Abstract
Spilanthes spp. are used as traditional herbal medicines in Africa and India to treat malaria. Yet, to date, there is no data on active constituents or most effective extraction methods for this indication. The isolated alkylamides, spilanthol and undeca-2E-ene-8,10-diynoic acid isobutylamide, found in S. acmella Murr., were shown to have IC50s of 16.5 μg/mL and 41.4 μg/mL on Plasmodium falciparum strain PFB and IC50s of 5.8 μg/mL and 16.3 μg/mL for the chloroquine resistant P. falciparum K1 strain, respectively. Further investigations revealed that at relatively low concentrations, spilanthol and the water extract of S. acmella reduced the parasitemia 59% and 53% in mice infected with P. yoelii yoelii 17XNL at 5 mg/kg and 50 mg/kg, respectively. Unexpectedly, the 95% ethanol extract of S. acmella was less effective (36% reduction in parasitemia) at 50 mg/kg. These results provide the first evidence supporting S. acmella against malaria and demonstrating active constituents in S. acmella against P. falciparum.
Keywords: Plasmodium falciparum; alkylamides; deca-2E,6Z,8E-trienoic acid isobutylamide; malaria; traditional medicine; phytotherapy
INTRODUCTION
The majority of countries coping with malaria spend less than US $10 per capita annually on healthcare, resulting in a situation where drug costs of 50 cents or less are economic deterrents to treatment (White, 2004). Perhaps due to such economics, and the lack of access to healthcare facilities, medicinal plant preparations remain a well utilized option for the treatment of malaria by the rural poor (Spelman, 2009). Spilanthes acmella Murr. (Asteraceae; syn. Blainvillea acmella (L.) Philipson) is one such plant from the traditional pharmacopoeia that is reported to be useful in the treatment of malaria. A related species, S. oleracea L., is a component of a formula known as Malarial-5, produced and sanctioned by the National Institute of Public Health in Mali for the treatment of malaria, relying primarily on ethnobotanical indications as evidence for treatment (Keita et al., 1990).
Several bioactive compounds have thus far been isolated and characterized from S. acmella which includes alkylamides and flavonoids. The N-alkylamides are fatty acid derivatives and have been identified in several species of Spilanthes (Greger, 1988). Early work found spilanthol, also known as affinin or deca-2E,6Z,8E-trienoic acid isobutyl amide, a local anesthetic, as the main lipidic component (Gerber, 1903). More recent work has found acetylenic alkylamides such as undeca-2E-en-8,10-diynoic acid isobutylamide (UDA) in lower quantities (Bae et al., 2010). However, these compounds and the extracts of S. acmella, have never been assessed for antiplasmodial activity.
MATERIALS AND METHODS
Plant Material and Extractions
Cultivation of S. acmella took place in Williams, OR at Horizon Herbs. Fresh, whole plants of S. acmella were harvested. Species was verified by Richard Cech (Horizon Herbs, Williams, OR) and voucher specimens were submitted to the University of North Carolina Herbarium in Chapel Hill, NC (accession numbers 583423 and 583424). The plants were one year old at time of harvest. A typical protocol for the manufacture of dry root ethanolic extracts was followed and has been described previously (Spelman et al., 2009b). After extraction, extracts were concentrated to 10 mg/mL by speedvac for in vitro and in vivo experiments.
Isolation of spilanthol
A CombiFlash system (Teledyne, Lincoln, Nebraska, USA) with a 130 g C18 column was used for the separation. A gradient of isocratic separation was conducted with mobile phase composition of 50% A and 50% B, where A = 1% acetic acid (Fisher Chemical, Pittsburgh, PA, USA) in nanopure water and B = HPLC grade acetonitrile (Pharmaco-AAPER, Shelbyville, KT, USA). A flow rate of 0.2 mL/min was used, the injection volume was 10 μL, detection wavelength 229 nm and the total analysis time was 10 min. An ion trap mass spectrometer with electrospray ionization source (LCQ Advantage, ThermoFisher, San Jose, CA) was employed for quantification of spilanthol content and for identification of other alkylamides and has been published previously. The purity of the spilanthol was estimated to be 84% spilanthol and 13% spilanthol isomers, and an alkylamide of (MH+) of 236 m/z at 2.2% previously identified as deca-2E,6Z,8E-trienenoic acid 2-methylbutylamide (Bae et al., 2010). UDA was purchased from Chromadex (Santa Ana, CA, USA).
