Abstract
Objective
Recent scientific findings have reinvigorated interest in examining the role of γ-aminobutyric acid (GABA), the major inhibitory central nervous system neurotransmitter, in chronic pain conditions. Decreased inhibitory neurotransmission is a proposed mechanism in the pathophysiology of chronic pain syndromes such as fibromyalgia (FM). The purpose of this study was to test the hypothesis that decreased levels of insular and anterior cingulate GABA would be present in FM patients, and that the concentration of this neurotransmitter would be correlated with pressure–pain thresholds.
Methods
Sixteen FM patients and 17 age- and sex-matched healthy controls underwent pressure–pain testing and a 3T proton magnetic resonance spectroscopy session in which the right anterior insula, right posterior insula, anterior cingulate, and occipital cortex were examined in subjects at rest.
Results
GABA levels in the right anterior insula were significantly lower in FM patients compared with healthy controls (mean ± SD 1.17 ± 0.24 arbitrary institutional units versus 1.42 ± 0.32 arbitrary institutional units; P = 0.016). There was a trend toward increased GABA levels in the anterior cingulate of FM patients compared with healthy controls (P = 0.06). No significant differences between groups were detected in the posterior insula or occipital cortex (P > 0.05 for all comparisons). Within the right posterior insula, higher levels of GABA were positively correlated with pressure–pain thresholds in the FM patients (Spearman's rho = 0.63; P = 0.02).
Conclusion
Diminished inhibitory neurotransmission resulting from lower concentrations of GABA within the right anterior insula may play a role in the pathophysiology of FM and other central pain syndromes.
Fibromyalgia (FM) is the prototypical “central” chronic pain syndrome and is known to cause significant distress and disability in patients. FM is challenging to treat clinically and affects 2–4% of the US population (1). Several studies have suggested that a central nervous system–based processing problem causes the widespread pain sensitivity in these individuals (2). To date, neuroimaging studies have largely used functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and voxel-based morphometry techniques to look at differences in neuronal function and structure in the brains of patients with FM (2). There is increasing interest in the application of proton magnetic resonance spectroscopy (1H-MRS) to identify in vivo neurochemical markers that may contribute to these changes (3).
We and other investigators have previously used 1H-MRS to identify elevations of Glx (a combined measure of glutamate and glutamine) in FM patients within brain areas shown to be important in pain processing, including the right insula (4,5). Since glutamate is the main excitatory neurotransmitter in the central nervous system, these findings suggest that there may be an up-regulation in the excitatory activity that contributes to the generalized hyperalgesic state in central chronic pain syndromes such as FM.
In the central nervous system, γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter, and its role in pain processing has been recognized for some time (6). Of direct relevance to our study is the finding that lowering GABA levels within the insula enhances pain in an animal model (7). Studies in humans also show proof of concept that GABA plays a critical role in pain transmission, whereby baclofen, a GABAB agonist, has demonstrated effectiveness in preclinical models of acute and chronic pain (8). However, the failure of baclofen as well as benzodiazepines (which primarily bind to the GABAA receptor) to be effective analgesics in clinical trials has diminished interest regarding the role of GABA in chronic pain. Moreover, until recently, there was no way to directly test whether GABA plays a role in chronic pain states by measurement of this neurotransmitter using conventional 1H-MRS techniques, due to signal overlap and low signal intensity.
Several recent scientific findings have reinvigorated interest in examining the role of GABA in chronic pain. The drug sodium oxybate (also referred to as γ-hydroxybutyrate) has been recently shown to be highly effective in treating the pain, fatigue, and sleep difficulties in patients with FM (9). Moreover, recent advances in MR spectroscopy editing techniques now allow for direct quantification of GABA using a conventional 3T magnet (10). Therefore, in the present study, we investigated whether GABA levels in brain regions involved in pain processing (i.e., the pain matrix, which includes the anterior and posterior insula and anterior cingulate) are reduced in patients with FM compared to age- and sex-matched pain-free controls. We hypothesized that reduced GABA concentrations could result in excitation and hyperactivity in the insula, contributing to the hyperalgesia that is thought to be partially responsible for the symptoms seen in FM.
