induced ROS and DNA strand breaks will need to be evaluated in further genetic and proteomic studies in order to exploit DATS’ antioxidant potential in breast cells.
As fresh garlic contains about 900-1100 μg of DATS per gram, it may be possible to consume concentrations of DATS necessary to bring about anticarcinogenic cellular responses (Yu et al., 1989). Assuming 100% bioavailability in an ideal one-compartment pharmacokinetic mathematical model under steady state conditions, the ideal average person would need to consume 7-8 garlic cloves or 3-4 tablespoons of minced garlic to attain a 60 μM circulating dose of DATS. However, realistic human pharmacokinetic studies are needed to determine if biologically effective doses can be achieved in the blood plasma and reach target organs through dietary or pharmacological administration (Powolny and Singh, 2008).
5. Conclusion
In conclusion, we presented novel evidence of the inhibition of acute cellular carcinogenesis initiation by singular exposure of BaP in MCF-10A human breast epithelial cells by the garlic organosulfur compound, DATS. The mechanisms of DATS inhibition of early precancerous activities of BaP, include inhibition of cell proliferation, attenuation of S-phase cell cycle transition, suppression of ROS production, and inhibition of DNA strand break formation, all within 24 hours of exposure. In these studies, DATS did not produce a linear dose response with increasing concentration, and thus the biological consequences of high and low doses of DATS may elicit varying results. As a prominent OSC in garlic, DATS contributes to the effectiveness of garlic as a chemopreventive agent, and may be effective as an isolated agent for inhibiting environmentally induced carcinogenesis. Further studies are needed to elucidate the potential dietary prevention of human breast cancer with DATS.
Highlights.
BaP induces changes in cell viability and cell growth in MCF-10A cells
BaP induces peroxide formation and DNA strand breaks in MCF-10A cells
DATS inhibits BaP-induced cell proliferation and cell cycle changes
DATS inhibits BaP-induced peroxide formation in MCF-10A cells
DATS inhibits BaP-induced DNA strand breaks in MCF-10A cells
Acknowledgements
This study is a continuance of the legacy of Dr. Ronald D. Thomas in his search to inhibit environmental and dietary induced carcinogenesis with garlic and biblically recorded plants. We would like to acknowledge the FAMU College of Pharmacy and Pharmaceutical Sciences faculty and staff, especially Drs. Karam Soliman, Barack Abonyo, Carl Goodman, R. Renee Reams and Tracy Womble for their assistance and use of their supplies and equipment. In addition, we would like to acknowledge and thank the Florida Education Fund's McKnight Doctoral Program for assisting in the funding of the doctoral education of Yasmeen Nkrumah-Elie. The research conducted here was funded in part by NIH Programs RCMI Grant # 5G12RR003020-25.
Abbreviations
- BaP
benzo(a)pyrene
- CoTx
concomitant treatment
- DATS
diallyl trisulfide
- DMSO
dimethylsulfoxide
- GSH
glutathione
- OSC
organosulfide compound
- PAH
polycyclic aromatic hydrocarbon
- PBS
phosphate buffered saline
- PreTx
pre-treatment
- ROS
reactive oxygen species
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboyade-Cole A, Darling-Reed S, Oriaku E, McCaskill M, Thomas R. Diallyl sulfide inhibits PhIP-induced cell death via the inhibition of DNA strand breaks in normal breast epithelial cells. Oncol Rep. 2008;20:319–323. [PMC free article] [PubMed] [Google Scholar]
- Ali M, Thompson M, Afzal M. Garlic and onions: their effect on eicosandoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids. 2000;62:55–73. doi: 10.1054/plef.1999.0124. [DOI] [PubMed] [Google Scholar]
- Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. 9C-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–5386. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- Augusti KT, Mathew PT. Lipid lowering effect of allicin (diallyl disulfide oxide) on long-term feeding in normal rats. Experientia. 1974;30:468–470. doi: 10.1007/BF01926297. [DOI] [PubMed] [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- Brodnitz MH, Pascale JV, van Derslice L. Flavor components of garlic extract. J Agric Food Chem. 1971;19:273–275. [Google Scholar]
- Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associate genes by benzo(a)pyrene 3,6-quinone and benzo(a)pyrene 1,6-quinone in MCF-10A human mammary epithelial cells. Toxicol Appl Pharmacol. 2007;221:203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Davis JW, Liu KJ, Hudson LG, Shi H, Monske ML, Burchiel SW. Benzo[a]pyrene Quinones Increase Cell Proliferation, Generate ROS, and Transactivate the Epidermal Growth Factor Receptor in Breast Epithelial Cells. Cancer Res. 2003;63:7825–7833. [PubMed] [Google Scholar]
- Caruso JA, Reiners JJ, Emond J, Shultz T, Tainsky MA, Alaoui-Jamali M, Batist G. Genetic alteration of chromosome 8 is a common feature of human mammary epithelial cell lines transformed in vitro with benzo[a]pyrene. Mutat Res. 2001;473:85–99. doi: 10.1016/s0027-5107(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Challier B, Perarnau JM, Viel JF. Garlic, onion, and cereal fibre as protective factors for breast cancer: A French case-control study. Eur J Epidemiol. 1998;14:737–747. doi: 10.1023/a:1007512825851. [DOI] [PubMed] [Google Scholar]
- Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AT. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor NRF2: Effect of chemical structure and stress signals. Free Radic Biol Med. 2004;27:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Corzo-Martinez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. [Google Scholar]
- Edwards NT. Polycyclic aromatic hydrocarbons (PAHs) in the environment: a review. J Environ Qual. 1983;12:427–441. [Google Scholar]
- Fukao T, Hosono T, Misawa A, Seki T, Ariga T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol. 2004;42:743–749. doi: 10.1016/j.fct.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hanelt S, Helbig R, Hartmann A, Lang M, Seidel A, Speit G. A comparative investigation of DNA adducts, DNA strand breaks and gene mutations induced by benzo[a]pyrene and ( )-anti-benzo[a]pyrene -7,8-diol 9,10-oxide in cultured human cells. Mutat Res. 1997;390:179–188. doi: 10.1016/s0165-1218(97)00019-0. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28528. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Stan SD, Hahm ER, Xiao D, Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related-checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic. Mol Cancer Ther. 2007;6:1249–1261. doi: 10.1158/1535-7163.MCT-06-0477. [DOI] [PubMed] [Google Scholar]
- Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki Y, Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem. 2005;280:41487–41493. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, Fraumeni JF. Allium vegetables and the risk of prostate cancer: A population-based study. J Natl Cancer Inst. 2002;94:1648–1651. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247–265. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Patel SJ, Ray SK. Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-Sy5Y cells. Apoptosis. 2007;12:671–684. doi: 10.1007/s10495-006-0024-x. [DOI] [PubMed] [Google Scholar]
- Keshava C, Divi RL, Whipkey DL, Frye BL, McCanlies E, Kuo M, Poirier MC, Weston A. Induction of CYP1A1 and CYP1B1 and formation of carcinogen-DNA adducts in normal human mammary epithelial cells treated with benzo[a]pyrene. Cancer Lett. 2005a;221:213–224. doi: 10.1016/j.canlet.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Keshava C, Whipkey D, Weston A. Transcriptional signatures of environmentally relevant exposures in normal human mammary epithelial cells: benzo[a]pyrene. Cancer Lett. 2005b;221:201–211. doi: 10.1016/j.canlet.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Kim YA, Xiao D, Xiao H, Powolyn AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon SA, Stampfer MR, Bartley J. Production of oxidative DNA damage during the metabolic activation of benzo[a]pyrene in human mammary epithelial cells correlates with cell killing. Proc of the Natl Acad Sci USA. 1988;85:4365–68. doi: 10.1073/pnas.85.12.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li HQ, Wang Y, Xu HX, Fan W, Wang M, Sun P, Xie X. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J (Engl) 2004;117:1155–1160. [PubMed] [Google Scholar]
- Li X, Yue Y, Zhou Y, Fan Y, Fan C, Huang Y, Wu F, Liu Y. An oil-free microemulsion for intravenous delivery of diallyl trisulfide: Formulation and evaluation. Int J Pharm. 2011;407:158–166. doi: 10.1016/j.ijpharm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu YY. Isolation of diallyl trisulfide inducible differentially expressed genes in human gastric cancer cells by modified cDNA representation difference analysis. DNA Cell Biol. 2002;21:771–780. doi: 10.1089/104454902320908423. [DOI] [PubMed] [Google Scholar]
- Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther. 2009;8:2175–85. doi: 10.4161/cbt.8.22.9882. [DOI] [PubMed] [Google Scholar]
- Mei X, Wang MC, Xu HX, Pan XP, Gao CY, Han N, Fu MY. Garlic and gastric cancer—The effect of garlic on nitrite and nitratein gastric juice. Acta Nutr Sin. 1982;4:53–58. [Google Scholar]
- Morris JJ, Seifter E. The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses. 1992;38:177–184. doi: 10.1016/0306-9877(92)90090-y. [DOI] [PubMed] [Google Scholar]
- Nishino H, Iwashima A, Itakura Y, Matsuura H, Fuwa T. Antitumor-promoting activity of garlic extracts. Oncology. 1989;46:277–280. doi: 10.1159/000226731. [DOI] [PubMed] [Google Scholar]
- Pliskova M, Vondracek J, Vejtesek B, Kozubik A, Machala M. Deregulation of cell proliferation by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells reflects both genotoxic and nongenotoxic events. Toxicol Sci. 2005;83:246–256. doi: 10.1093/toxsci/kfi040. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promega Technical Bulletin: CellTiter 96 AQueous One Solution Cell Proliferation Assay: Instructions for Use of Products G3580, G3581, and G3582. Promega; Madison, WI: 2005. pp. 1–2. [Google Scholar]