Biological assays-in vitro and in vivo.
The IC50s of spilanthol and UDA were determined against the chloroquine-resistant K1 strain and the mildly chloroquine-resistant strain PFB of Plasmodium falciparum using a previously established method (Desjardins et al., 1979). The growth inhibition was assessed in triplicate by comparison of the radioactivity incorporated into the treated culture with that in control culture from the same plate. The in vivo experiments were carried out with 5 week old male and female Swiss mice weighing 23.2 g (±0.67) (Elevage Janvier, La Genest Saint Isle, France) were housed in standard environmental conditions (24±1.8 C, 55% humidity). The experiments were conducted in accordance with the EEC Directive of 1986 (86/609/EEC) on laboratory animals. Briefly, extracts of Spilanthes acmellla and spilanthol (25 mg/kg and 2.5 mg/kg twice daily, respectively) in a PBS methylcellulose solution (1%) were injected intraperitonealy into each animal 2 hours after inoculation with 107 Plasmodium yoelii yoelii parasites. Injections were continued twice a day for 4 days and experimental groups consisted of 5 mice (1 male and 4 females) per group. Parasitaemia was monitored daily by microscopic examination of Giemsa stained-thin blood smears.
Statistical analysis
Data is expressed as the mean ± SEM and comparison of means was conducted using a two tailed t-test for paired data when differences were observed. The mean values were considered significantly different if p < or = 0.01.
RESULTS AND DISCUSSION
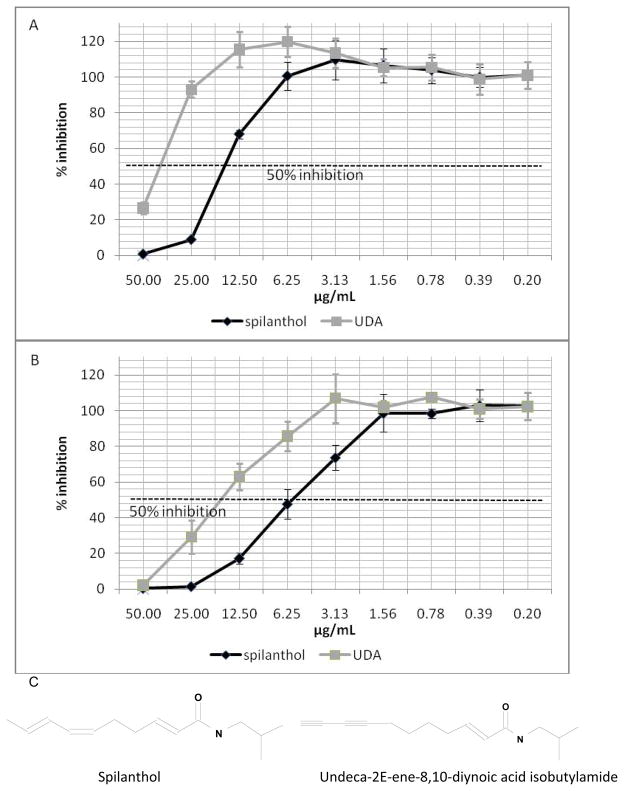
Figure 1 illustrates the IC50s for the tested alkylamides, spilanthol and UDA on P. falciparum in vitro. For the Brazilian mildy chloroquine resistant strain PFB (Figure 1A), the IC50s for spilanthol and UDA are 16.5 and 41.4 μg/mL, respectively. While for the Thailanese chloroquine resistant strain K1 (Figure 1B), the effect of the alkylamides is significantly greater, with IC50s of 5.8 and 16.3 μg/mL, respectively.