PATIENTS AND METHODS
Participants
Sixteen female patients with FM (mean ± SD age 37.2 ± 12.8 years) and 17 age- and sex-matched healthy control subjects (mean ± SD age 36.1 ± 11.7 years; P = 0.79) were studied. All participants gave written informed consent, and the study protocol was approved by the University of Michigan Institutional Review Board.
All participants with FM met the following inclusion criteria: 1) a diagnosis of FM for at least 1 year, according to the American College of Rheumatology 1990 criteria for FM (11); 2) continued presence of pain for more than 50% of each day; 3) limited introduction of any new medications or treatments for control of FM symptoms during the study; 4) age between 18 years and 75 years; 5) female; and 6) right-handed. Participants with FM were excluded if they had any of the following exclusion criteria: 1) current use or history of use of opioid or narcotic analgesics; 2) history of substance abuse; 3) presence of concurrent autoimmune or inflammatory disease that causes pain; 4) concurrent participation in other therapeutic trials; 5) pregnant or nursing; and/or 6) severe psychiatric illnesses, including current major depression.
All healthy control subjects were 1) between age 18 years and 75 years, 2) female, and 3) right-handed. Exclusion criteria for healthy controls were as follows: 1) having met the American College of Rheumatology 1990 criteria for FM; 2) having any chronic medical illness, including psychiatric disorders; and 3) current pregnancy.
Magnetic resonance spectroscopy
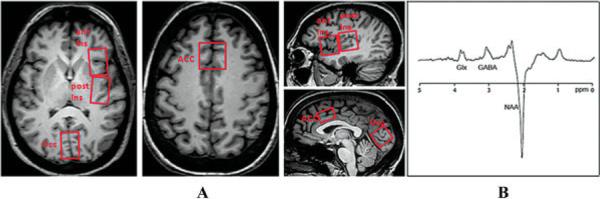
The brains of all subjects were imaged on a Philips Achieva 3T system, using an 8-channel receive head coil. We performed T1-weighted 3-dimensional MPRAGE imaging with an isotropic voxel resolution of (0.9 mm)3. MR spectra were acquired from 3.0 cm × 2.0 cm × 3.0 cm volumes of the right anterior insula, right posterior insula, anterior cingulate, and the occipital midline cortex (Figure 1). The right insula was chosen for detailed study, as we have previously demonstrated elevations in the levels of Glx in the right insular region (4). The occipital cortex was selected as a control region.
Figure 1.
Voxel placement and resulting spectrum on magnetic resonance spectroscopy. A, Axial and sagittal T1-weighted brain images obtained from fibromyalgia patients show single-voxel placements (boxed areas) for the right anterior insula (ant Ins), right posterior insula (post Ins), anterior cingulate (ACC), and occipital cortex (Occ). B, A representative proton magnetic resonance spectroscopy spectrum from the posterior insula is shown, as determined using the multi-echo single-voxel point-resolved spectroscopy technique edited for γ-aminobutyric acid (GABA). The peak for the combined measure of glutamine and glutamate (Glx) is resolved at 3.8 parts per million, and the peak for GABA is resolved at 3.0 ppm, with an inverted N-acetylaspartate (NAA) peak at 2.0 ppm.
Single-voxel point-resolved spectroscopy (PRESS) spectra (time to recovery [TR]/time to echo [TE] 2,000/35 msec) were acquired using VAPOR water suppression with 32 averages and a scan time of ~1 minute for each voxel. For the quantification of GABA, spectroscopy experiments using the Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) technique were performed, using the following parameters: TE 68 msec (TE1 15 msec, TE2 53 msec), TR 1.8 seconds, 256 transients of 2,000 data points, spectral width 2 kHz, frequency-selective editing pulses (14 msec) applied at 1.9 parts per million (the ON mode) and 7.46 ppm (the OFF mode). Refocusing was performed using the amplitude-modulated pulse GTST1203 (length 7 msec, bandwidth 1.2 kHz). The results from the conventional PRESS technique were analyzed using LCModel. The MEGA-PRESS results were analyzed using in-house postprocessing software in Matlab, with Gaussian curve fitting to the GABA and inverted N-acetylaspartate (NAA) peaks.