- Rivlin RS. Historical perspectives on the use of garlic. J Nutr. 2001;131:951S–954S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- Sadikovic B, Rodenhiser DI. Benzopyrene exposure disrupts DNA methylation and growth dynamics in breast cancer cells. Toxicol Appl Pharmacol. 2006;216:458–468. doi: 10.1016/j.taap.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer. 1997;29:152–156. doi: 10.1080/01635589709514617. [DOI] [PubMed] [Google Scholar]
- Seki T, Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Ogihara J, Ariga T. Anticancer effects of diallyl trisulfide derived from garlic. Asia Pac J Clin Nutr. 2008;17:249–52. [PubMed] [Google Scholar]
- Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Sigma Technical Bulletin: PeroxiDetect Kit For the Determination of Aqueous and Lipid Hydroperoxides. Sigma-Aldrich; St. Louis, MO: 2005. [Google Scholar]
- Sigounas G, Hairr JW, Cooke CD, Owen JR, Asch AS, Weidner DA, Wiley JE. Role of benzo[a]pyrene in generation of clustered DNA damage in human breast tissue. Free Radic Biol Med. 2010;49:77–87. doi: 10.1016/j.freeradbiomed.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Siriwardhana N, Wang H-CR. Precancerous carcinogenesis of human breast epithelial cells by chronic exposure to benzo[a]pyrene. Mol Carcinog. 2008;47:338–348. doi: 10.1002/mc.20392. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131–134. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. 1994;139:1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- Tampio M, Markkanen P, Puttonen KA, Hagelberg E, Heikkinen H, Huhtinen K, Loikkanen J, Hirvonen MR, Vahakangas KH. Induction of PUMA-alpha and down regulation of PUMA-beta expression is associated with benzo(a)pyrene-induced apoptosis in MCF-7 cells. Toxicol Lett. 2009;188:214–222. doi: 10.1016/j.toxlet.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Tannheimer S, Barton S, Ethier S, Burchiel S. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human epithelial cells. Carcinogenesis. 1997;18:1177–1182. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Lauer FT, Lane J, Burchiel SW. Factors influencing elevation of intracellular Ca2+ in the MCF-10A human mammary epithelial cell line by carcinogenic polycyclic aromatic hydrocarbons. Mol Carcinog. 1998;25:48–54. doi: 10.1002/(sici)1098-2744(199905)25:1<48::aid-mc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tsao S-M, Yin M-C. In vitro activity of garlic oil and four diallyl sulfides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J Antimicrob Chemother. 2001;47:665–70. doi: 10.1093/jac/47.5.665. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang HC, Yang JH, Hsieh SC, Sheen LY. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J Agric Food Chem. 2010a;58:7096–7103. doi: 10.1021/jf100613x. [DOI] [PubMed] [Google Scholar]
- Wang Y-B, Qin J, Zheng X-Y, Bai Y, Yang K, Xie L-P. Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomedicine. 2010b;17:363–368. doi: 10.1016/j.phymed.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Weisberger JH. Antimutagenesis and anticarcinogenesis from the past to the future. Mutat Res. 2001;480:23–35. doi: 10.1016/s0027-5107(01)00166-x. [DOI] [PubMed] [Google Scholar]
- Wu C-C, Sheen LY, Chen HW, Kuo WW, Tsai SJ, Lii CK. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem. 2002;50:378–383. doi: 10.1021/jf010937z. [DOI] [PubMed] [Google Scholar]
- Wu C-C, Chung J-G, Tsai S-J, Yang JH, Sheen LY. Differential affects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol. 2004;42:1937–1947. doi: 10.1016/j.fct.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Wu P-P, Liu K-C, Huang W-W, Chueh F-S, Ko Y-C, Chiu T-H, Lin J-P, Kuo J-H, Yang J-S, Chung J-G. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine. 2011;18:672–676. doi: 10.1016/j.phymed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Brisson M, Lazo JS, Singh SV. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc 25C. Oncogene. 2005;24:6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh S. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- Xiao D, Singh SV. Diallyl trisulfide, a garlic-derived organosulfide, causes G2/M arrest in PC-3 human prostate cancer cells by promoting proteasome-mediated degradation of Cdc25C phosphatase. Proc Am Assoc Cancer Res. 2003;44:197–202. [Google Scholar]
- Xiao D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533–540. doi: 10.1093/carcin/bgi228. [DOI] [PubMed] [Google Scholar]
- Yu FL, Bender W, Fang Q, Ludeke A, Welch B. Prevention of chemical carcinogen DNA binding and inhibition of nuclear RNA polymerase activity by organosulfur compounds as the possible mechanisms for their anticancer initiation and proliferation effects. Cancer Detect Prev. 2003;27:370–379. doi: 10.1016/s0361-090x(03)00135-1. [DOI] [PubMed] [Google Scholar]
- Yu TH, Wu CM, Liou YC. Volatile compounds from garlic. J Agric Food Chem. 1989;37:725–730. [Google Scholar]
- Zhang ZM, Yang XY, Deng SH, Xu W, Gao HQ. Anti-tumor effects of polybutylcyanoacrylate nanoparticles of diallyl trisulfide on orthotopic transplantation tumor model of hepatocellular carcinoma in BALB/c nude mice. Chin Med J (Engl) 2007;120:1336–1342. [PubMed] [Google Scholar]