Figure 1. Spilanthol and undeca-2E-ene-8,10-diynoic acid isobutylamide(UDA) in vitro inhibition of P.falciparum strains.
1A Spilanthol and UDA show IC50son the P. falciparum strain PFB at 16.5 and 41.4 μg/mL.
1B Spilanthol and UDA demonstrate IC50s of5.8 and 16.3 μg/mL on the chloroquine resistantstrain P. falciparum K1.
1C . Molecular structures of spilanthol and UDA.
Growth inhibition was determined by comparison of the radioactivity incorporated into the treated culture with that in control culture from the same plate. Chloroquine served as a positive control (IC50s: PFB–28.4 nM; K1–100 nM). Values are mean± S.E.M.of experiments performed in triplicate.
Because Spilanthes remedies are commonly prepared as tea, further studies into S. acmella were performed in vivo on P. yoellii yoelii-infected mice using a whole plant galenic extract (an extract of herb/vegetable origin) of 100% water (10 mg/mL). In addition, another form of traditional remedy, a fresh plant ethanolic extract (10 mg/mL, 70% ethanol final volume) was also utilized. Ethanol extracts have been shown to contain ten times the concentrations of spilanthol as compared to aqueous extracts (Bae et al., 2010). As seen in Figure 2, the control group had an average parasitemia of 17.7% (± 3.3) five days after infection. A significant reduction of parasitemia by treatment with spilanthol (5 mg/kg) and S. acmella water extract (50 mg/kg) was observed with parasitemia decreased to 7.3% ± 1.4 and 8.4% ± 1.7, respectively (p < 0.001). The average parasitemia of the Spilanthes acmella ethanol extract (50 mg/kg) group after 5 days was 11.3% ± 2.0 (p = 0.01). Thus, isolated spilanthol and water extract exhibited the highest activity, 59% and 53% reductions in parasitemia, while the ethanol extract showed a 36% reduction in parasitemia under these experimental conditions.
Figure 2. Spilanthol and extracts of whole plant S. acmella reduce parasitemia in Plasmodium y. yoelli-infected mice.
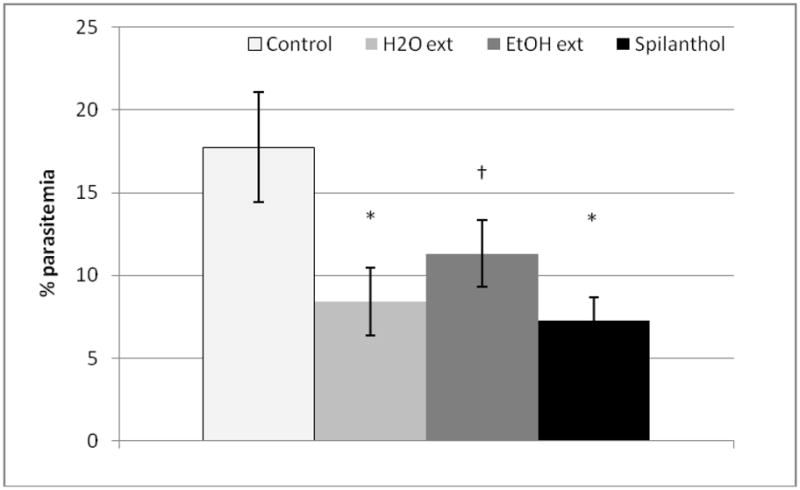
Spilanthol, water extract, or (70%) ethanol extract of Spilanthes acmella inhibit parasitemia as compared to the control group. Mice were inoculated with P. yoelli yoelli 17XNLand treatment started 2 hours later. Treatments (twice daily spilanthol 2.5mg/kg; water extract 25 mg/kg; ethanol extract 25mg/kg) were given two times aday for four days. Parasitaemia was determined 5 days after infection by microscopic examination of Giemsa stained-thin blood smears. Values are mean± S.E.M. (n = 5 for each group). †p = 0.01; *p< 0.001
This suggests that in addition to spilanthol, there may be water soluble constituents that are also active against Plasmodium. Common hydrophilic phytochemicals have previously been shown to potentiate known antimalarials (Soh et al., 2009). Moreover, there is likely multiple modes of activity for the observed effect of Spilanthes extract. For example, the treatments could have also induced immunological activity contributing to a reduction in parasitemia. Recent studies on alkylamides structurally similar to spilanthol, and UDA specifically, demonstrate immunological activity, particularly cytokine modulation, at concentrations below 1.5 μM (Matthias et al., 2008; Spelman et al., 2009a). Further investigations are necessary to determine the viability of this traditional medicine, and its lead compounds, for the treatment of malaria.