We measured GABA relative to the NAA signal in the edited spectra (12) to calculate a ratio based on the concentration of NAA, as generated by the MEGA-PRESS technique. After calculating this GABA:NAA ratio, we then multiplied this ratio by the NAA concentration, determined from LCModel analysis of a short-TE PRESS spectrum of the same voxel, which provides a concentration of NAA (in arbitrary institutional units [AIU]) relative to the values for water. Correction for cerebral spinal fluid was performed for each voxel, using statistical parameter mapping with the program SPM (Wellcome Trust Centre for Neuroimaging). Metabolite concentrations were used only for statistical analysis from the LCModel if the Cramér-Rao bounds were less than 20%.
Experimental pain
Pressure–pain tenderness was assessed prior to the MRS session using a previously described method (13). Briefly, discrete pressure stimuli were applied to the subject's left thumbnail using a stimulation device, which eliminates any direct examiner–subject interaction. Pain intensity ratings were recorded on the Gracely Box Scale questionnaire using a random presentation paradigm (13). During the testing, stimulus pressures were determined interactively: a computer program continuously adjusted stimulus pressure levels to produce the same response distribution in each subject. In 6 cases, pressure–pain testing was performed more than 3 months prior to imaging.
Statistical analysis
Pressure–pain thresholds and GABA levels were analyzed using PASW Statistics version 18. We performed 2-sample t-tests to determine differences in GABA levels between groups (FM patients versus healthy controls) for the 4 brain regions. We next calculated Spearman's correlations between pressure–pain thresholds and GABA levels from each of the interrogated brain regions. P values less than or equal to 0.05 were considered significant.
RESULTS
Comparison of brain GABA levels between FM patients and healthy controls
Due to an inadequate signal-to-noise ratio, the spectra determined by MEGA-PRESS were not assessed in the anterior cingulate of 2 FM patients and in the occipital cortex of 3 FM patients. Moreover, GABA spectra were unavailable for the occipital cortex of 2 control subjects. To gauge reproducibility, we remeasured the anterior cingulate GABA concentration 5 times in the brain of 1 subject, with a resulting coefficient of variance of 15%.
As shown in Figure 2, FM patients demonstrated lower levels of GABA within the right anterior insula (mean ± SD 1.17 ± 0.24 AIU) compared to healthy controls (1.42 ± 0.32 AIU; P = 0.016). Conversely, there was an unanticipated trend toward higher GABA levels in the anterior cingulate of FM patients (1.46 ± 0.25 AIU) compared to healthy controls (1.27 ± 0.27 AIU; P = 0.06) (Figure 2). There were no significant differences in the mean level of GABA between the FM patients and the healthy controls in either the right posterior insula or the occipital cortex.
Figure 2.
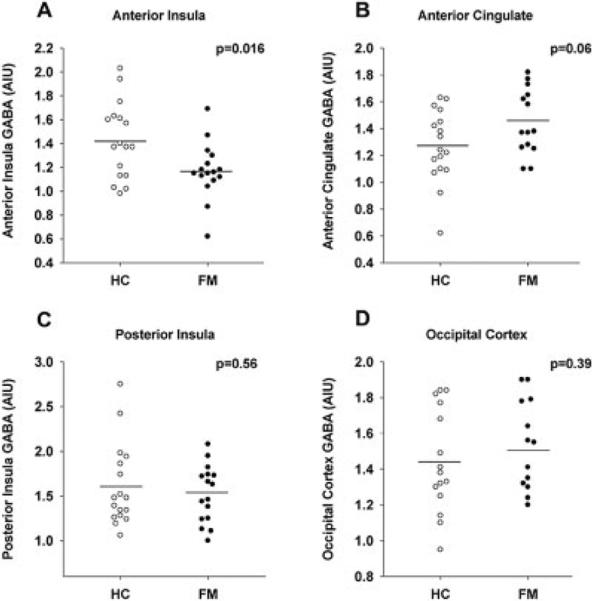
Levels of γ-aminobutyric acid (GABA) within the right anterior insula (A), anterior cingulate (B), right posterior insula (C), and occipital cortex (D) of individual patients with fibromyalgia (FM) compared with healthy controls (HC). FM patients have reduced concentrations of GABA in the right anterior insula, with a trend toward elevated concentrations of GABA in the anterior cingulate, as compared to healthy controls. There are no between-group differences in the right posterior insula or occipital cortex. Circles represent individual subjects; horizontal bars indicate the mean. AIU = arbitrary institutional units.