Acknowledgments
This publication was made possible by grant number 1R15AT001466-01 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health and the Marie Curie Foundation which provided an early stage training fellowship for KS at the Muséum National d’Histoire Naturelle, Paris and the Laboratorie of Pharmacognosy, Université Paris 11. Additional support was provided by the National Science Foundation under grant number 0420292. Special thanks to Nadja Cech for providing the spilanthol and S. acmella extracts.
Abbreviations
- K1
a strain of Plasmodium falciparum originating from Tailand
- PFB
a strain of Plasmodium falciparum originating from Brazil
- UDA
undeca-2E-en-8,10-diynoic acid isobutylamide
Footnotes
CONFLICT OF INTEREST
None reported.
References
- Bae SS, Ehrmann BM, Ettefagh KA, Cech NB. A validated liquid chromatography-electrospray ionization-mass spectrometry method for quantification of spilanthol in Spilanthes acmella (L.) Murr. Phytochem Anal. 2010;5:438–43. doi: 10.1002/pca.1215. [DOI] [PubMed] [Google Scholar]
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710 –718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber E. Ueber die chemischen Bestandteile der Parakresse (Spilanthes olearacea, Jacquin) Arch Pharm. 1903;241:270–89. [Google Scholar]
- Greger H. Comparitive phytochemistry of the alkylamides. In: Lam J, Breteler H, Arnason T, Hansen L, editors. Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC) v7. Elsevier; Amersterdam; New York: 1988. pp. 159–78. [Google Scholar]
- Keita A, Doumbo O, Koita N, Diallo D, Guindo M, Traore AK. Etude preliminaire sur la faisabilite d’un protocole d’essai clinique. Bull Med Trad Pharm. 1990;4:139–46. [Google Scholar]
- Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53–8. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Soh PN, Witkowski B, Olagnier D, Nicolau ML, Garcia-Alvarez MC, Berry A, Benoit-Vical F. In vitroand in vivo properties of ellagic acid in malaria treatment. Antimicrob Agents Chemother. 2009;53:1100–6. doi: 10.1128/AAC.01175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman K. “Silver Bullet” Drugs Vs. Traditional Herbal Remedies: Perspectives on Malaria. HG J Am Bot Counc. 2009;84:44–55. [Google Scholar]
- Spelman K, Iiams-Hauser K, Cech NB, Taylor EW, Smirnoff N, Wenner CA. Role for PPARγ in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int Immunopharmacol. 2009;9:1260–1264. doi: 10.1016/j.intimp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Spelman K, Wetschler MH, Cech NB. Comparison of alkylamide yield in ethanolic extracts prepared from fresh versus dry Echinacea purpurea utilizing HPLC-ESI-MS. J Pharm Biomed Anal. 2009;49:1141–9. doi: 10.1016/j.jpba.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–92. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]