Positive correlation of posterior insula GABA levels with pressure–pain thresholds
Pressure–pain thresholds were obtained from 13 of the 16 patients with FM. There was a significant positive correlation between slightly intense pressure–pain thresholds and the right posterior insula GABA levels in the FM patients (Spearman's rho = 0.63; P = 0.02) (Figure 3). Patients with FM who have lower levels of GABA within the right posterior insula have higher sensitivity (i.e., lower thresholds) to experimentally induced pressure–pain. No significant correlations were detected in the right posterior insula of the healthy controls or in any other brain regions for either the FM patients or healthy controls.
Figure 3.
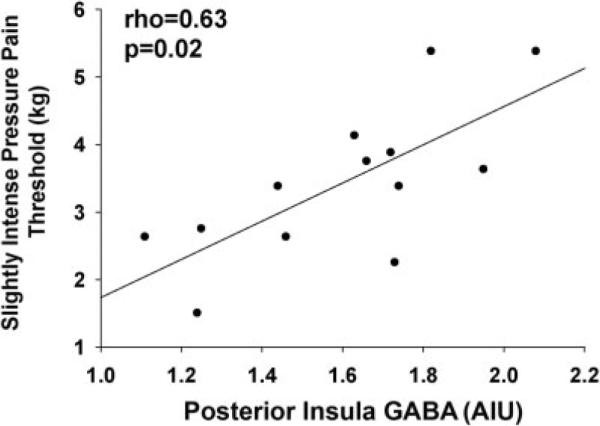
Levels of γ-aminobutyric acid (GABA) within the right posterior insula are positively correlated with pressure–pain thresholds in patients with fibromyalgia. Results are shown as a scatterplot of GABA concentrations in relation to slightly intense pressure–pain thresholds in individual patients (solid circles); the regression line is also shown. Correlations were assessed using Spearman's rho. AIU = arbitrary institutional units.
DISCUSSION
Our results suggest that GABA may play a critical role in the pathophysiology of FM. The right anterior insular levels of GABA were lower in the FM group compared to the healthy control group, and there was a positive correlation between the right posterior insular GABA levels and sensitivity to experimental pain in FM patients. Moreover, there was a trend toward higher GABA levels in the anterior cingulate of FM patients compared to healthy controls. These results suggest that relatively reduced levels of GABA in the insular regions are associated with FM symptoms.
The anterior insula is recognized as an area of the brain that is involved with the affective dimension of pain and emotional regulation, whereas the posterior insula is proposed to be involved more with sensory perception and processing of pain (14,15). Since the levels of GABA were lower in the anterior insula of the FM patients, this could suggest that GABA may be involved in the modulation of the affective dimension of pain in this patient population. Furthermore, the correlation of posterior insula GABA levels and experimental pain in FM patients may indicate that this inhibitory brain neurotransmitter may contribute to “setting the gain” on pain sensitivity in FM. A reduced level of GABA in the insula may reflect lack of inhibitor response and may therefore lead to heightened neuronal activity, which results in amplified nociceptive response.
We also demonstrated a trend toward increased GABA levels in the anterior cingulate in FM patients compared with healthy controls. The anterior cingulate has been implicated as an important pain-processing region of the brain in a number of fMRI studies. As both glutamatergic activity and GABAergic activity have been shown to contribute to brain metabolism and neuronal activity in functional imaging, it is unknown what portion of the increased activity seen on fMRI or PET imaging techniques is due to excitatory or inhibitory mechanisms (16). If this trend toward higher GABA levels in FM patients is indeed true, this may indicate modulation on the part of the anterior cingulate to inhibit neuronal response to chronic pain.
These alterations in GABA levels may represent differences in concentrations of GABA in the presynaptic terminals, synaptic vesicles, or in GABA uptake mechanisms. Although our 1H-MRS method cannot discern these possibilities, our findings are consistent with the notion of reduced GABAergic neurotransmission in pain-processing regions in FM. If this concept proves to be true, one would predict that patients with reduced insular levels of GABA may be more responsive to pharmacologic interventions that would enhance GABAergic neurotransmission. This hypothesis is consistent with the positive results of a recent phase III clinical trial of sodium oxybate, which acts as a GABA agonist, for the treatment of FM (9).
Given the cross-sectional design of this study, we cannot determine whether the reduced concentrations of GABA are causing the pain in FM or whether the reductions are a consequence of the pathologic processes of the disease. Moreover, the number of subjects included in the study was relatively small; however, our cohort is comparable to those used in studies of other functional and advanced neuroimaging techniques in FM. We acknowledge that the measurements of GABA and other metabolites by 1H-MRS represent the brain tissue volume, and this technique does not differentiate between intracellular and extracellular components. Edited measurements of GABA in 1H-MRS do not excite a pure GABA signal and are limited by coediting of the macromolecular signal. Although it is possible that the effects we observed were driven by changes in this macromolecular component, this study was based on prior observations of a strong association between central pain processing and GABAergic function, and we therefore interpret our observations as representing actual GABA levels.
We also did not collect information on the menstrual cycle or depression symptoms of any of the subjects, both of which may serve as potential mediators in any findings related to the role of GABA. In 6 of the FM patients, pressure testing was performed more than 3 months prior to imaging, which somewhat limits the observed correlation between the right posterior insula GABA levels and the pressure–pain thresholds. One final limitation is the relatively large size of the MRS voxels. The prescribed voxel size is required to achieve adequate signal-to-noise ratio for quantification of GABA using the MEGA-PRESS technique at 3T field strength.
In conclusion, we find that GABA alteration in the right insula is a potential pathologic mediator in FM. Prior findings indicating the presence of allodynia and hyperalgesia in these patients may therefore be explained by the alteration in inhibitory neurotransmission within the insula and possibly the anterior cingulate. The application of the MEGA-PRESS technique allows for the study of inhibitory GABAergic neurotransmission. These studies need to be replicated in larger cohorts of FM patients, as well as in patients with other chronic pain syndromes.
Acknowledgments
Ms Lowe owns stock or stock options in Philips Medical. Dr. Clauw has received consulting fees, speaking fees, and/or honoraria from Cypress Biosciences, Eli Lilly, Forest Laboratories, Jazz Pharmaceuticals, Merck, Pierre Fabre Pharmaceuticals USA, Pfizer, and UCB (more than $10,000 each) as well as a one-time royalty from Lilly for the knowfibro.com Web site and has received grant support from Forest Laboratories and Merck. Dr. Harris has received consulting fees from Pfizer (less than $10,000).
Supported by the US Department of the Army (grant DAMD-W81XWH-07-2-0050) and the Dana Foundation (Award in Brain and Immuno-Imaging).
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Foerster had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Foerster, Petrou, Edden, Sundgren, Schmidt-Wilcke, Harte, Clauw, Harris.
Acquisition of data. Foerster, Petrou, Edden, Sundgren, Lowe, Harris.
Analysis and interpretation of data. Foerster, Petrou, Edden, Sundgren, Harte, Harris.
REFERENCES
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Harris RE, Clauw DJ. How do we know that the pain in fibromyalgia is “real”? Curr Pain Headache Rep. 2006;10:403–7. doi: 10.1007/s11916-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 3.Borsook D, Moulton EA, Schmidt KF, Becerra LR. Neuroimaging revolutionizes therapeutic approaches to chronic pain. Mol Pain. 2007;3:25. doi: 10.1186/1744-8069-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valdes M, Collado A, Bargallo N, Vazquez M, Rami L, Gomez E, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 2010;62:1829–36. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- 6.Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, IV, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–20. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, et al. The effects of GABAB agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90:217–26. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 9.Russell IJ, Holman AJ, Swick TJ, Alvarez-Horine S, Wang YG, Guinta D, for the Sodium Oxybate 06-008 FM Study Group Sodium oxybate reduces pain, fatigue, and sleep disturbance and improves functionality in fibromyalgia: results from a 14-week, randomized, double-blind, placebo-controlled study. Pain. 2011;152:1007–17. doi: 10.1016/j.pain.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Edden RA, Barker PB. Spatial effects in the detection of γ-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–82. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 12.Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain. 2005;9:325–35. doi: 10.1016/j.ejpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–90. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 15.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 16.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–93. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]